Lung Cancer Screening—Trends and Current Studies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Lung Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 20 April 2024).
- Li, C.; Lei, S.; Ding, L.; Xu, Y.; Wu, X.; Wang, H.; Zhang, Z.; Gao, T.; Zhang, Y.; Li, L. Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. 2023, 136, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Branstetter, S.; Lazarus, P.; Muscat, J.E. Smoking, Lung Cancer Stage, and Prognostic Factors-Findings from the National Lung Screening Trial. Int. J. Environ. Res. Public Health 2024, 21, 400. [Google Scholar] [CrossRef] [PubMed]
- Shiels, M.S.; Graubard, B.I.; McNeel, T.S.; Kahle, L.; Freedman, N.D. Trends in smoking-attributable and smoking-unrelated lung cancer death rates in the United States, 1991–2018. J. Natl. Cancer Inst. 2024, 116, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.P.; Teare, M.D.; Stevens, J.; Archer, R. Risk Prediction Models for Lung Cancer: A Systematic Review. Clin. Lung Cancer 2016, 17, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Świątkowska, B. Identification of susceptibility pathways for the role of chromosome 15q25.1 in modifying lung cancer risk. Nat. Commun. 2018, 9, 3221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Zhang, L.; He, J.; Bo, J.; Wang, J.; Ding, B.; Ren, M. Common immunological and prognostic features of lung and bladder cancer via smoking-related genes: PRR11 gene as potential immunotherapeutic target. J. Cell. Mol. Med. 2024, 28, e18384. [Google Scholar] [CrossRef]
- Pass, H.; Ball, D.; Scagliotti, G. IASLC Thoracic Oncology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for Lung Cancer US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef]
- Aberle, D.R.; Adams, A.M.; Berg, C.D.; Black, W.C.; Clapp, J.D.; Fagerstrom, R.M.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Oudkerk, M. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Hirales Casillas, C.E.; Flores Fernández, J.M.; Padilla Camberos, E.; Herrera López, E.J.; Leal Pacheco, G.; Martínez Velázquez, M. Current status of circulating protein biomarkers to aid the early detection of lung cancer. Future Oncol. 2014, 10, 1501–1513. [Google Scholar] [CrossRef]
- Oken, M.M.; Hocking, W.G.; Kvale, P.A.; Andriole, G.L.; Buys, S.S.; Church, T.R. PLCO Project Team. Screening by chest radiograph and lung cancer mortality: The Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA 2011, 306, 1865–1873. [Google Scholar] [CrossRef]
- Can Lung Cancer Be Found Early? Available online: https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/detection.html (accessed on 29 April 2024).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 30 April 2024).
- Program Badań w Kierunku Wykrywania Raka Płuca. Available online: https://www.gov.pl/web/zdrowie/program-badan-w-kierunku-wykrywania-raka-pluca (accessed on 29 April 2024).
- World Health Organization. Toolkit for Delivering the 5A’s and 5R’s Brief Tobacco Interventions in Primary Care; World Health Organization: Geneva, Switzerland, 2014; Available online: https://www.who.int/publications/i/item/toolkit-for-delivering-5as-and-5rs-brief-tobacco-interventions-in-primary-care (accessed on 30 April 2024).
- Coleman, T. ABC of smoking cessation. Cessation interventions in routine health care. BMJ 2004, 328, 631–633. [Google Scholar] [PubMed]
- Nian, T.; Guo, K.; Liu, W.; Deng, X.; Hu, X.; Xu, M.; E, F.; Wang, Z.; Song, G.; Yang, K.; et al. Non-pharmacological interventions for smoking cessation: Analysis of systematic reviews and meta-analyses. BMC Med. 2023, 21, 378. [Google Scholar] [CrossRef] [PubMed]
- Czy Leki Takie jak Wareniklina i Cytyzyna (Częściowi Agoniści Receptorów Nikotynowych) Mogą Pomóc Rzucić Palenie i czy Powodują Niepożądane Skutki? Biblioteka Cochrane. 2022. Available online: https://www.cochrane.org/pl/CD006103/TOBACCO_czy-leki-takie-jak-wareniklina-i-cytyzyna-czesciowi-agonisci-receptorow-nikotynowych-moga-pomoc (accessed on 4 May 2024).
