Charity-Provided Community-Based PSA Testing for Assessment of Prostate Cancer Risk in the UK: Clinical Implications and Future Opportunities
Simple Summary
Abstract
1. Background
2. Materials and Methods
2.1. Data Collection
2.2. PSA Risk Stratification
2.3. Outcome Data
2.4. Data Analysis
3. Results
3.1. Risk Group Analysis
3.2. PSA Risk Stratification (As Green, Amber, and Red) and Riskman Score Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, E.H.; Burn, E.; Barclay, N.L.; Delmestri, A.; Man, W.Y.; Golozar, A.; Serrano, À.R.; Duarte-Salles, T.; Cornford, P.; Prieto Alhambra, D.; et al. Incidence, Prevalence, and Survival of Prostate Cancer in the UK. JAMA Netw. Open 2024, 7, e2434622. [Google Scholar] [CrossRef] [PubMed]
- Dee, E.C.; Todd, R.; Ng, K.; Aidoo-Micah, G.; Amen, T.B.; Moon, Z.; Vince, R., Jr.; Muralidhar, V.; Mutsvangwa, K.; Funston, G.; et al. Racial disparities in prostate cancer in the UK and the USA: Similarities, differences and steps forwards. Nat. Rev. Urol. 2024, 22, 223–234. [Google Scholar] [CrossRef]
- Chandran, A.; van Harten, M.; Singh, D.; Vilaseca, J.; Patasius, A.; Tupikowski, K.; Amorín, Á.G.; Galvin, D.; López, H.; Salazar, J.P.; et al. Risk-stratified Approach to Implementing Population-based Prostate Cancer Screening in Five Pilot Sites in the European Union: A Protocol for the PRAISE-U Project. Eur. Urol. Open Sci. 2024, 70, 8–17. [Google Scholar] [CrossRef]
- UK National Screening Committee. Prostate Cancer Screening Recommendations. Available online: https://view-health-screening-recommendations.service.gov.uk/prostate-cancer/ (accessed on 25 January 2025).
- Wang, M.C.; Papsidero, L.D.; Kuriyama, M.; Valenzuela, L.A.; Murphy, G.P.; Chu, T.M. Prostate antigen: A new potential marker for prostatic cancer. Prostate 1981, 2, 89–96. [Google Scholar] [CrossRef]
- Catalona, W.J. History of the discovery and clinical translation of prostate-specific antigen. Asian J. Urol. 2014, 1, 12–14. [Google Scholar] [CrossRef]
- Heijnsdijk, E.A.; der Kinderen, A.; Wever, E.M.; Draisma, G.; Roobol, M.J.; de Koning, H.J. Overdetection, overtreatment and costs in prostate-specific antigen screening for prostate cancer. Br. J. Cancer 2009, 101, 1833–1838. [Google Scholar] [CrossRef]
- Lophatananon, A.; Muir, K.R.; Gnanapragasam, V.J. The efficacy of different biomarkers and endpoints to refine referrals for suspected prostate cancer: The TARGET study (Tiered integrAted tests for eaRly diaGnosis of clinically significant ProstatE Tumours). BMC Med. 2024, 22, 440. [Google Scholar] [CrossRef]
- Barry, M.J.; Fowler, F.J., Jr.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J. Urol. 1992, 148, 1549–1557; discussion 1564. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Suspected Cancer: Recognition and Referral. Available online: https://www.nice.org.uk/guidance/ng12/chapter/Recommendations-organised-by-site-of-cancer#urological-cancers (accessed on 13 May 2025).
