CT and MRI Key Features of Benign Tumors and Tumor-like Lesions of the Tongue: A Pictorial Review
Simple Summary
Abstract
1. Introduction
2. Benign vs. Malignant Tongue Lesions: Diagnostic Imaging Features
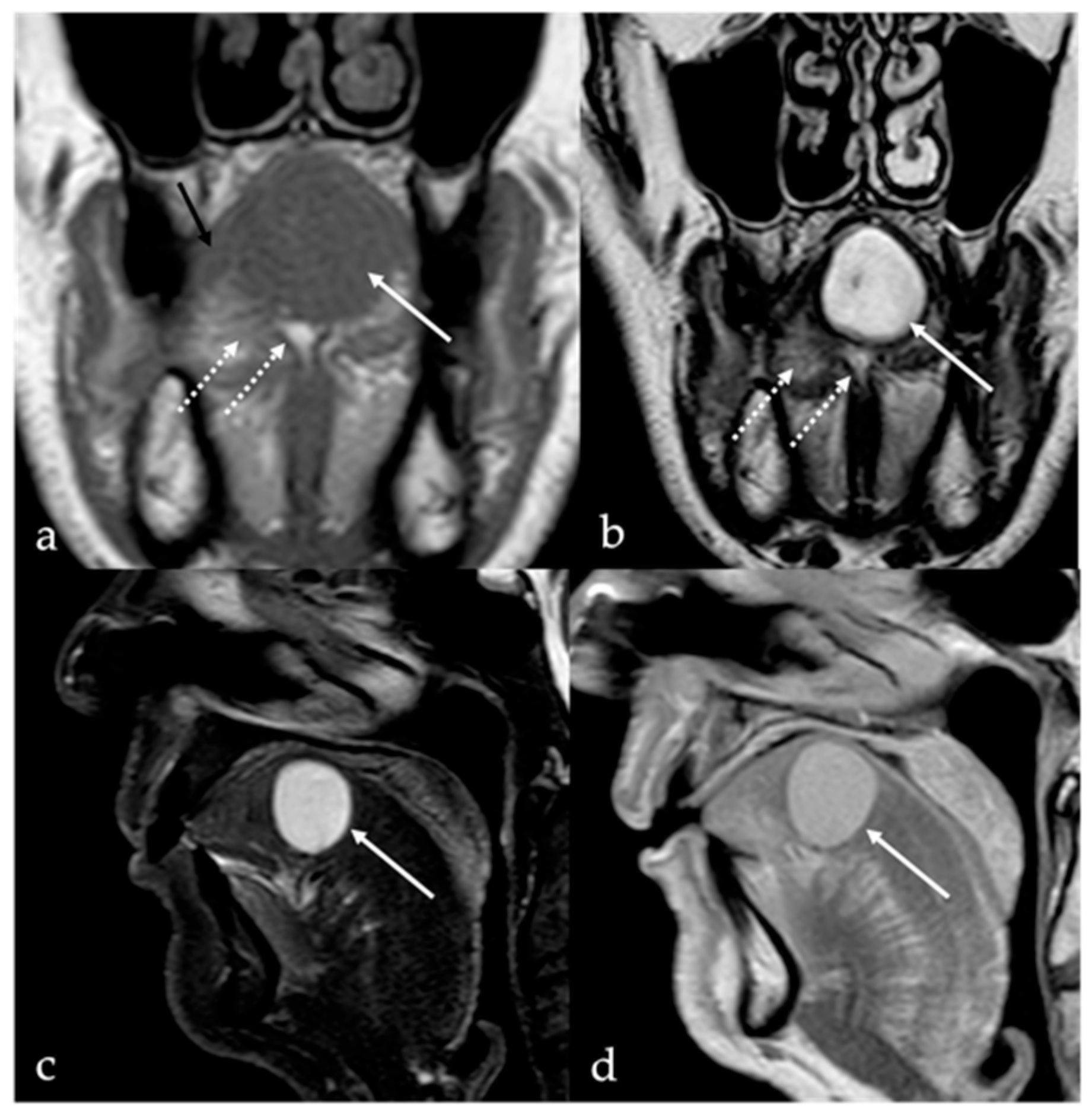
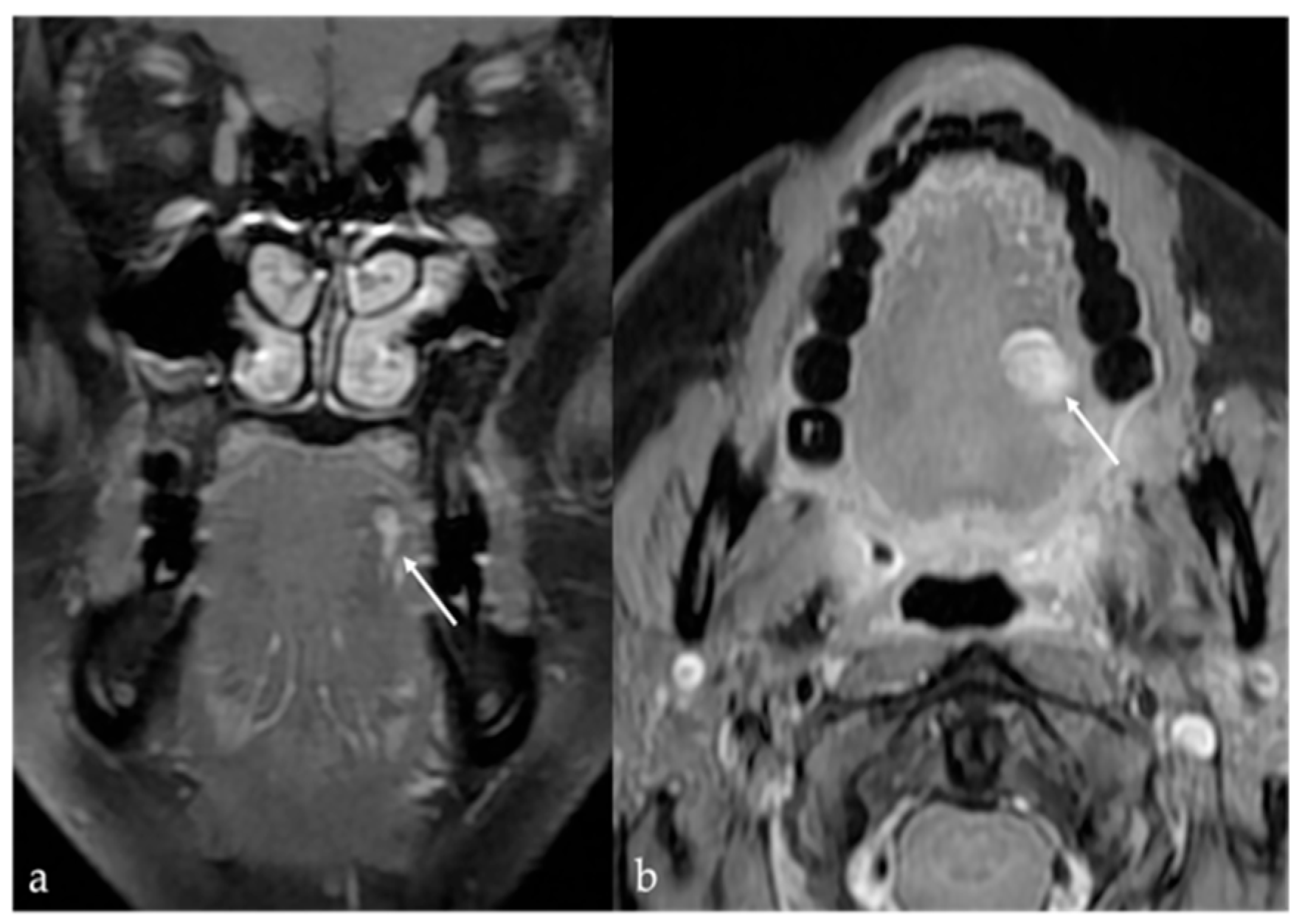
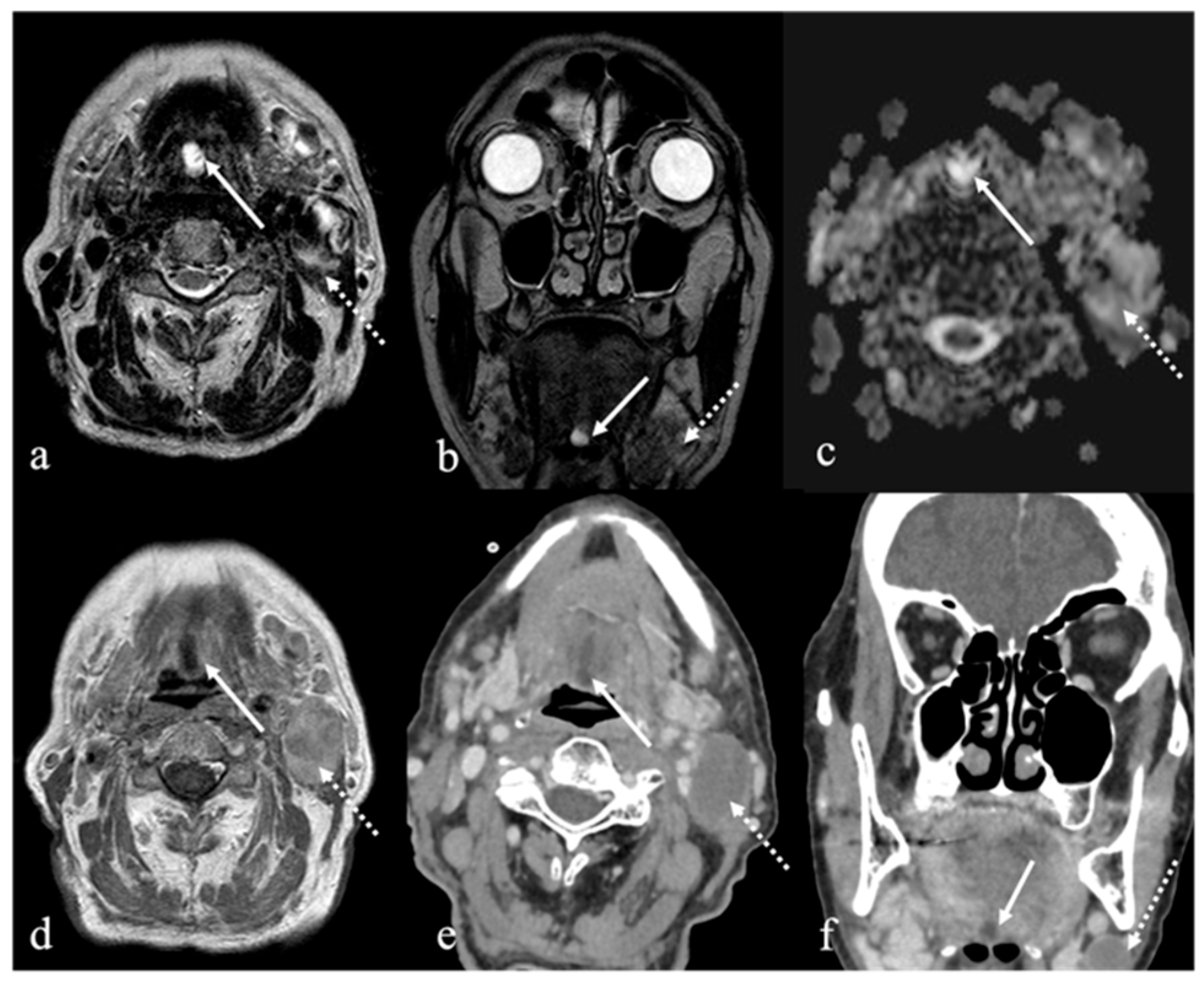