- Livingstone-Banks, J.; Fanshawe, T.R.; Thomas, K.H.; Theodoulou, A.; Hajizadeh, A.; Hartman, L.; Lindson, N. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst. Rev. 2023, 5, CD006103. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.A.; Fedewa, S.A.; Henley, S.J.; Pollack, L.A.; Jemal, A. Proportion of Never Smokers Among Men and Women with Lung Cancer in 7 US States. JAMA Oncol. 2021, 7, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, J.; Govindan, R. Lung cancer in never smokers: A review. J. Clin. Oncol. 2007, 25, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Steffensen, I.; Miguel, R.T.D.; Babic, T.; Carlone, J. A Systematic Review Investigating Associations Between E-Cigarette Use among Former Cigarette Smokers and Relapse to Smoking Cigarettes. INQUIRY J. Health Care Organ. Provis. Financ. 2023, 60, 00469580231214457. [Google Scholar] [CrossRef] [PubMed]
- Shehata, S.A.; Toraih, E.A.; Ismail, E.A.; Hagras, A.M.; Elmorsy, E.; Fawzy, M.S. Vaping, Environmental Toxicants Exposure, and Lung Cancer Risk. Cancers 2023, 15, 4525. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.H.J.; Kong, S.L.; Tai, J.A.; Poh, H.M.; Yao, F.; Sia, Y.Y.; Lim, E.K.H.; Takano, A.M.; Tan, D.S.-W.; Javed, A.; et al. Experimental and bioinformatics considerations in cancer application of single cell genomics. Comput. Struct. Biotechnol. J. 2021, 19, 343–354. [Google Scholar] [CrossRef]
- Tong, F.; Zhou, Y.; Xu, Y.; Chen, Y.; Yudintceva, N.; Shevtsov, M.; Gao, H. Supramolecular nanomedicines based on host-guest interactions of cyclodextrins. Exploration 2023, 3, 20210111. [Google Scholar] [CrossRef]
- Lv, Y.; Xiao, C.; Ma, J.; Zhou, D.; Wu, W.; Yang, C. Solvent and guest-binding-controlled chiroptical inversion of molecular devices based on pseudo[1]catenane-type pillar[5]arene derivatives. Chin. Chem. Lett. 2024, 35, 108757. [Google Scholar] [CrossRef]
- Yan, M.; Wu, S.; Wang, Y.; Liang, M.; Wang, M.; Hu, W.; Zhou, J. Recent Progress of Supramolecular Chemotherapy Based on Host–Guest Interactions. Adv. Mater. 2024, 36, 2304249. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiong, M.; Rong, Q.; Zhang, X.M.; Zhang, B. Nucleic acid sensors in vivo: Challenges and opportunities. VIEW 2023, 4, 20220064. [Google Scholar] [CrossRef]
