Role of Quantitative CEUS in the Diagnosis of Peripheral Pulmonary Lesions: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
- ○
- Study design (retrospective, prospective, or ambispective);
- ○
- Number of patients and number of lesions;
- ○
- Key CEUS parameters analyzed (e.g., arrival time (AT), time difference of arrival (TDOA), washout time (WOT), enhancement pattern, perfusion homogeneity);
- ○
- Diagnostic criteria for malignancy (e.g., AT ≥ 10 s, lesion-lung AT difference ≥ 2.5 s, washout pattern, logistic regression models incorporating multiple features);
- ○
- Sensitivity, specificity, and diagnostic accuracy;
- ○
- Additional outcomes, such as biopsy success rate and procedure complications.
2.7. Study Risk of Bias Assessment
2.8. Effect Measures
2.9. Synthesis Methods
2.10. Synthesis Methods, Reporting Bias Assessment, and Certainty Assessment
3. Results
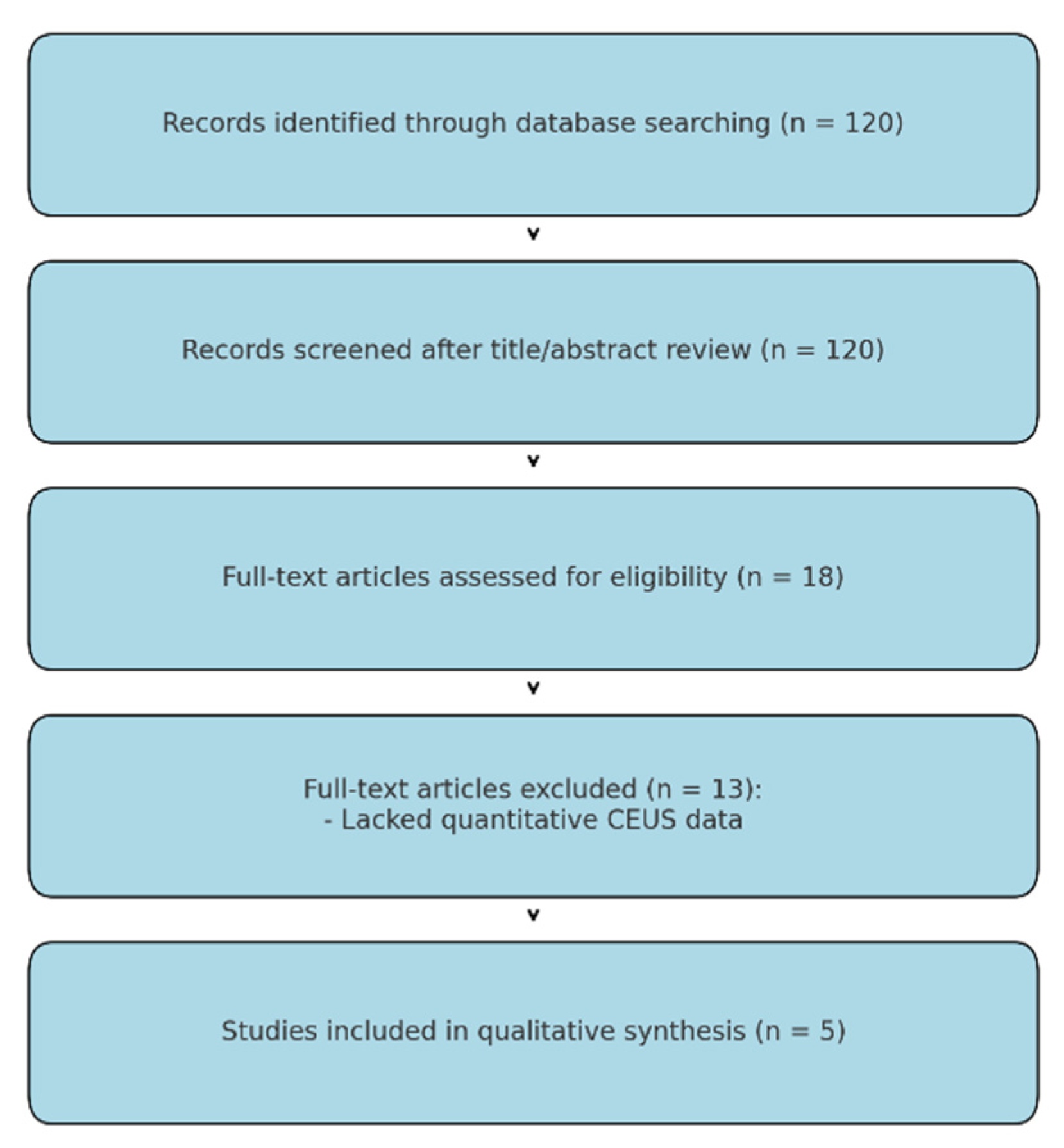
3.1. Study Identification
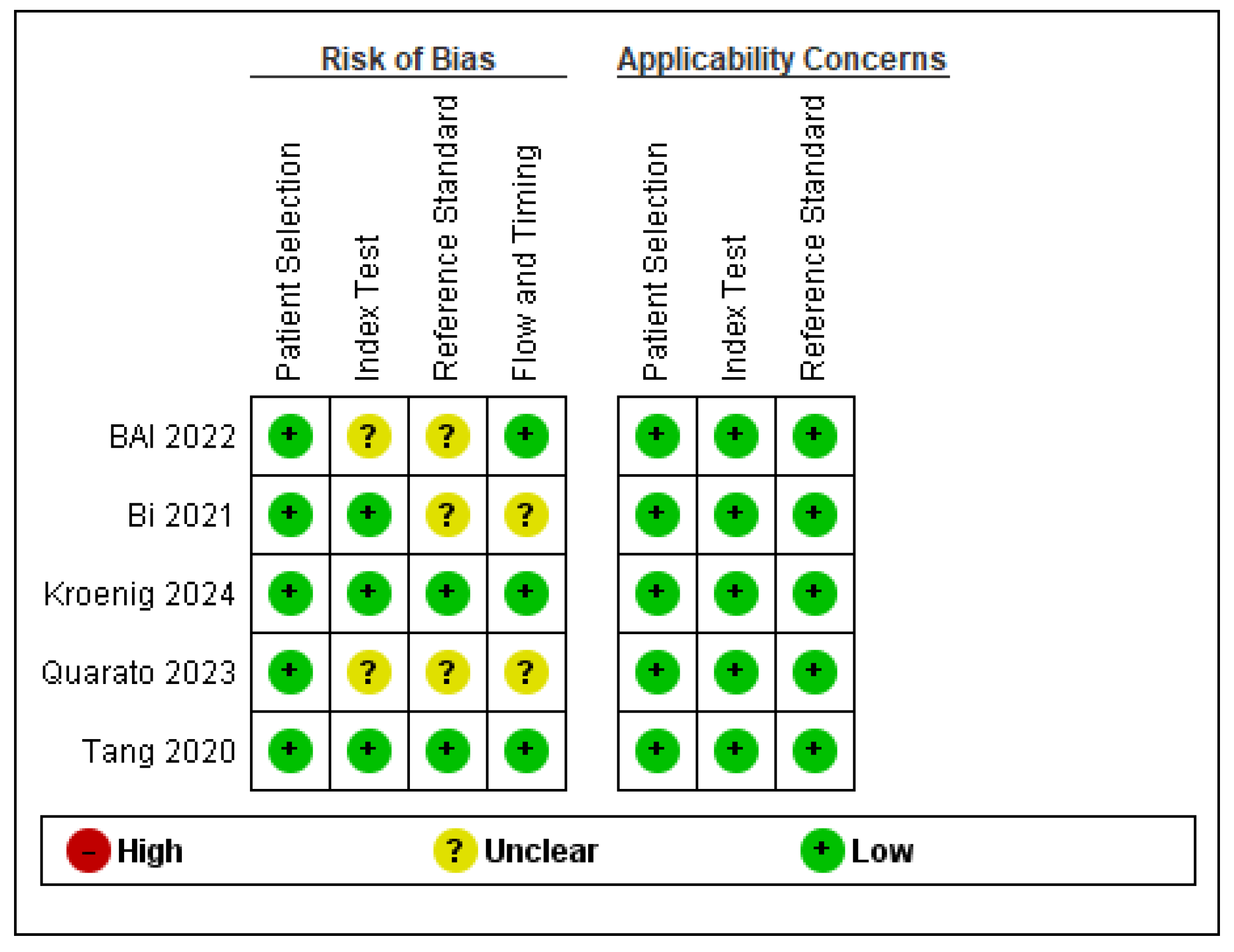
3.2. Quality Assessment
3.3. Study Characteristics
4. Synthesis of Findings
5. Discussion
6. Limitations of the Current Evidence Base
7. Future Directions
8. Conclusions
- -
- When used in tandem with ROSE, CEUS-guided biopsy can achieve success rates approaching 98%, significantly higher than conventional ultrasound-guided approaches.
- -
- Time difference of arrival (∆AT/TDOA) consistently emerges as a useful discriminator between benign and malignant processes, with different proposed numeric thresholds (2.05–2.5 s) providing strong sensitivity and specificity.
- -
- Multi-parameter models that integrate B-mode features (lesion shape, angle with chest wall, vascular sign) and CEUS features (arrival time difference, basic intensity, presence of non-enhancing regions) can achieve near-perfect discrimination, with AUC values > 0.95 in both developmental and external validation cohorts.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stone, E.; Leong, T.L. Contemporary Concise Review 2021: Pulmonary nodules from detection to intervention. Respirology 2022, 27, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, L.; Liao, X.; Tang, S.; Li, Z. Ultrasound-guided percutaneous needle biopsies of peripheral pulmonary lesions: Diagnostic efficacy and risk factors for diagnostic failure. Ann. Palliat. Med. 2021, 10, 9772–9783. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.S.; Lee, G.; Roh, J.; Chung, H.S.; Jeong, Y.J. Feasibility of Ultra-Low-Dose CT for Bronchoscopy of Peripheral Lung Lesions. Medicina 2020, 56, 479. [Google Scholar] [CrossRef]
