Effects of DHA Oral Supplementation on Plasma Resolvin D1 and D2 Levels in Naïve Breast Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Intervention: DHA Oral Supplementation
2.3. Sample Collection and Measurement of Plasma Resolvin D1 and D2
2.4. Measurement of Plasma Resolvins
2.5. Statistical Analyses and Sample Size Calculation
3. Results
3.1. Patient’s Characteristics at Baseline
3.2. Baseline Plasma Resolvin D1 and D2 Levels Among Breast Cancer Patients and Controls
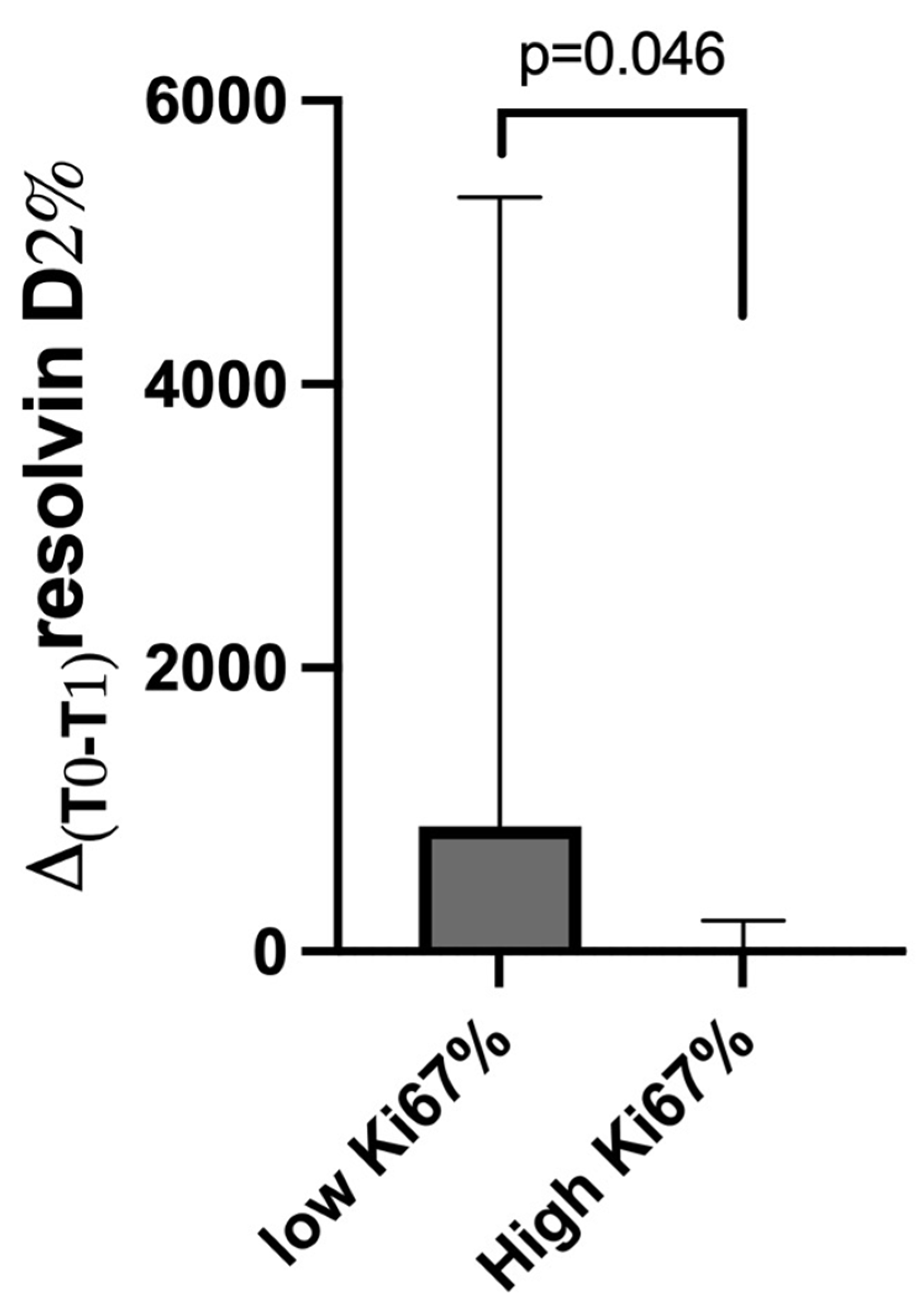
3.3. Changes from Baseline (T0) in Plasma Resolvin D1 and D2 Levels After DHA Oral Supplementation (T1) Between Patients and Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valente, S.; Roesch, E. Breast Cancer Survivorship. J. Surg. Oncol. 2024, 130, 8–15. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/News-Room/Fact-Sheets/Detail/Breast-Cancer (accessed on 1 April 2025).