- Yan, M.; Wang, Y.; Chen, J.; Zhou, J. Potential of nonporous adaptive crystals for hydrocarbon separation. Chem. Soc. Rev. 2023, 52, 6075–6119. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, W.; Yang, W.; Xi, F.; He, H.; Liang, M.; Dong, Q.; Hou, J.; Wang, M.; Yu, G.; et al. Separation of benzene and toluene associated with vapochromic behaviors by hybrid[4]arene-based co-crystals. Nat. Commun. 2024, 15, 1260. [Google Scholar] [CrossRef]


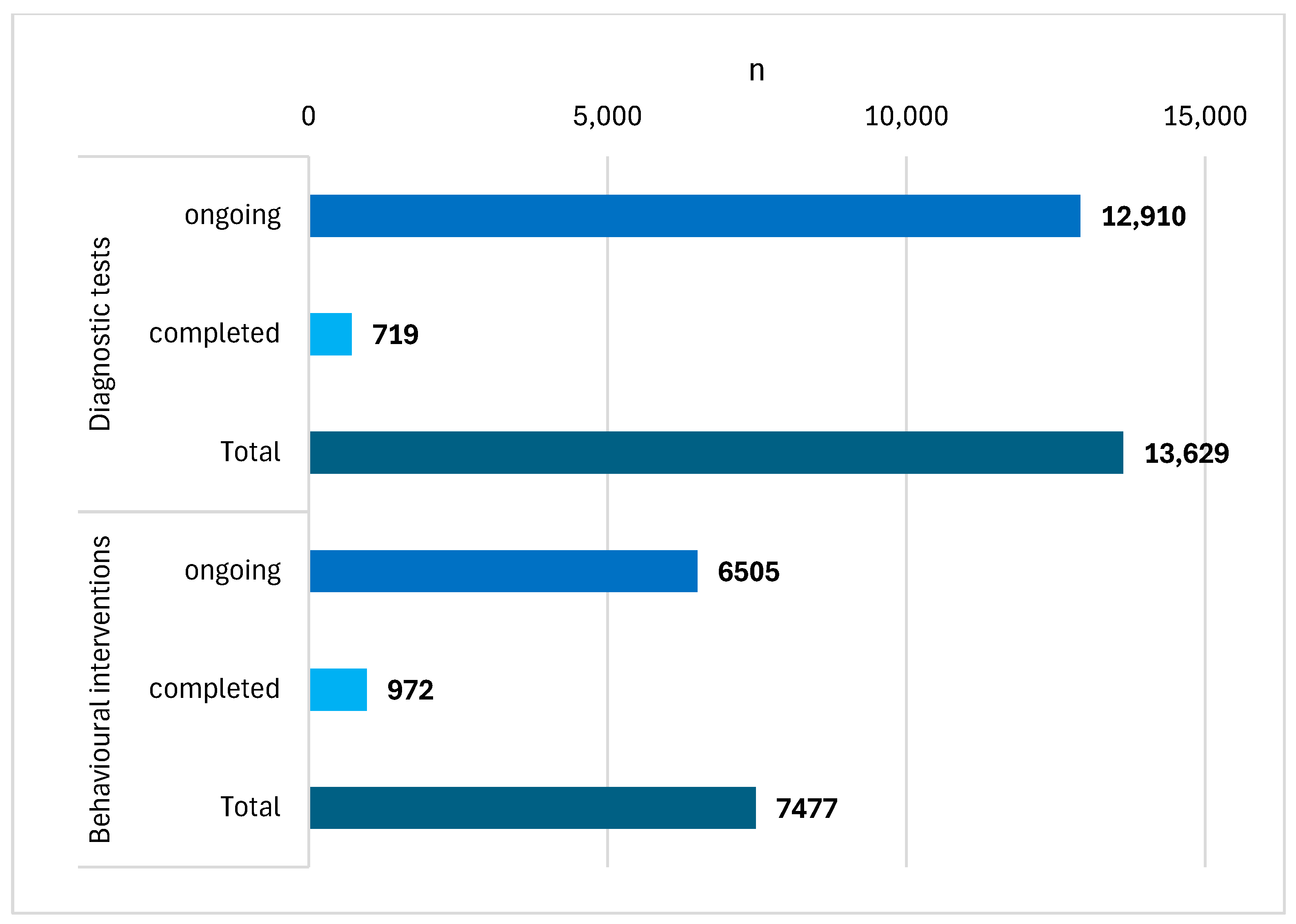
| Time Frame: 2019–31 March 2024 | |||||||
|---|---|---|---|---|---|---|---|
| No. | Group | Title | Years | n | Status | Trial | Population |
| (1) | Diagnostic | A Multicentre Clinical Trial of Sputum DNA Testing for Lung Cancer in China | 2022–2023 | 659 | completed | observational | b.o. |
| (2) | tests | AI for Lung Cancer Risk Definition in Computed Tomography Screening Programs | 2023- | 650 | ongoing | observational | smokers, aged 50–75 |
| (3) | Feasibility of Cell-Free DNA Liquid Biopsy in Screening High-Risk Patients for Lung Cancer | 2022- | 100 | ongoing | interventional | smokers, aged 50–80 | |
| (4) | Evaluation of a Scalable Decision Support and Shared Decision Making Tool for Lung Cancer Screening | 2020- | 12,000 | ongoing | interventional | people meeting the inclusion criteria for LDCT screening | |
| (5) | Breathomics: May it Become an Affordable, New Tool for Early Diagnosis and Screening of Lung Cancer? | 2021–2023 | 60 | completed | observational | adults, clinical group and control group | |
| (6) | Structuring of a Lung Cancer Screening Program Including Clinical, Radiological and Biological Phenotyping Useful for the Development of Individualized Risk Prediction Tools: PREVALUNG ETOILE | 2023- | 160 | ongoing | interventional | aged 45–75, lung diseases, meeting inclusion criteria for lung cancer screening | |
| (7) | Behavioural | Inpatient Smokers and LDCT Screening Part 2 | 2019–2020 | 128 | completed | interventional | people meeting the inclusion criteria for LDCT screening |
| (8) | interventions | The Impact of Picture Narrative Format on Print Lung Screening Communication Outcomes | 2021 | 326 | completed | interventional | people aged 49–75 |
| (9) | Health Opportunities and Promoters of Equitable Screening for Lung Cancer | 2023- | 2000 | ongoing | observational | smokers, aged 50–80 | |
| (10) | Proactive Outreach and Shared Decision Making in Improving Lung Cancer Screening Rates in Primary Care Patients | 2019- | 2355 | ongoing | interventional | smokers, aged 55–80 | |