- Fink, N.; Sperl, J.I.; Rueckel, J.; Stüber, T.; Goller, S.S.; Rudolph, J.; Escher, F.; Aschauer, T.; Hoppe, B.F.; Ricke, J.; et al. Artificial intelligence-based automated matching of pulmonary nodules on follow-up chest CT. Eur. Radiol. Exp. 2025, 9, 48. [Google Scholar] [CrossRef]
- Sathekge, C.; Maes, J.; Maes, A.; Van de Wiele, C. FDG PET/CT for Staging Lung Carcinoma: An Update. Semin. Nucl. Med. 2025, 55, 167–174. [Google Scholar] [CrossRef]
- Li, S.; Yu, L.L.; Li, L.; Tang, X.M.; He, P.; Gu, P. Ultrasound-guided core-needle biopsy for peripheral pulmonary lesions: A systematic review and meta-analysis. Clin. Radiol. 2023, 78, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Matsumoto, Y.; Furuse, H.; Uchimura, K.; Imabayashi, T.; Tsuchida, T. Diagnostic utility of bronchoscopy for newly emerging peripheral pulmonary lesions after pulmonary resection. Transl. Lung Cancer Res. 2025, 14, 798–809. [Google Scholar] [CrossRef]
- Boccatonda, A.; Ianniello, E.; D’Ardes, D.; Cocco, G.; Giostra, F.; Borghi, C.; Schiavone, C. Can Lung Ultrasound be Used to Screen for Pulmonary Embolism in Patients with SARS-CoV-2 Pneumonia? Eur. J. Case Rep. Intern. Med. 2020, 7, 001748. [Google Scholar] [CrossRef]
- Boccatonda, A.; Andreetto, L.; Vicari, S.; Campello, E.; Simioni, P.; Ageno, W. The Diagnostic Role of Lung Ultrasound and Contrast-Enhanced Ultrasound in Pulmonary Embolism. Semin. Thromb. Hemost. 2024, 50, 842–850. [Google Scholar] [CrossRef]
- Giangregorio, F.; Mosconi, E.; Debellis, M.G.; Provini, S.; Esposito, C.; Mendozza, M.; Raccanelli, R.; Maresca, L.; Cinquini, S.; Tursi, F. Clinical utility of bedside Contrast-Enhanced Ultrasound (CEUS) in the diagnosis of pneumonia in elderly patients: Comparison with clinical, -radiological and ultrasound diagnosis. Multidiscip. Respir. Med. 2024, 19, 967. [Google Scholar] [CrossRef]
- Safai Zadeh, E.; Beutel, B.; Dietrich, C.F.; Keber, C.U.; Huber, K.P.; Görg, C.; Trenker, C. Perfusion Patterns of Peripheral Pulmonary Lesions in COVID-19 Patients Using Contrast-Enhanced Ultrasound (CEUS): A Case Series. J. Ultrasound Med. 2021, 40, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.; Sperandeo, G.; Varriale, A.; Filabozzi, P.; Decuzzi, M.; Dimitri, L.; Vendemiale, G. Contrast-enhanced