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast Cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Harris, M.A.; Savas, P.; Virassamy, B.; O’Malley, M.M.R.; Kay, J.; Mueller, S.N.; Mackay, L.K.; Salgado, R.; Loi, S. Towards Targeting the Breast Cancer Immune Microenvironment. Nat. Rev. Cancer 2024, 24, 554–577. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, L.; Zhang, Y.; Nakata, Y.; Chan, H.L.; Morey, L. Epigenetic Mechanisms in Breast Cancer Therapy and Resistance. Nat. Commun. 2021, 12, 1786. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Kohli, P.; Levy, B.D. Resolvins and Protectins: Mediating Solutions to Inflammation. Br. J. Pharmacol. 2009, 158, 960–971. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving Lipid Mediators Resolvin D1, Resolvin D2, and Maresin 1 Are Critical in Modulating T Cell Responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, K.M.; Howard, I.V.; Southan, F.; Bayer, R.L.; Torres, K.L.; Serhan, C.N.; Panigrahy, D. Exploring the Unique Role of Specialized Pro-Resolving Mediators in Cancer Therapeutics. Prostaglandins Other Lipid Mediat. 2024, 178, 106944. [Google Scholar] [CrossRef]
- Mattoscio, D.; Isopi, E.; Lamolinara, A.; Patruno, S.; Medda, A.; De Cecco, F.; Chiocca, S.; Iezzi, M.; Romano, M.; Recchiuti, A. Resolvin D1 Reduces Cancer Growth Stimulating a Protective Neutrophil-Dependent Recruitment of Anti-Tumor Monocytes. J. Exp. Clin. Cancer Res. 2021, 40, 129. [Google Scholar] [CrossRef]
- Serhan, C.N.; Bäck, M.; Chiurchiù, V.; Hersberger, M.; Mittendorfer, B.; Calder, P.C.; Waitzberg, D.L.; Stoppe, C.; Klek, S.; Martindale, R.G.; et al. Expert Consensus Report on Lipid Mediators: Role in Resolution of Inflammation and Muscle Preservation. FASEB J. 2024, 38, e23699. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Imbimbo, G.; Salerno, G.; Lionetto, L.; De Luca, A.; Costanzo, M.L.; Simmaco, M.; Muscaritoli, M.; Amabile, M.I. Assessment of Plasma Resolvin Levels in Women with Breast Cancer and Their Associations with Disease Presentation and Immunohistochemical Characteristics. Lipids Health Dis. 2024, 23, 396. [Google Scholar] [CrossRef]