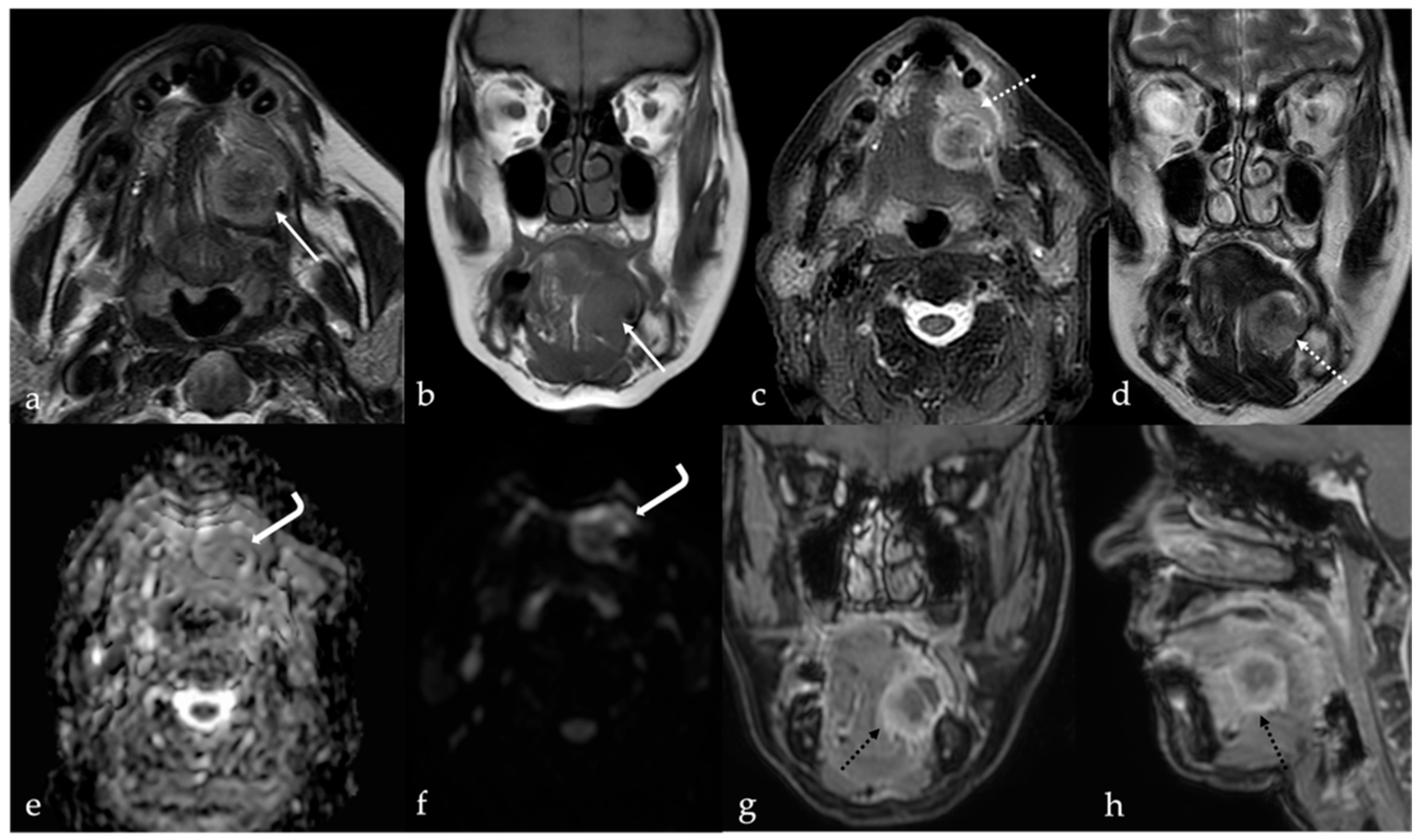
3. Schwannoma
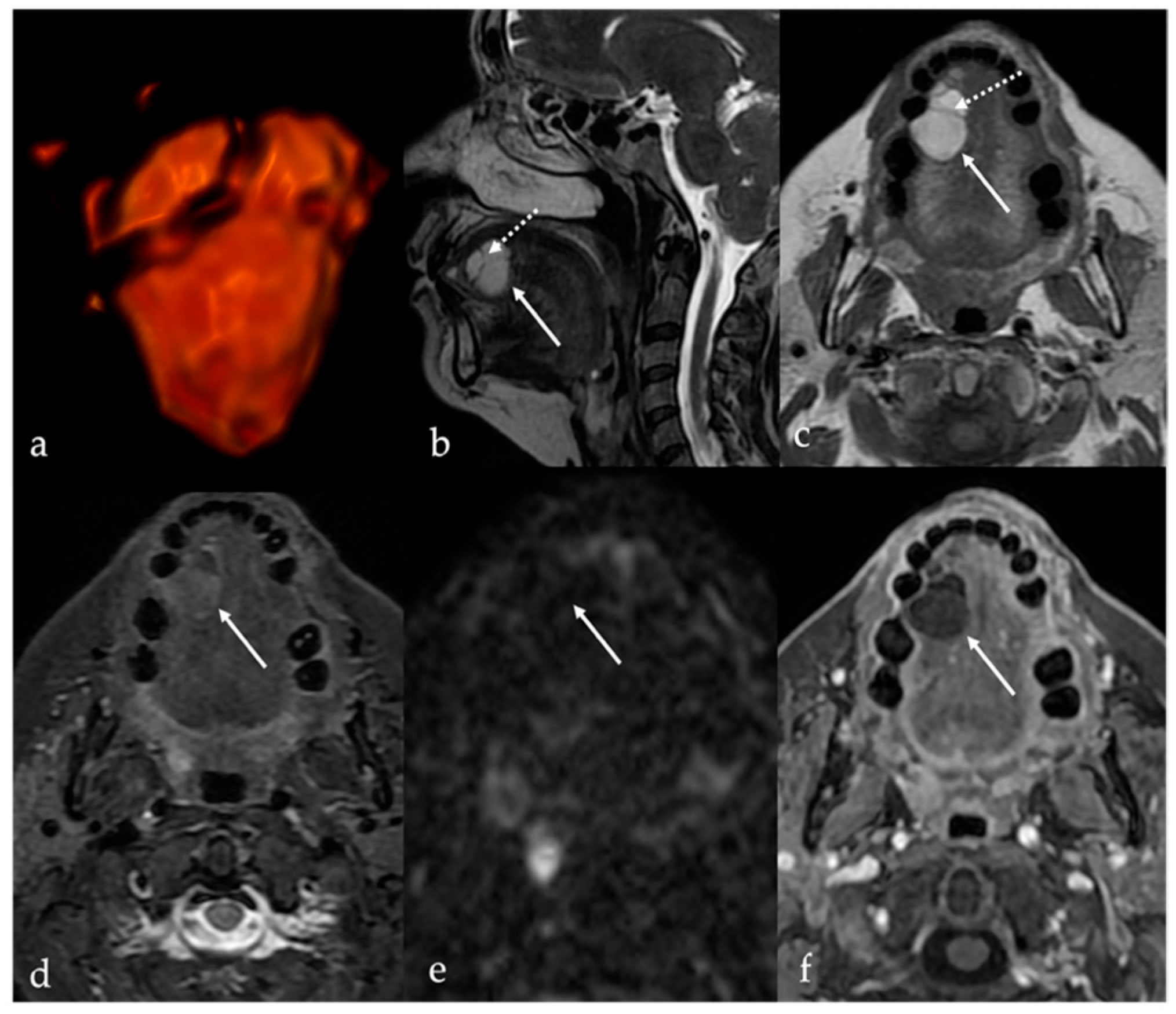