ultrasound (CEUS) for the study of peripheral lung lesions: A preliminary study. Ultrasound Med. Biol. 2006, 32, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Caremani, M.; Benci, A.; Lapini, L.; Tacconi, D.; Caremani, A.; Ciccotosto, C.; Magnolfi, A.L. Contrast enhanced ultrasonography (CEUS) in peripheral lung lesions: A study of 60 cases. J. Ultrasound 2008, 11, 89–96. [Google Scholar] [CrossRef]
- Sartori, S.; Postorivo, S.; Vece, F.D.; Ermili, F.; Tassinari, D.; Tombesi, P. Contrast-enhanced ultrasonography in peripheral lung consolidations: What’s its actual role? World J. Radiol. 2013, 5, 372–380. [Google Scholar] [CrossRef]
- Yu Qing, D.; Jing, B.; Bing, W.; Song, W.; Fei, Z.Q.; Kun, Y.; Wei, Y. Differential Diagnosis of Pathological Type of Peripheral Lung Cancer with Multimodal Contrast-Enhanced Ultrasound. Ultrasound Med. Biol. 2024, 50, 1485–1493. [Google Scholar] [CrossRef]
- Badiu, S.M.; Gheorghe, E.C.; Nicolau, C.; Săftoiu, A. Quantitative time intensity curve analysis of contrast-enhanced ultrasound (CEUS) examinations for the assessment of focal liver lesions. Med. Ultrason. 2024, 26, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Safai Zadeh, E.; Keber, C.U.; Dietrich, C.F.; Westhoff, C.C.; Günter, C.; Beutel, B.; Alhyari, A.; Trenker, C.; Görg, C. Perfusion Patterns of Peripheral Pulmonary Granulomatous Lesions Using Contrast-Enhanced Ultrasound (CEUS) and Their Correlation with Immunohistochemically Detected Vascularization Patterns. J. Ultrasound Med. 2022, 41, 565–574. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Huang, H.; Zhou, X.; Xian, M. Application of quantitative contrast-enhanced ultrasound for evaluation and guiding biopsy of peripheral pulmonary lesions: A preliminary study. Clin. Radiol. 2020, 75, 79.E19–79.E24. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, J.; Gu, F.F.; Li, Z.R.; Song, Y.S.; Long, J.J.; Zhang, S.Z.; Xu, T.T.; Tang, Y.J.; Gu, J.Y.; et al. Evaluating the efficacy of percutaneous puncture biopsy guided by contrast-enhanced ultrasound for peripheral pulmonary lesions. World J. Clin. Cases 2024, 12, 3791–3799. [Google Scholar] [CrossRef]
- Li, Q.; Nie, F.; Yang, D.; Dong, T.; Liu, T. Contrast-enhanced ultrasound (CEUS)—A new tool for evaluating blood supply in primary peripheral lung cancer. Clin. Hemorheol. Microcirc. 2023, 83, 61–68. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar]