| (11) | Shared Decision Making in Rural Primary Care Lung Cancer Screening and Smoking Cessation | 2020–2023 | 118 | completed | interventional | smokers, aged 50–80 | |
| (12) | Designing an Implementation Strategy for Lung Screening and Smoking Cessation Treatment in Community Health Centres | 2022–2023 | 9 | completed | interventional | personnel working with at-risk patients | |
| (13) | CONNECT: Smoking Cessation and Lung Cancer Screening | 2019–2023 | 99 | completed | interventional | smokers scheduled for LDCT, aged 55–80 | |
| (14) | A Study to Develop a Strategy to Increase Lung Cancer Screening in Women Who May Be at Risk for Lung Cancer | 2021- | 150 | ongoing | interventional | women meeting the inclusion criteria for LDCT screening who had no lesions on mammography | |
| (15) | Awareness, Information, and Resources for Lung Cancer Screening Program for Community-Partnered Lung Cancer Screening | 2020–2022 | 292 | completed | interventional | adults | |
| (16) | Screening and Multiple Intervention on Lung Epidemics | 2019- | 2000 | ongoing | interventional | smokers, aged 55–75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerw, A.; Deptała, A.; Partyka, O.; Pajewska, M.; Wiśniewska, E.; Sygit, K.; Wysocki, S.; Cipora, E.; Konieczny, M.; Banaś, T.; et al. Lung Cancer Screening—Trends and Current Studies. Cancers 2024, 16, 2691. https://doi.org/10.3390/cancers16152691
Czerw A, Deptała A, Partyka O, Pajewska M, Wiśniewska E, Sygit K, Wysocki S, Cipora E, Konieczny M, Banaś T, et al. Lung Cancer Screening—Trends and Current Studies. Cancers. 2024; 16(15):2691. https://doi.org/10.3390/cancers16152691
Chicago/Turabian StyleCzerw, Aleksandra, Andrzej Deptała, Olga Partyka, Monika Pajewska, Ewa Wiśniewska, Katarzyna Sygit, Sławomir Wysocki, Elżbieta Cipora, Magdalena Konieczny, Tomasz Banaś, and et al. 2024. "Lung Cancer Screening—Trends and Current Studies" Cancers 16, no. 15: 2691. https://doi.org/10.3390/cancers16152691
APA StyleCzerw, A., Deptała, A., Partyka, O., Pajewska, M., Wiśniewska, E., Sygit, K., Wysocki, S., Cipora, E., Konieczny, M., Banaś, T., Małecki, K., Grochans, E., Grochans, S., Cybulska, A. M., Schneider-Matyka, D., Bandurska, E., Ciećko, W., Drobnik, J., Pobrotyn, P., ... Kozlowski, R. (2024). Lung Cancer Screening—Trends and Current Studies. Cancers, 16(15), 2691. https://doi.org/10.3390/cancers16152691