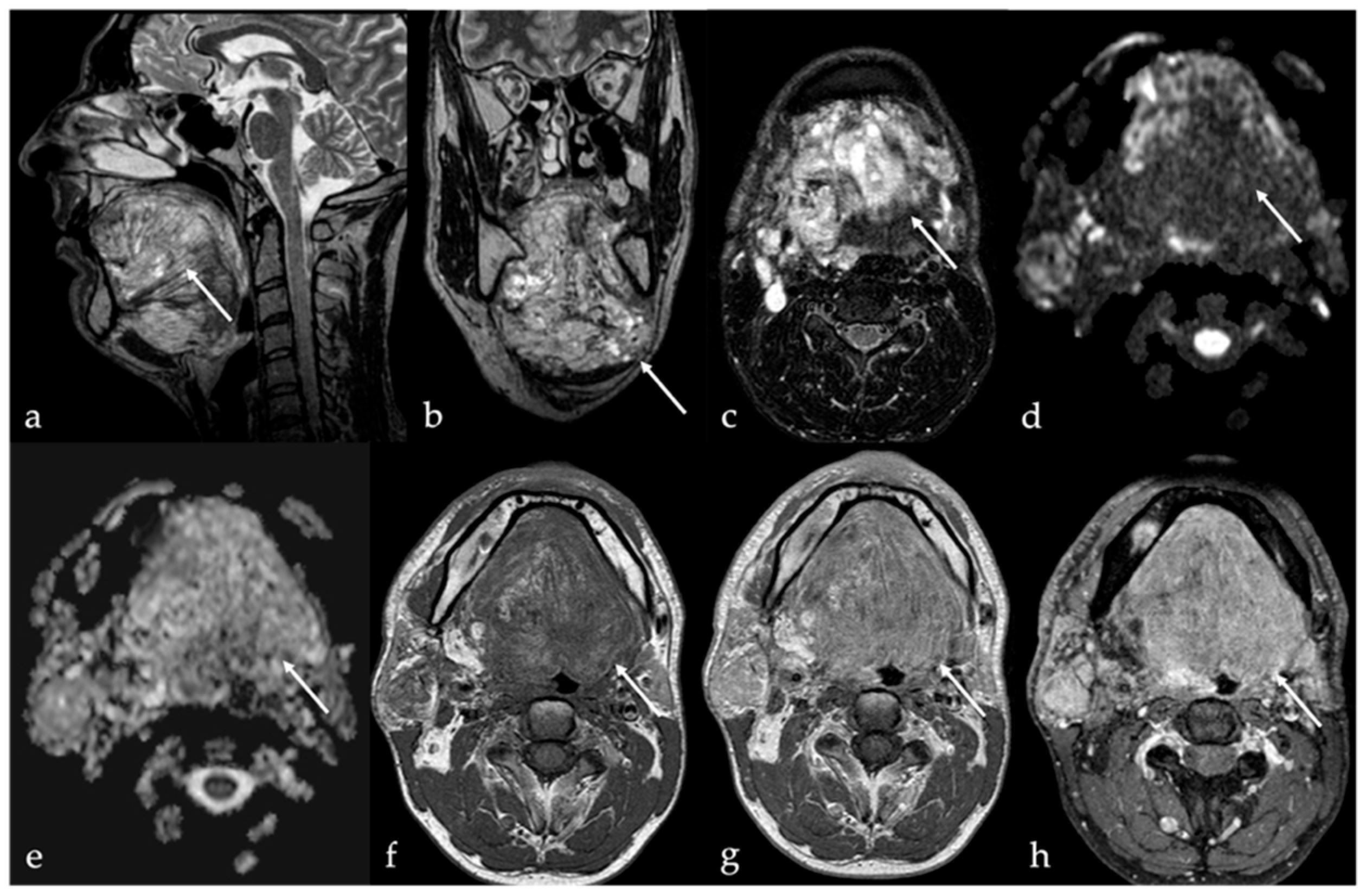
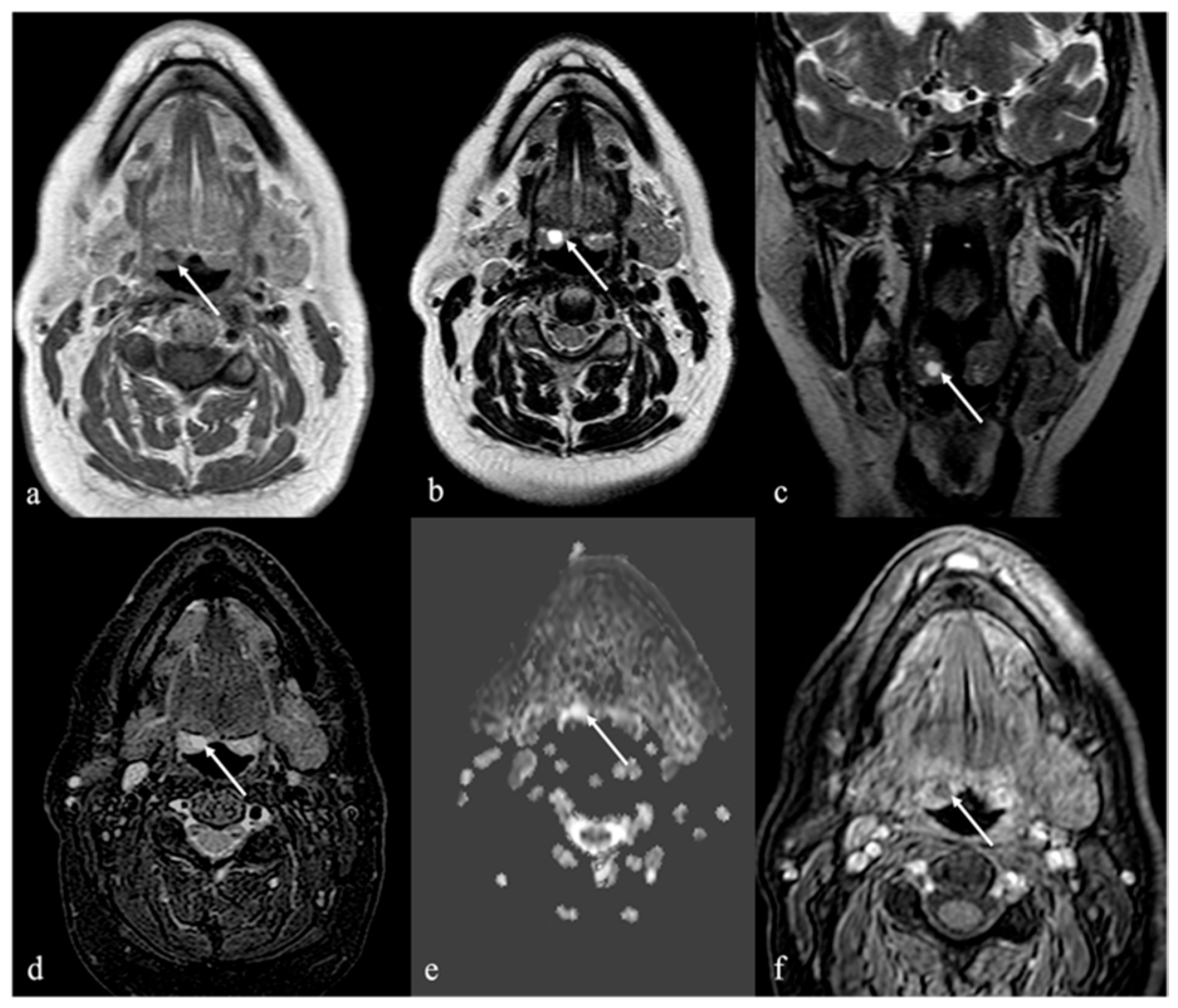
4. Lipoma and Angiomyolipoma