- Deyama, S.; Ishikawa, Y.; Yoshikawa, K.; Shimoda, K.; Ide, S.; Satoh, M.; Minami, M. Resolvin D1 and D2 Reverse Lipopolysaccharide-Induced Depression-Like Behaviors Through the mTORC1 Signaling Pathway. Int. J. Neuropsychopharmacol. 2017, 20, 575–584. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Mazzucco, S.; Biolo, G.; Farcomeni, A.; Ramaccini, C.; Antonaroli, S.; Monti, M.; Muscaritoli, M. Effect of Oral Docosahexaenoic Acid (DHA) Supplementation on DHA Levels and Omega-3 Index in Red Blood Cell Membranes of Breast Cancer Patients. Front. Physiol. 2017, 8, 549. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Lionetto, L.; Spagnoli, A.; Ramaccini, C.; De Luca, A.; Simmaco, M.; Monti, M.; Muscaritoli, M. DHA Oral Supplementation Modulates Serum Epoxydocosapentaenoic Acid (EDP) Levels in Breast Cancer Patients. Oxid. Med. Cell. Longev. 2019, 2019, 1280987. [Google Scholar] [CrossRef]
- Arun, B.; Couch, F.J.; Abraham, J.; Tung, N.; Fasching, P.A. BRCA-Mutated Breast Cancer: The Unmet Need, Challenges and Therapeutic Benefits of Genetic Testing. Br. J. Cancer 2024, 131, 1400–1414. [Google Scholar] [CrossRef] [PubMed]
- Mas, E.; Croft, K.D.; Zahra, P.; Barden, A.; Mori, T.A. Resolvins D1, D2, and Other Mediators of Self-Limited Resolution of Inflammation in Human Blood Following n-3 Fatty Acid Supplementation. Clin. Chem. 2012, 58, 1476–1484. [Google Scholar] [CrossRef]