- Whiting, P.; Rutjes, A.W.; Reitsma, J.B.; Bossuyt, P.M.; Kleijnen, J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 2003, 3, 25. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, T.; Liu, W.; Li, Z.; Zheng, H.; Li, X. Application value of contrast-enhanced ultrasound in the diagnosis of peripheral pulmonary focal lesions. Medicine 2022, 101, e29605. [Google Scholar] [CrossRef]
- Bi, K.; Xia, D.M.; Fan, L.; Ye, X.F.; Zhang, Y.; Shen, M.J.; Chen, H.W.; Cong, Y.; Zhu, H.M.; Tang, C.H.; et al. Development and Prospective Validation of an Ultrasound Prediction Model for the Differential Diagnosis of Benign and Malignant Subpleural Pulmonary Lesions: A Large Ambispective Cohort Study. Front. Oncol. 2021, 11, 656060. [Google Scholar] [CrossRef] [PubMed]
- Quarato, C.M.I.; Feragalli, B.; Lacedonia, D.; Rea, G.; Scioscia, G.; Maiello, E.; Di Micco, C.; Borelli, C.; Mirijello, A.; Graziano, P.; et al. Contrast-Enhanced Ultrasound in Distinguishing between Malignant and Benign Peripheral Pulmonary Consolidations: The Debated Utility of the Contrast Enhancement Arrival Time. Diagnostics 2023, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xie, Q.; Wang, J.; Zhai, X.; Lin, H.; Zheng, X.; Wei, G.; Tang, Y.; Zeng, F.; Chu, Y.; et al. Time Difference of Arrival on Contrast-Enhanced Ultrasound in Distinguishing Benign Inflammation From Malignant Peripheral Pulmonary Lesions. Front. Oncol. 2020, 10, 578884. [Google Scholar] [CrossRef]
- Kroenig, J.; Görg, C.; Prosch, H.; Von Schumann, L.; Westhoff, C.C.; Alhyari, A.; Koenig, F.R.M.; Findeisen, H.; Safai Zadeh, E. Perfusion Patterns of Peripheral Pulmonary Metastasis Using Contrast-Enhanced Ultrasound (CEUS) and Their Correlation with Immunohistochemically Detected Vascularization Pattern. Cancers 2024, 16, 3365. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, H.; Trenker, C.; Figiel, J.; Greene, B.H.; Görg, K.; Görg, C. Vascularization of Primary, Peripheral Lung Carcinoma in CEUS—A Retrospective Study (n = 89 Patients). Ultraschall Med. 2019, 40, 603–608. [Google Scholar] [CrossRef]
- Findeisen, H.; Görg, C.; Hartbrich, R.; Dietrich, C.F.; Görg, K.; Trenker, C.; Safai Zadeh, E. Contrast-enhanced ultrasound is helpful for differentiating benign from malignant parietal pleural lesions. J. Clin. Ultrasound 2022, 50, 90–98. [Google Scholar] [CrossRef]