5. Vascular Malformations and Tumors
5.1. Venous Malformations
5.2. Lymphatic Malformations
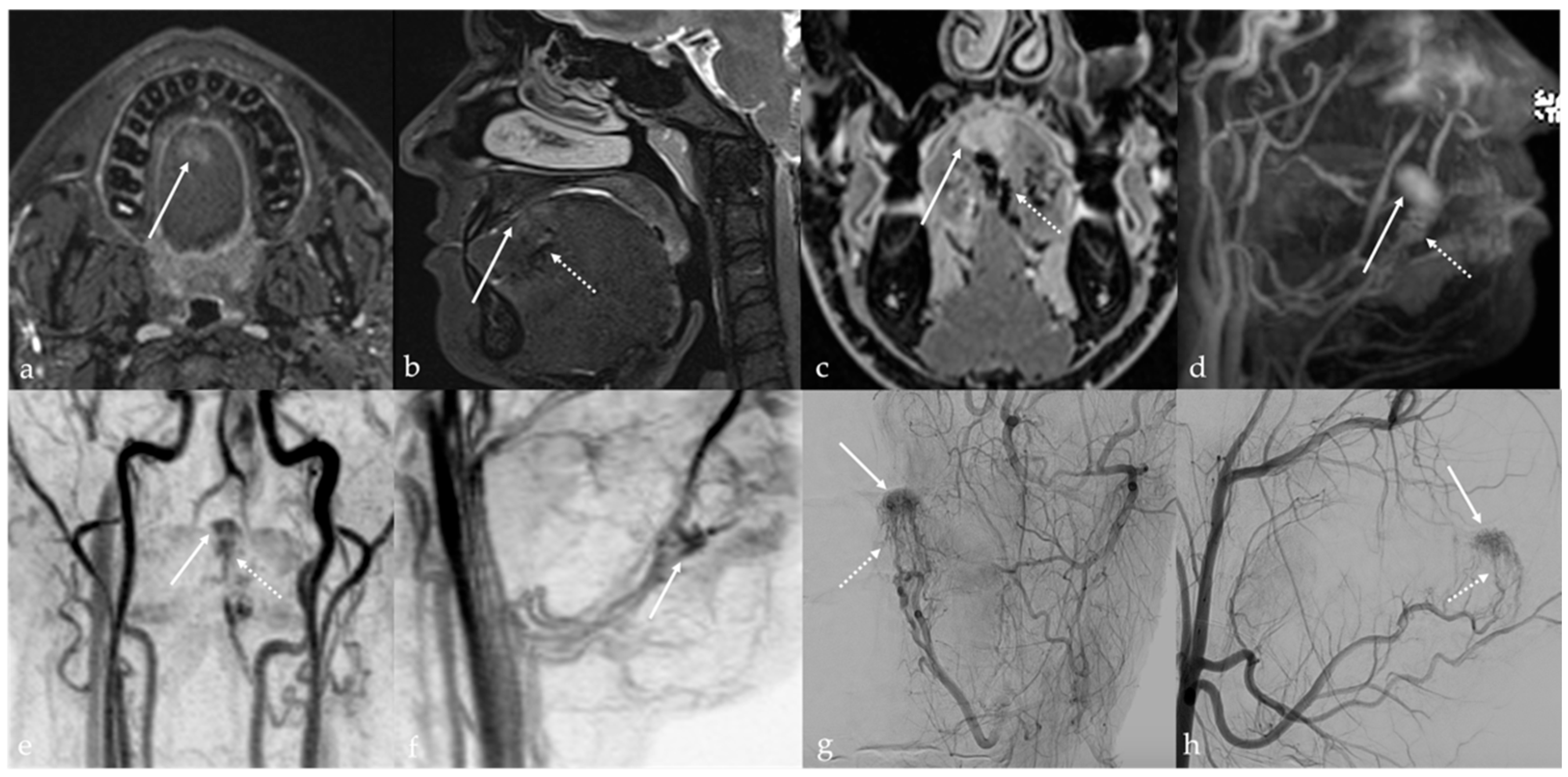