- Sulciner, M.L.; Serhan, C.N.; Gilligan, M.M.; Mudge, D.K.; Chang, J.; Gartung, A.; Lehner, K.A.; Bielenberg, D.R.; Schmidt, B.; Dalli, J.; et al. Resolvins Suppress Tumor Growth and Enhance Cancer Therapy. J. Exp. Med. 2018, 215, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.; Johansson, A.; Nordenskjöld, A.; Iftimi, A.; Yau, C.; Perez-Tenorio, G.; Benz, C.; Nordenskjöld, B.; Stål, O.; Esserman, L.J.; et al. Assessment of 25-Year Survival of Women With Estrogen Receptor-Positive/ERBB2-Negative Breast Cancer Treated With and Without Tamoxifen Therapy: A Secondary Analysis of Data From the Stockholm Tamoxifen Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2114904. [Google Scholar] [CrossRef]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, Y.-J.; Bae, S.J.; Baek, S.H.; Kook, Y.; Cha, Y.J.; Lee, J.W.; Son, B.H.; Ahn, S.H.; Lee, H.J.; et al. Ki-67, 21-Gene Recurrence Score, Endocrine Resistance, and Survival in Patients With Breast Cancer. JAMA Netw. Open 2023, 6, e2330961. [Google Scholar] [CrossRef] [PubMed]