- Shen, M.; Bi, K.; Cong, Y.; Chen, H.; Zhang, Y.; Zhu, H.; Wang, Y. Application of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Benign and Malignant Subpleural Pulmonary Lesions. J. Ultrasound Med. 2022, 41, 1147–1157. [Google Scholar] [CrossRef]
- Sperandeo, M.; Rea, G.; Grimaldi, M.A.; Trovato, F.; Dimitri, L.M.; Carnevale, V. Contrast-enhanced ultrasound does not discriminate between community acquired pneumonia and lung cancer. Thorax 2017, 72, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Guagnano, M.T.; D’Ardes, D.; Cipollone, F.; Vetrugno, L.; Schiavone, C.; Piscaglia, F.; Serra, C. The Role of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Malignant and Benign Subpleural Lung Lesions. J. Clin. Med. 2024, 13, 2302. [Google Scholar] [CrossRef] [PubMed]
- Quarato, C.M.I.; Cipriani, C.; Dimitri, L.; Lacedonia, D.; Graziano, P.; Copetti, M.; De Cosmo, S.; Simeone, A.; Scioscia, G.; Foschino Barbaro, M.; et al. Assessing value of contrast-enhanced ultrasound vs. conventional transthoracic ultrasound in improving diagnostic yield of percutaneous needle biopsy of peripheral lung lesions. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yuan, Z.F.; Gan, K.H.; Zhong, Y.; Huang, J.X.; Huang, W.J.; Xie, Y.H.; Pei, X.Q. Contrast-enhanced Imaging in Peripheral Pulmonary Lesions: The Role in US-guided Biopsies. Radiol. Cardiothorac. Imaging 2024, 6, e230234. [Google Scholar] [CrossRef]
| Author(s) | Population Size | Study Type | Key Diagnostic Tools | Diagnostic Accuracy/AUC | Main Conclusion |
|---|---|---|---|---|---|
| Bai et al. [23] | 80 | Prospective comparative | CEUS + ROSE | Biopsy success: 97.62% (CEUS) vs. 84% (conventional) | CEUS improves biopsy yield; ΔAT helps discriminate lesions |
| Bi et al. [24] | 812 | Ambispective cohort (development + validation) | B-mode + CEUS (multi-parameter model) | AUC: 0.974 (dev), 0.980 (val); sens: ~93–95%, spec: ~92% | Multi-parametric model significantly outperforms single thresholds |
| Quarato et al. [25] | 317 | Retrospective | CEUS (AT, WOT) | AT <10 s: 47.6% accuracy, WOT >300 s: 53.6% accuracy | AT and WOT alone are unreliable discriminators |
| Tang et al. [26] | 96 | Retrospective | CEUS (TDOA) | AUC: 0.894 for TDOA | TDOA is a robust parameter to distinguish malignancy |