- Nam, R.K.; Satkunavisam, R.; Chin, J.L.; Izawa, J.; Trachtenberg, J.; Rendon, R.; Bell, D.; Singal, R.; Sherman, C.; Sugar, L.; et al. Next-generation prostate cancer risk calculator for primary care physicians. Can. Urol. Assoc. J. J. L’association Urol. Can. 2018, 12, E64–E70. [Google Scholar] [CrossRef]
- StataCorp LLC. StataCorp; Stata Statistical Software: Release 17; StataCorp LLC: College Station, TX, USA, 2021. [Google Scholar]
- Teo, C.H.; Ng, C.J.; Booth, A.; White, A. Barriers and facilitators to health screening in men: A systematic review. Soc. Sci. Med. 2016, 165, 168–176. [Google Scholar] [CrossRef]
- Lilja, H.; Ulmert, D.; Vickers, A.J. Prostate-specific antigen and prostate cancer: Prediction, detection and monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Prcic, A.; Begic, E.; Hiros, M. Actual Contribution of Free to Total PSA Ratio in Prostate Diseases Differentiation. Med. Arch. 2016, 70, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Filella, X.; Alcover, J.; Molina, R.; Rodríguez, A.; Carretero, P.; Ballesta, A.M. Free and total PSA in the diagnosis of prostate cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 1997, 18, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Lillard Jr, J.W.; Moses, K.A.; Mahal, B.A.; George, D.J. Racial disparities in Black men with prostate cancer: A literature review. Cancer 2022, 128, 3787–3795. [Google Scholar] [CrossRef]
- Mahal, B.A.; Gerke, T.; Awasthi, S.; Soule, H.R.; Simons, J.W.; Miyahira, A.; Halabi, S.; George, D.; Platz, E.A.; Mucci, L.; et al. Prostate Cancer Racial Disparities: A Systematic Review by the Prostate Cancer Foundation Panel. Eur. Urol. Oncol. 2022, 5, 18–29. [Google Scholar] [CrossRef]
- Beyer, K.; Leenen, R.; Venderbos, L.D.F.; Helleman, J.; Denijs, F.; Bramer, W.; Vasilyeva, V.; Briers, E.; Rivas, J.G.; Chloupkova, R.; et al. Health Policy for Prostate Cancer Early Detection in the European Union and the Impact of Opportunistic Screening: PRAISE-U Consortium. J. Pers. Med. 2024, 14, 84. [Google Scholar] [CrossRef]
- Shipe, M.E.; Deppen, S.A.; Farjah, F.; Grogan, E.L. Developing prediction models for clinical use using logistic regression: An overview. J. Thorac. Dis. 2019, 11, S574–S584. [Google Scholar] [CrossRef]
- Prostate Cancer UK. TRANSFORM Trial. Available online: https://prostatecanceruk.org/research/transform-trial (accessed on 25 January 2025).
- Ahmed, H.U.; El-Shater Bosaily, A.; Brown, L.C.; Gabe, R.; Kaplan, R.; Parmar, M.K.; Collaco-Moraes, Y.; Ward, K.; Hindley, R.G.; Freeman, A.; et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet 2017, 389, 815–822. [Google Scholar] [CrossRef]
- Vassy, J.L.; Dornisch, A.M.; Karunamuni, R.; Gatzen, M.; Kachulis, C.J.; Lennon, N.J.; Brunette, C.A.; Danowski, M.E.; Hauger, R.L.; Garraway, I.P.; et al. From a genomic risk model to clinical trial implementation in a learning health system: The ProGRESS Study. medRxiv 2024. [Google Scholar] [CrossRef]
- Seibert, T.M.; Fan, C.C.; Wang, Y.; Zuber, V.; Karunamuni, R.; Parsons, J.K.; Eeles, R.A.; Easton, D.F.; Kote-Jarai, Z.; Al Olama, A.A.; et al. Polygenic hazard score to guide screening for aggressive prostate cancer: Development and validation in large scale cohorts. BMJ 2018, 360, j5757. [Google Scholar] [CrossRef]
- Kachuri, L.; Hoffmann, T.J.; Jiang, Y.; Berndt, S.I.; Shelley, J.P.; Schaffer, K.R.; Machiela, M.J.; Freedman, N.D.; Huang, W.-Y.; Li, S.A.; et al. Genetically adjusted PSA levels for prostate cancer screening. Nat. Med. 2023, 29, 1412–1423, Erratum in Nat. Med. 2025, 31, 697. [Google Scholar] [CrossRef] [PubMed]
- Grönberg, H.; Eklund, M.; Picker, W.; Aly, M.; Jäderling, F.; Adolfsson, J.; Landquist, M.; Haug, E.S.; Ström, P.; Carlsson, S.; et al. Prostate Cancer Diagnostics Using a Combination of the Stockholm3 Blood Test and Multiparametric Magnetic Resonance Imaging. Eur. Urol. 2018, 74, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Proteomedix. Proclarix® Next Generation Blood Test for Prostate Cancer. Available online: https://www.proteomedix.com/en/ (accessed on 25 January 2025).
- Randox Health. Advanced PSA Test. Available online: https://randoxhealth.com/en-GB/in-clinic/advanced-psa?srsltid=AfmBOoobr3ED02FtJrPNJX0ctdCPdj0VowWH0ObYZtAhu_jaoFRNfo0O (accessed on 25 January 2025).