- Schebb, N.H.; Kühn, H.; Kahnt, A.S.; Rund, K.M.; O’Donnell, V.B.; Flamand, N.; Peters-Golden, M.; Jakobsson, P.-J.; Weylandt, K.H.; Rohwer, N.; et al. Formation, Signaling and Occurrence of Specialized Pro-Resolving Lipid Mediators-What Is the Evidence so Far? Front. Pharmacol. 2022, 13, 838782. [Google Scholar] [CrossRef]
- Calder, P.C. Eicosapentaenoic and Docosahexaenoic Acid Derived Specialised Pro-Resolving Mediators: Concentrations in Humans and the Effects of Age, Sex, Disease and Increased Omega-3 Fatty Acid Intake. Biochimie 2020, 178, 105–123. [Google Scholar] [CrossRef]
- Fedirko, V.; McKeown-Eyssen, G.; Serhan, C.N.; Barry, E.L.; Sandler, R.S.; Figueiredo, J.C.; Ahnen, D.J.; Bresalier, R.S.; Robertson, D.J.; Anderson, C.W.; et al. Plasma Lipoxin A4 and Resolvin D1 Are Not Associated with Reduced Adenoma Risk in a Randomized Trial of Aspirin to Prevent Colon Adenomas. Mol. Carcinog. 2017, 56, 1977–1983. [Google Scholar] [CrossRef]
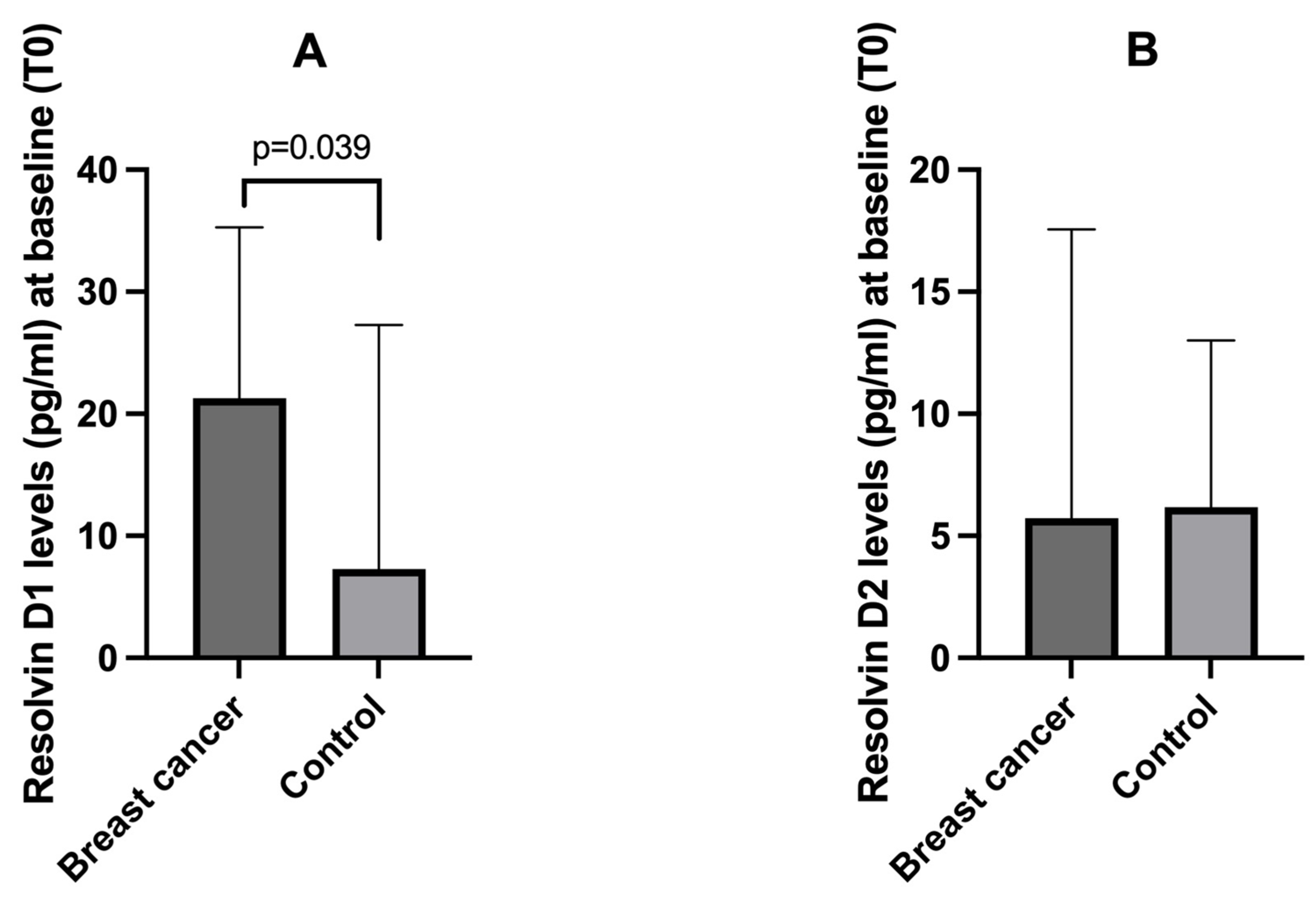
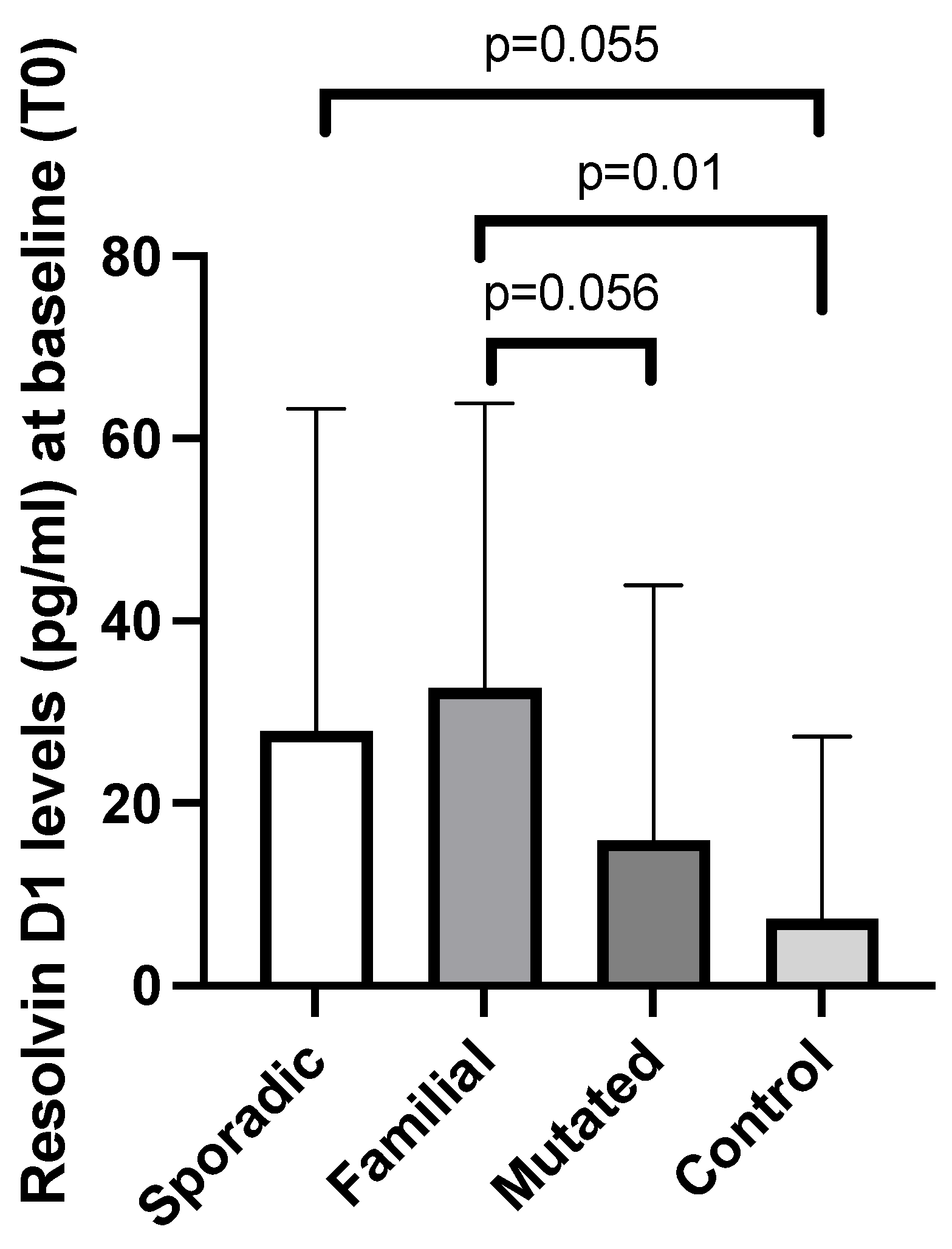
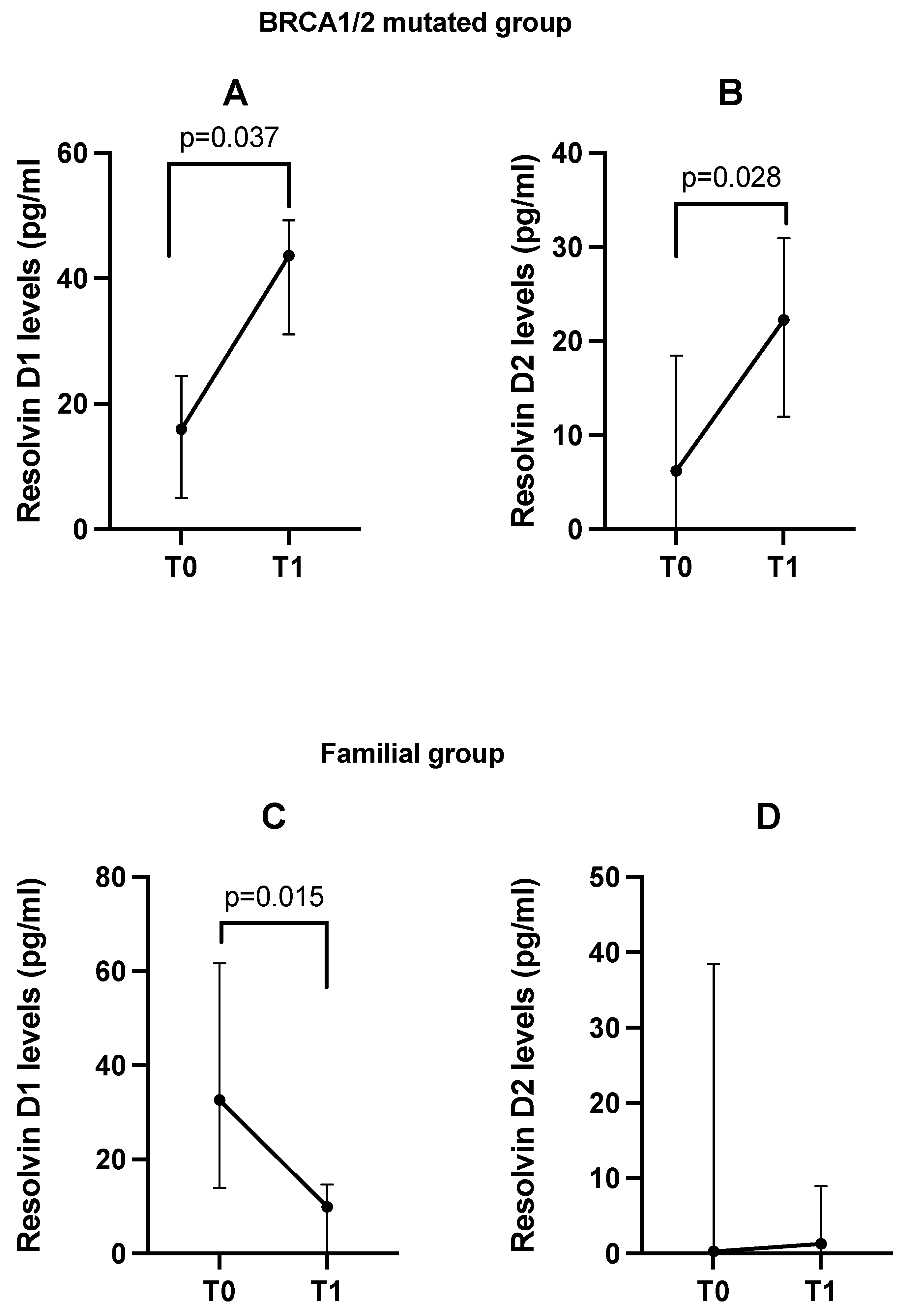
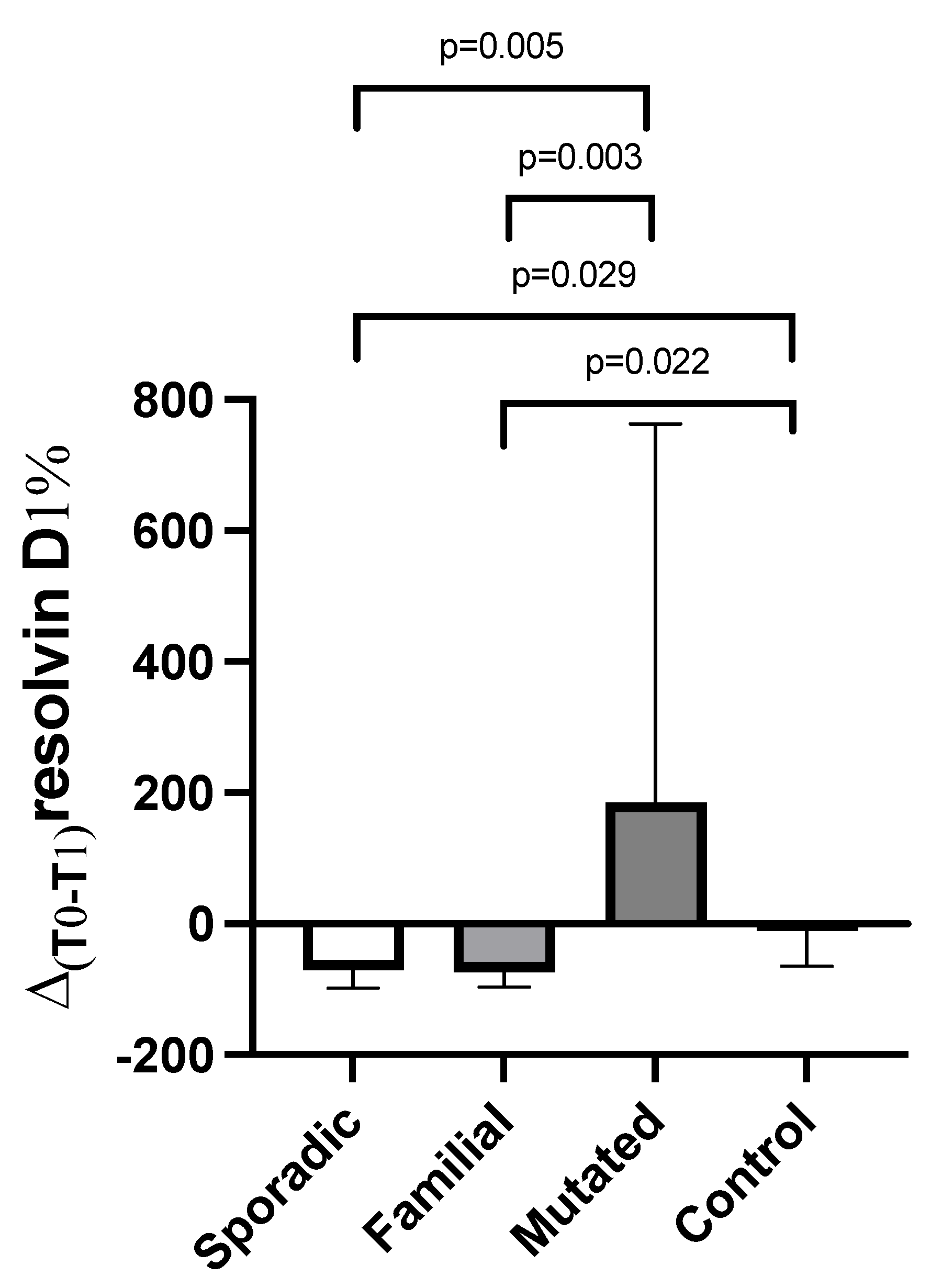
| Parameter | Breast Cancer Patients (N = 26) | Controls (N = 8) |