| Kroenig et al. [27] | 54 | Observational retrospective | CEUS (TE, EE, HE, DE) + CD34 staining | 92.6% BA pattern, 98.1% rapid washout (<120 s) | Metastases show BA supply and rapid washout, supporting CEUS utility |
| Reference | Study Design | Population and Sample | Main Ceus Parameters | Key Results |
|---|---|---|---|---|
| Bai Z et al. (2022) [23] | Prospective study | - 80 patients with peripheral pulmonary lesions - Divided into a conventional ultrasound group vs. a CEUS group, both using real-time ROSE | - Lesion enhancement - Arrival time (AT) - Lung arrival time (L-AT) - ΔAT (difference in arrival time) | - Biopsy success rate: 97.62% (CEUS) vs. 84% (conventional US) - Optimal ΔAT threshold = 2.05 s to distinguish benign vs. malignant - No significant complications in the CEUS group vs. 5.26% in the conventional group. |
| Bi K et al. (2021) [24] | Ambispective (retro-/prospective) | - Development: 592 patients (DC cohort, 2017–2018) - Validation: 220 patients (VC cohort, 2019) - 18 parameters from B-mode US and CEUS were collected | - Lesion-lung arrival time difference - AT ≥ 10 s (historical criterion) - Multivariate model (6 parameters) | - Final model with 6 parameters (B-mode + CEUS) with C-statistic: 0.974 (DC) and 0.980 (VC) - Superior to AT ≥ 10 s or difference ≥ 2.5 s - Sensitivity ~93–95% and specificity ~92%. |
| Quarato Cmi et al. (2023) [25] | Retrospective study | - 317 patients (215 men, 102 women; mean age 52) - Peripheral pulmonary lesions (benign/malignant) | - Arrival time (AT) - Enhancement pattern - Washout time (WOT) | - AT < 10 s vs. ≥ 10 s did not effectively differentiate benign vs. malignant (low sensitivity, 5.3%) - WOT > 300 s was not discriminative (accuracy ~53.6%) - Squamous cell carcinoma displayed a later AT than other subtypes, but significant difference only vs. undifferentiated carcinoma. |
| Tang M et al. (2020) [26] | Retrospective study | - 96 patients with peripheral pulmonary lesions undergoing biopsy - Comparison between conventional CEUS parameters and a new TDOA index | - TDOA (time difference of arrival) - Time-intensity curve (TIC) parameters | - TDOA was significantly higher in malignant lesions (p < 0.001) - AUC for TDOA = 0.894, outperforming conventional CEUS parameters |