5.3. Arteriovenous Malformations
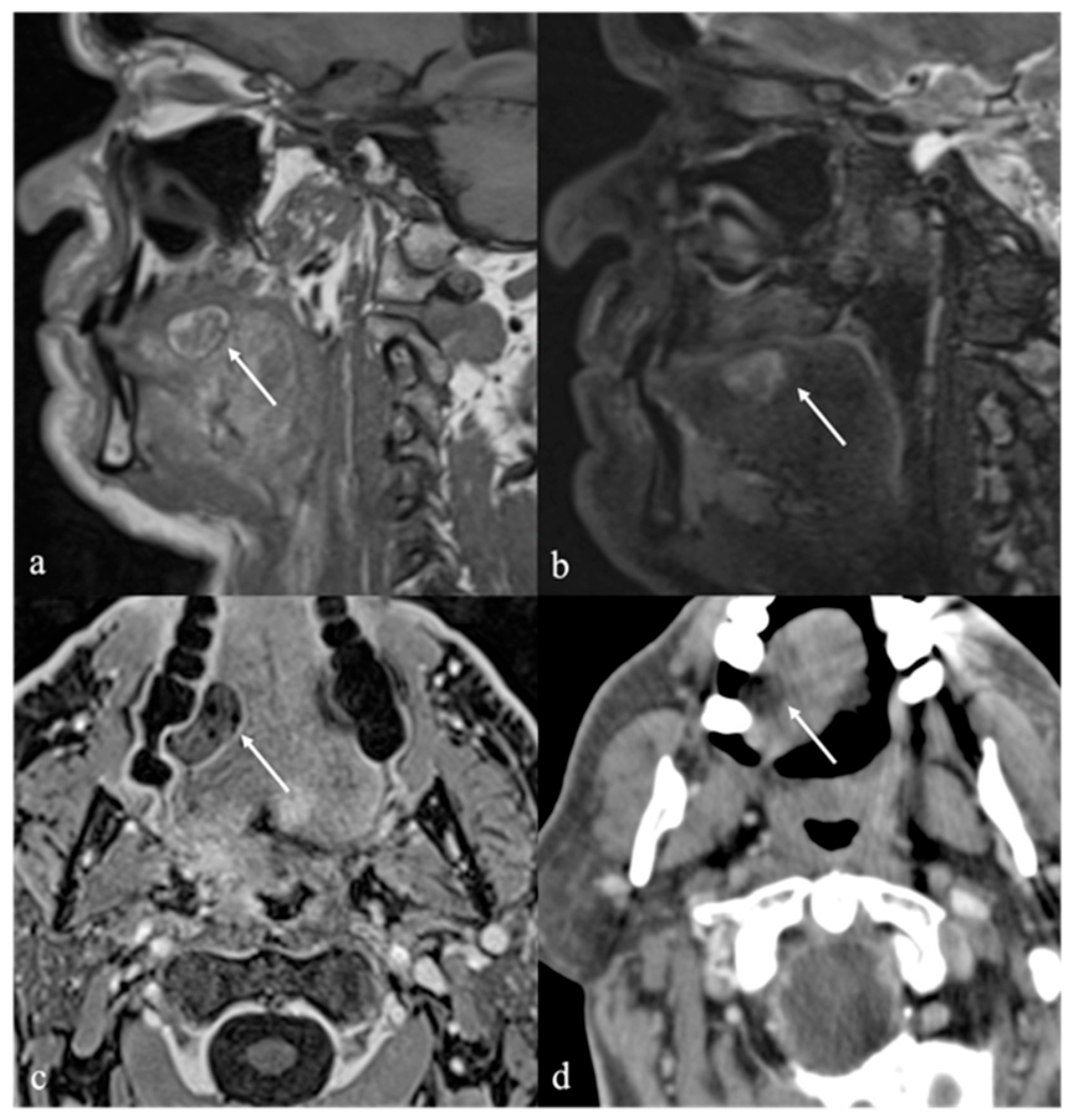
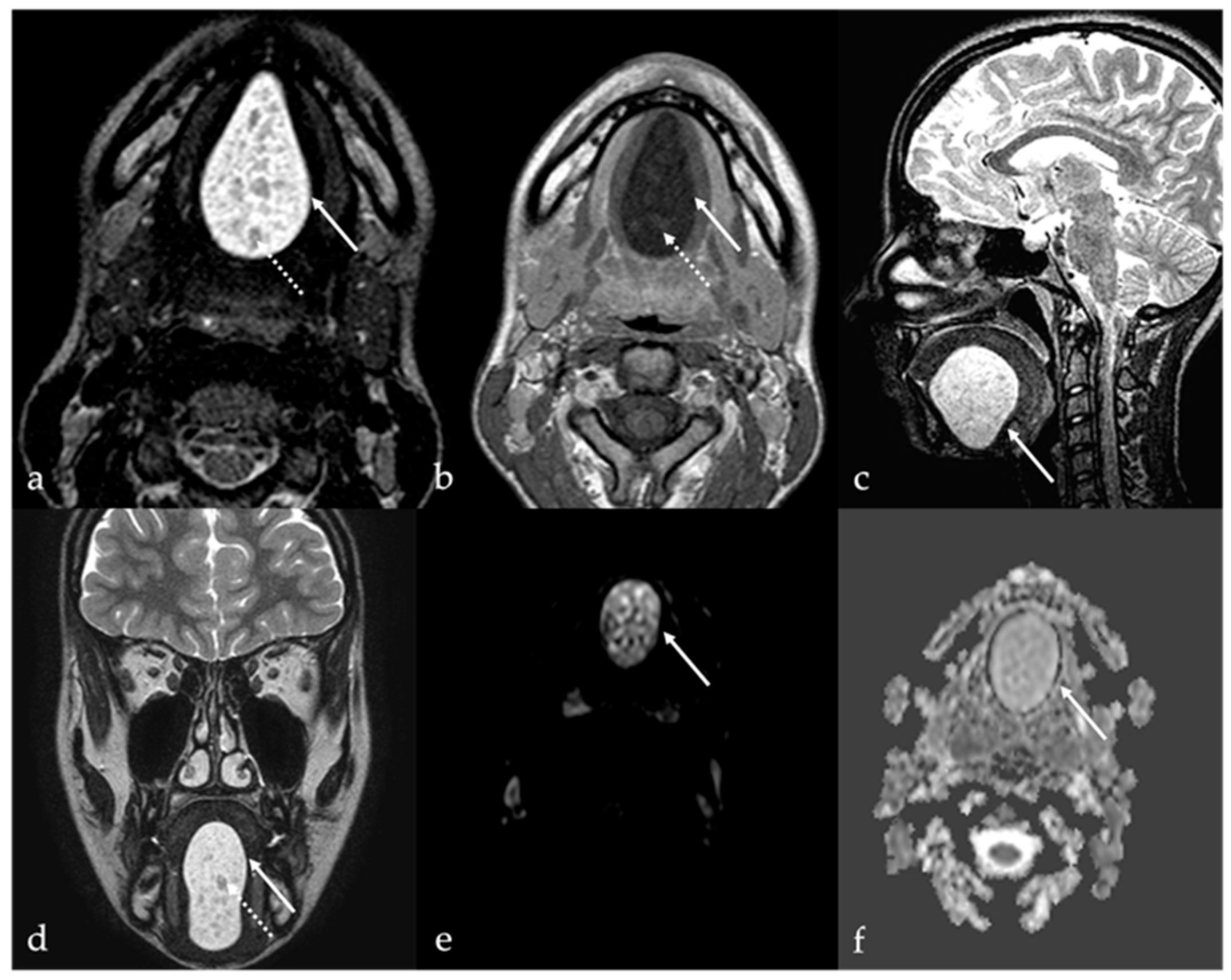