|---|---|---|
| Age, years | 47.4 ± 8.7 | 47.3 ± 5.4 |
| Actual weight, kg | 64.3 ± 12.8 | 59.1 ± 7.4 |
| Height, cm | 164.0 ± 6.9 | 160.3 ± 2.6 |
| BMI, kg/m2 | 23.9 ± 4.3 | 23.0 ± 2.2 |
| Type of disease presentation | ||
| Sporadic, n (%) | 7 (26.9) | / |
| Familial, n (%) | 9 (34.6) | / |
| Mutated, n (%) | 10 (38.5) | / |
| Immunohistochemistry | ||
| Luminal-A, n (%) | 11 (42.3) | / |
| Luminal-B, n (%) | 1 (3.8) | / |
| HER2+, n (%) | 8 (30.8) | / |
| Triple-negative, n (%) | 6 (23.1) | / |
| High Ki67% ( | 20 (76.9) | / |
| Main Comorbidities | ||
| Diabetes, n (%) | 1 (3.8) | 0 (0) |
| Dyslipidemia, n (%) | 3 (11.5) | 0 (0) |
| Resolvin D1 | Resolvin D2 | |||
|---|---|---|---|---|
| T0 | T1 | T0 | T1 | |
| Breast cancer | 21.3 (7.9; 41.4) | 14.6 (0.0; 40.9) | 5.3 (0.0; 22.1) | 8.9 (0.0; 26.9) |
| Controls | 7.3 (0.3; 23.8) | 17.9 (0.8; 30.8) | 6.2 (0.0; 10.7) | 9.9 (0.0; 27.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molfino, A.; Imbimbo, G.; Salerno, G.; Lionetto, L.; De Luca, A.; Simmaco, M.; Gallicchio, C.; Picconi, O.; Amabile, M.I.; Muscaritoli, M. Effects of DHA Oral Supplementation on Plasma Resolvin D1 and D2 Levels in Naïve Breast Cancer Patients. Cancers 2025, 17, 1694. https://doi.org/10.3390/cancers17101694