- Gudmundsson, J.; Besenbacher, S.; Sulem, P.; Gudbjartsson, D.F.; Olafsson, I.; Arinbjarnarson, S.; Agnarsson, B.A.; Benediktsdottir, K.R.; Isaksson, H.J.; Kostic, J.P.; et al. Genetic correction of PSA values using sequence variants associated with PSA levels. Sci. Transl. Med. 2010, 2, 62ra92. [Google Scholar] [CrossRef] [PubMed]
- Stockholm3. Finding Aggressive Prostate Cancer Earlier. Available online: https://www.stockholm3.com/ (accessed on 25 January 2025).

| Age | <1.50 | 1.50–2.49 | 2.50–2.99 | 3.00–3.99 | >4.00 |
| 40–49 | |||||
| 50–69 | |||||
| 70 and over |
| Age | Highest PSA(ng/mL) | Average PSA(ng/mL) | No of Tests |
|---|---|---|---|
| <30 | 6.6 | 0.79 | 217 |
| 30–39 | 20.6 | 0.81 | 1775 |
| 40–49 | 72.7 | 0.9 | 43,407 |
| 50–59 | 952 | 1.31 | 99,197 |
| 60–69 | 1803 | 2.1 | 104,277 |
| 70–79 | 12,463 | 3.04 | 68,584 |
| 80–89 | 361 | 3.44 | 9441 |
| 90–99 | 139 | 4.59 | 229 |
| >100 | 1.6 | 1.55 | 2 |
| All ages | - | - | 327,129 |
| Variables | Non-Prostate Cancer Group (N (%)) | Prostate Cancer Group (N (%)) |
|---|---|---|
| No = 1946 | No = 476 | |
| Age (years old) | range:34.0–90.0 | range:43.0–84.0 |
| mean 64.7 SD * (8.9) | mean 65.6 SD * (7.9) | |
| Unknown | 309 (15.9%) | 59 (12.4%) |
| PSA (ng/mL) | range:0.0–260.3 | range:0.0–242.0 |
| mean 5.8 SD * (8.0) | mean 13.9 SD * (23.4) | |
| Unknown | 302 (15.5%) | 58 (12.2%) |
| PSAft% | range: 0.0–22.8 | range: 0.0–9.6 |
| median 0.78 IQR ** (0.43–1.17) | median 0.77 IQR ** (0.29–1.35) | |
| 433 (22.3%) | 63 (13.2%) | |
| Ethnicity | ||
| African-Caribbean | 7 (0.4%) | 2 (0.4%) |
| Asian | 5 (0.3%) | 1 (0.2%) |
| Mixed Race | 8 (0.5%) | 1 (0.2%) |
| Other | 7 (0.4%) | 1 (0.2%) |
| White European | 1551 (93.7%) | 445 (93.5%) |