| Kroenig J et al. (2024) [27] | Retrospective study | - 54 patients with histologically proven peripheral pulmonary metastases - Included cases with immunohistochemical correlation (CD34) | - Enhancement time (TE): pulmonary-arterial (PA) or bronchial-arterial (BA) pattern - Extent and homogeneity of enhancement - Washout (<120 s or ≥120 s) | - 92.6% had a BA pattern, 98.1% exhibited a rapid washout - A “chaotic” vascular pattern correlated with tumor neo-angiogenesis (CD34) - Only 4 lesions (7.4%) showed a PA pattern. |
| Parameter | Description |
| AT (Arrival Time) | The time at which the contrast agent first appears in the target lesion. |
| L-AT (Lung Arrival Time) | The time at which the contrast agent first appears in the adjacent lung tissue. |
| ΔAT (Difference Between L-AT AND AT) | This parameter measures the discrepancy in arrival times between healthy lung and lesion; calculated as ΔAT = L-AT − AT. It helps assess differences in blood flow between normal lung and the lesion. |
| TDOA (Time Difference OF ARRIVAL) | Conceptually similar to ΔAT, it compares the time of contrast arrival in the lesion versus a chosen reference structure. TDOA is often used in evaluating perfusion delays indicative of malignancy. |
| WOT (Washout Time) | The time required for the contrast signal to disappear from the lesion (the washout phase). This can provide information on lesion vascularity and potential malignancy markers. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccatonda, A.; Brighenti, A.; Bakken, S.M.; D’Ardes, D.; Schiavone, C.; Piscaglia, F.; Serra, C. Role of Quantitative CEUS in the Diagnosis of Peripheral Pulmonary Lesions: A Systematic Review. Cancers 2025, 17, 1697. https://doi.org/10.3390/cancers17101697
Boccatonda A, Brighenti A, Bakken SM, D’Ardes D, Schiavone C, Piscaglia F, Serra C. Role of Quantitative CEUS in the Diagnosis of Peripheral Pulmonary Lesions: A Systematic Review. Cancers. 2025; 17(10):1697. https://doi.org/10.3390/cancers17101697
Chicago/Turabian StyleBoccatonda, Andrea, Alice Brighenti, Sofia Maria Bakken, Damiano D’Ardes, Cosima Schiavone, Fabio Piscaglia, and Carla Serra. 2025. "Role of Quantitative CEUS in the Diagnosis of Peripheral Pulmonary Lesions: A Systematic Review" Cancers 17, no. 10: 1697. https://doi.org/10.3390/cancers17101697
APA StyleBoccatonda, A., Brighenti, A., Bakken, S. M., D’Ardes, D., Schiavone, C., Piscaglia, F., & Serra, C. (2025). Role of Quantitative CEUS in the Diagnosis of Peripheral Pulmonary Lesions: A Systematic Review. Cancers, 17(10), 1697. https://doi.org/10.3390/cancers17101697