Molfino A, Imbimbo G, Salerno G, Lionetto L, De Luca A, Simmaco M, Gallicchio C, Picconi O, Amabile MI, Muscaritoli M. Effects of DHA Oral Supplementation on Plasma Resolvin D1 and D2 Levels in Naïve Breast Cancer Patients. Cancers. 2025; 17(10):1694. https://doi.org/10.3390/cancers17101694
Chicago/Turabian StyleMolfino, Alessio, Giovanni Imbimbo, Gerardo Salerno, Luana Lionetto, Alessandro De Luca, Maurizio Simmaco, Carmen Gallicchio, Orietta Picconi, Maria Ida Amabile, and Maurizio Muscaritoli. 2025. "Effects of DHA Oral Supplementation on Plasma Resolvin D1 and D2 Levels in Naïve Breast Cancer Patients" Cancers 17, no. 10: 1694. https://doi.org/10.3390/cancers17101694
APA StyleMolfino, A., Imbimbo, G., Salerno, G., Lionetto, L., De Luca, A., Simmaco, M., Gallicchio, C., Picconi, O., Amabile, M. I., & Muscaritoli, M. (2025). Effects of DHA Oral Supplementation on Plasma Resolvin D1 and D2 Levels in Naïve Breast Cancer Patients. Cancers, 17(10), 1694. https://doi.org/10.3390/cancers17101694