| White Other | 77 (4.7%) | 26 (5.5%) |
| Unknown | 291 (15%) | 0 (0%) |
| IPSS | ||
| Mild | 615 (47.5%) | 152 (43.8%) |
| Moderate | 578 (44.6%) | 165 (47.6%) |
| Severe | 102 (7.9%) | 30 (8.6%) |
| Unknown | 651 (33.5%) | 129 (27.1%) |
| Family history of prostate cancer | ||
| No | 1489 (95.4%) | 424 (94.9%) |
| Yes | 72 (4.6%) | 23 (5.1%) |
| Unknown | 385 (19.8%) | 29 (6.1%) |
| Group | Numbers | Mean PSA (ng/mL) | SD * | Min. | Max. |
|---|---|---|---|---|---|
| Green | 253 | 1.72 | 1.15 | 0.03 | 5.65 |
| Amber | 680 | 4.47 | 1.37 | 2.00 | 17.90 |
| Red | 1115 | 10.56 | 17.14 | 2.53 | 260.29 |
| Prostate Cancer Grade Group | No (%) | Mean PSA (ng/mL) | SD * |
|---|---|---|---|
| No cancer | 1644 (79.7) | 5.8 | 8.0 |
| Group 1 | 73(3.5) | 8.8 | 9.9 |
| Group 2 | 186 (9.0) | 10.8 | 18.6 |
| Group 3 | 78 (3.8) | 13.3 | 14.9 |
| Group 4 | 38 (1.8) | 23.9 | 36.0 |
| Group 5 | 43 (2.1) | 28.1 | 42.4 |
| Total | 2062 | 7.4 | 13.1 |
| Approach | Prostate Cancer Grade Group | Variables | Odd Ratios | 95% C.I. | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| GFCT risk stratification | Grade Group ≥ 1 | Age | 1.013 | 0.999 | 1.026 |
| Green (Reference) | 1.000 | ||||
| Amber | 2.757 | 1.233 | 6.166 | ||
| Red | 16.545 | 7.723 | 35.442 | ||
| Total no | 2050 | ||||
| Grade Group ≥ 3 | Age | 1.038 | 1.017 | 1.059 | |
| Green (Reference) | 1.000 | ||||
| Amber | 1.745 | 0.497 | 6.133 | ||
| Red | 15.222 | 4.804 | 48.228 | ||
| Total no | 1794 | ||||
| Riskman score | Grade Group ≥ 1 | Age | 0.997 | 0.983 | 1.011 |
| PSA | 1.153 | 1.124 | 1.183 | ||
| PSAft% * | 0.585 | 0.492 | 0.697 | ||
| Total no | 1920 | ||||
| Grade Group ≥ 3 | Age | 1.024 | 0.998 | 1.050 | |
| PSA | 1.158 | 1.117 | 1.200 | ||
| PSAft% * | 0.467 | 0.310 | 0.703 | ||
| Total no | 1371 | ||||
| Approach | Prostate Cancer Grade Group ≥ 1 | Prostate Cancer Grade Group ≥ 3 | ||||
|---|---|---|---|---|---|---|
| AUC | 95%C.I | AUC | 95%C.I | |||
| Lower | Upper | Lower | Upper | |||
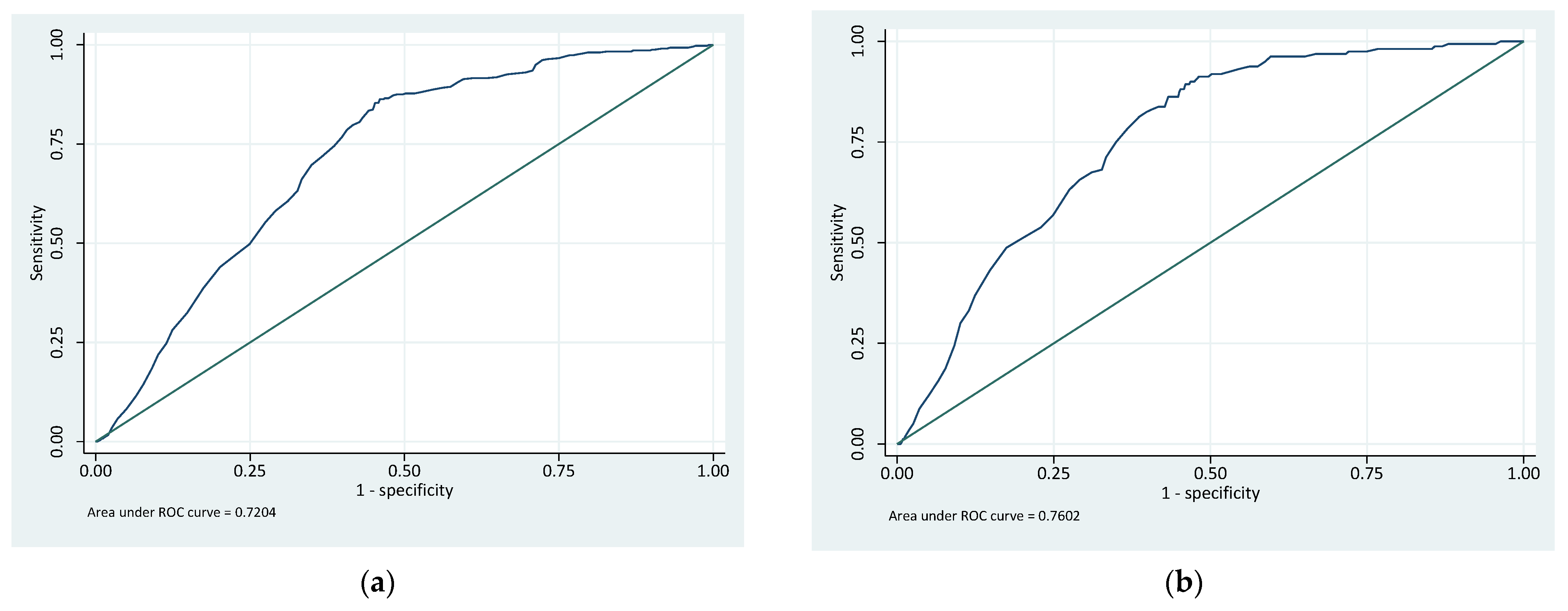
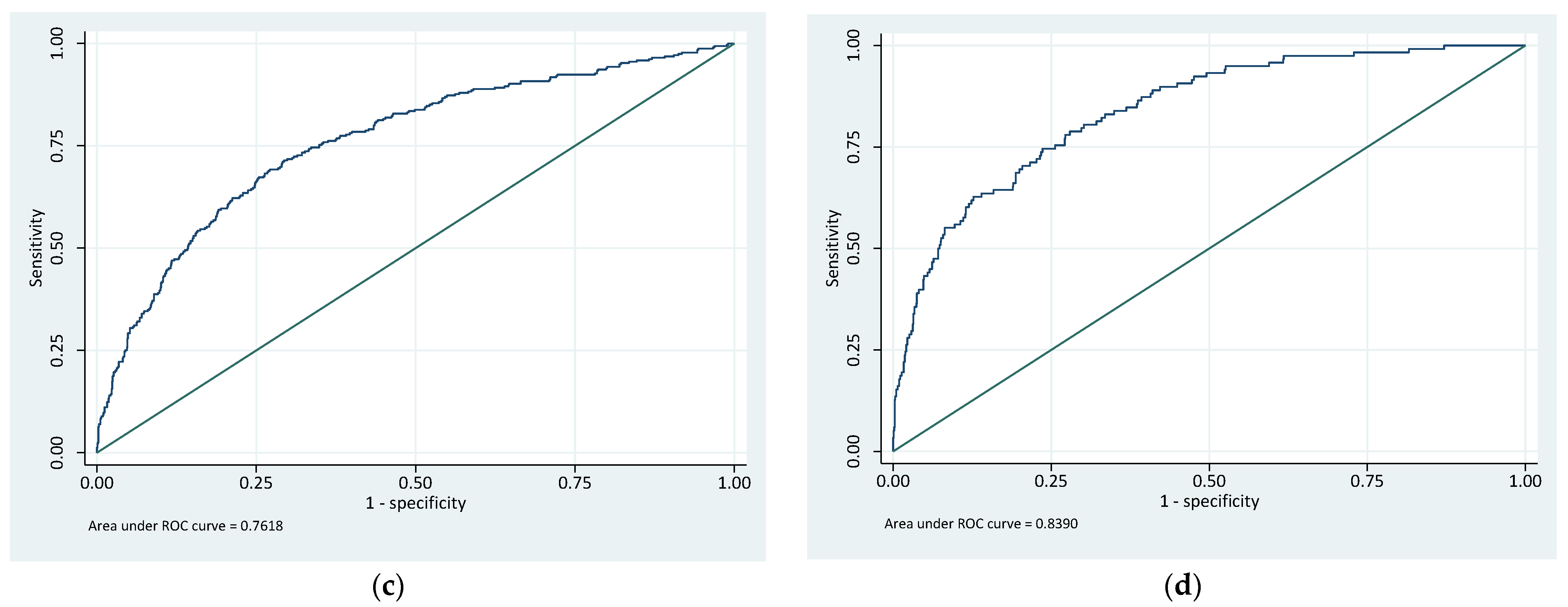
| GFCT risk stratification | 0.72 | 0.70 | 0.74 | 0.76 | 0.73 | 0.79 |
| Riskman | 0.76 | 0.74 | 0.79 | 0.84 | 0.80 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lophatananon, A.; Fulford, G.; Young, J.; Hart, S.; Brine, M.; Muir, K.R. Charity-Provided Community-Based PSA Testing for Assessment of Prostate Cancer Risk in the UK: Clinical Implications and Future Opportunities. Cancers 2025, 17, 1728. https://doi.org/10.3390/cancers17101728
Lophatananon A, Fulford G, Young J, Hart S, Brine M, Muir KR. Charity-Provided Community-Based PSA Testing for Assessment of Prostate Cancer Risk in the UK: Clinical Implications and Future Opportunities. Cancers. 2025; 17(10):1728. https://doi.org/10.3390/cancers17101728
Chicago/Turabian StyleLophatananon, Artitaya, Graham Fulford, Jon Young, Susan Hart, Matthew Brine, and Kenneth R. Muir. 2025. "Charity-Provided Community-Based PSA Testing for Assessment of Prostate Cancer Risk in the UK: Clinical Implications and Future Opportunities" Cancers 17, no. 10: 1728. https://doi.org/10.3390/cancers17101728
APA StyleLophatananon, A., Fulford, G., Young, J., Hart, S., Brine, M., & Muir, K. R. (2025). Charity-Provided Community-Based PSA Testing for Assessment of Prostate Cancer Risk in the UK: Clinical Implications and Future Opportunities. Cancers, 17(10), 1728. https://doi.org/10.3390/cancers17101728