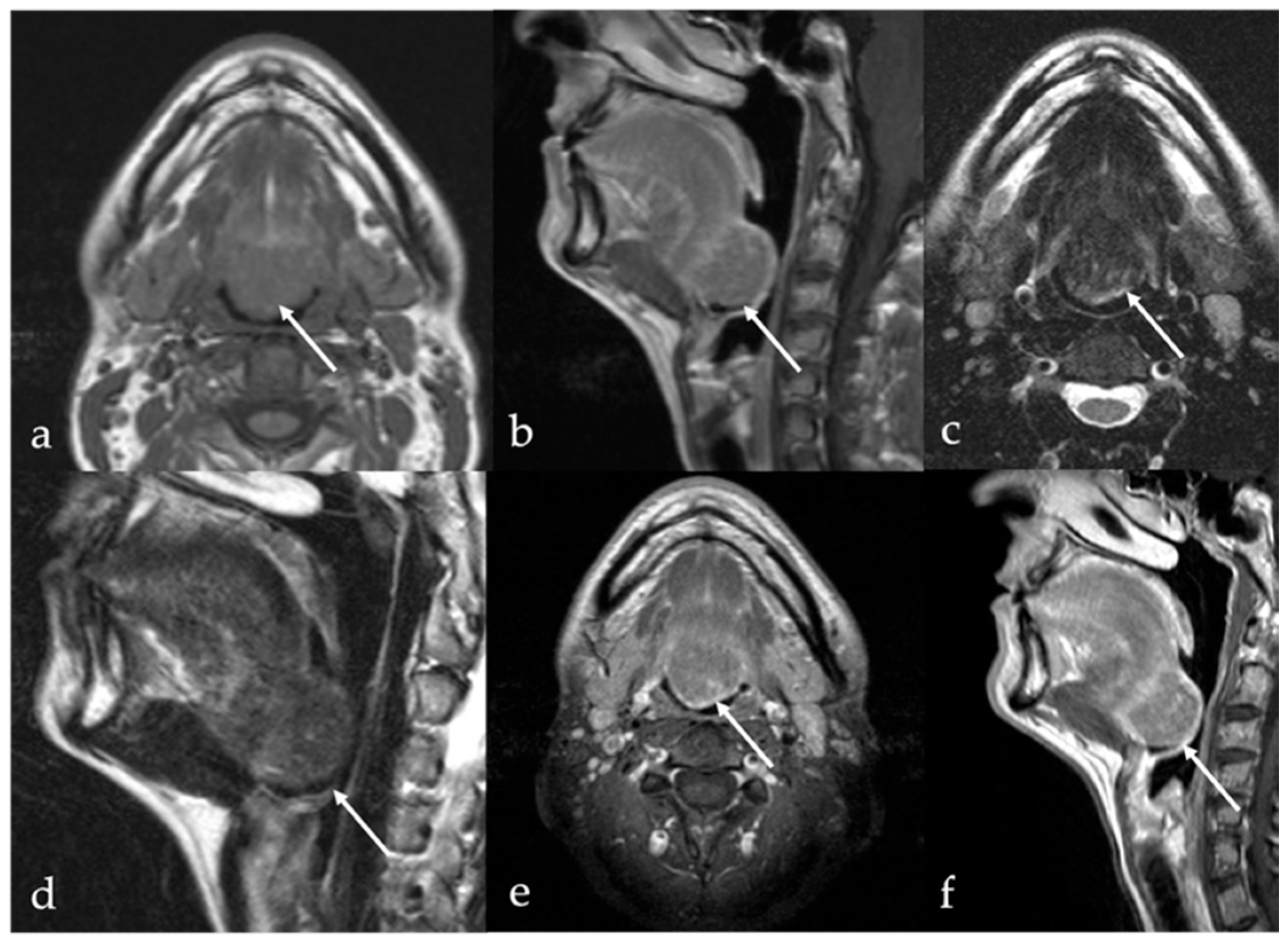
6. Dermoid Cysts
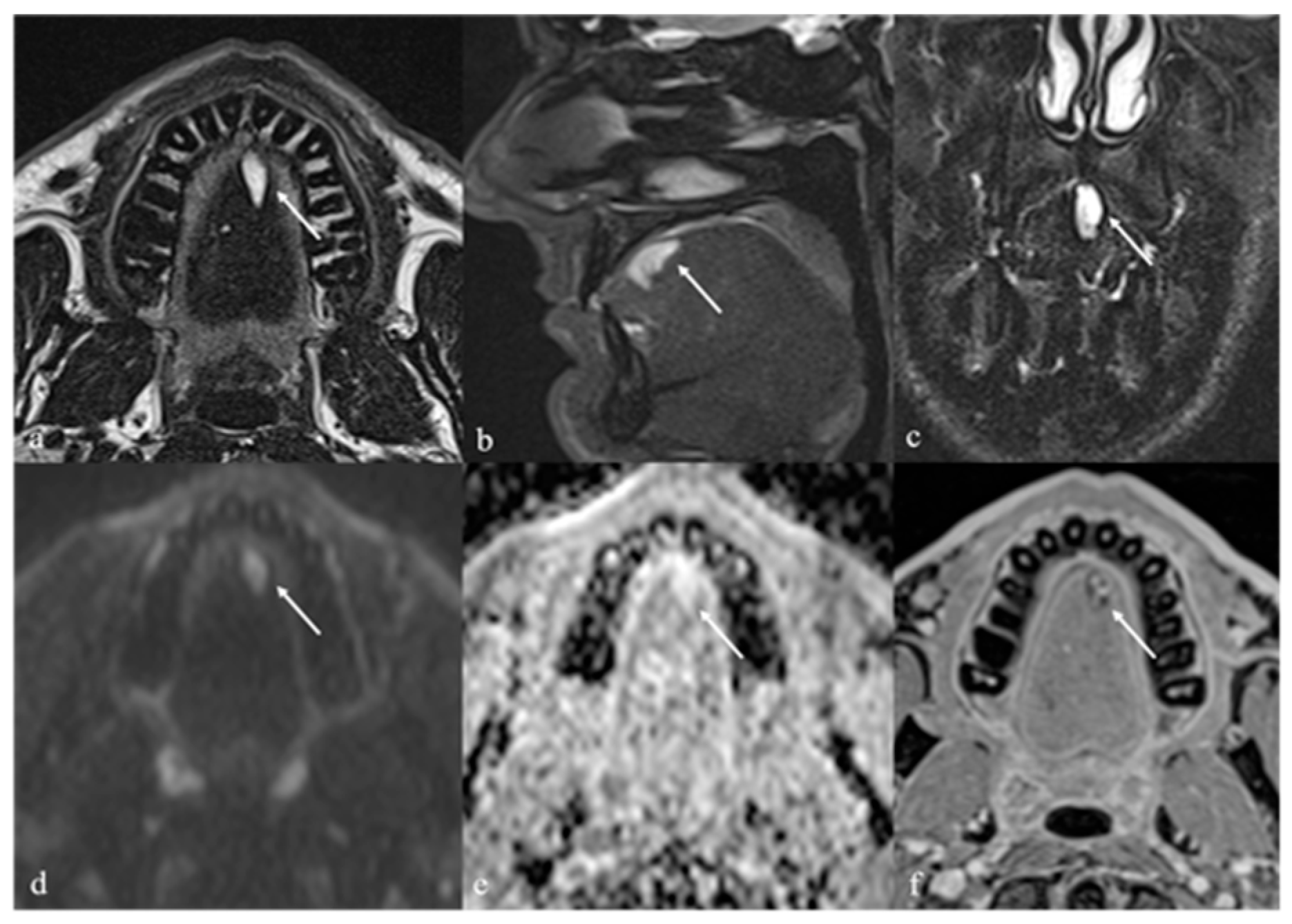
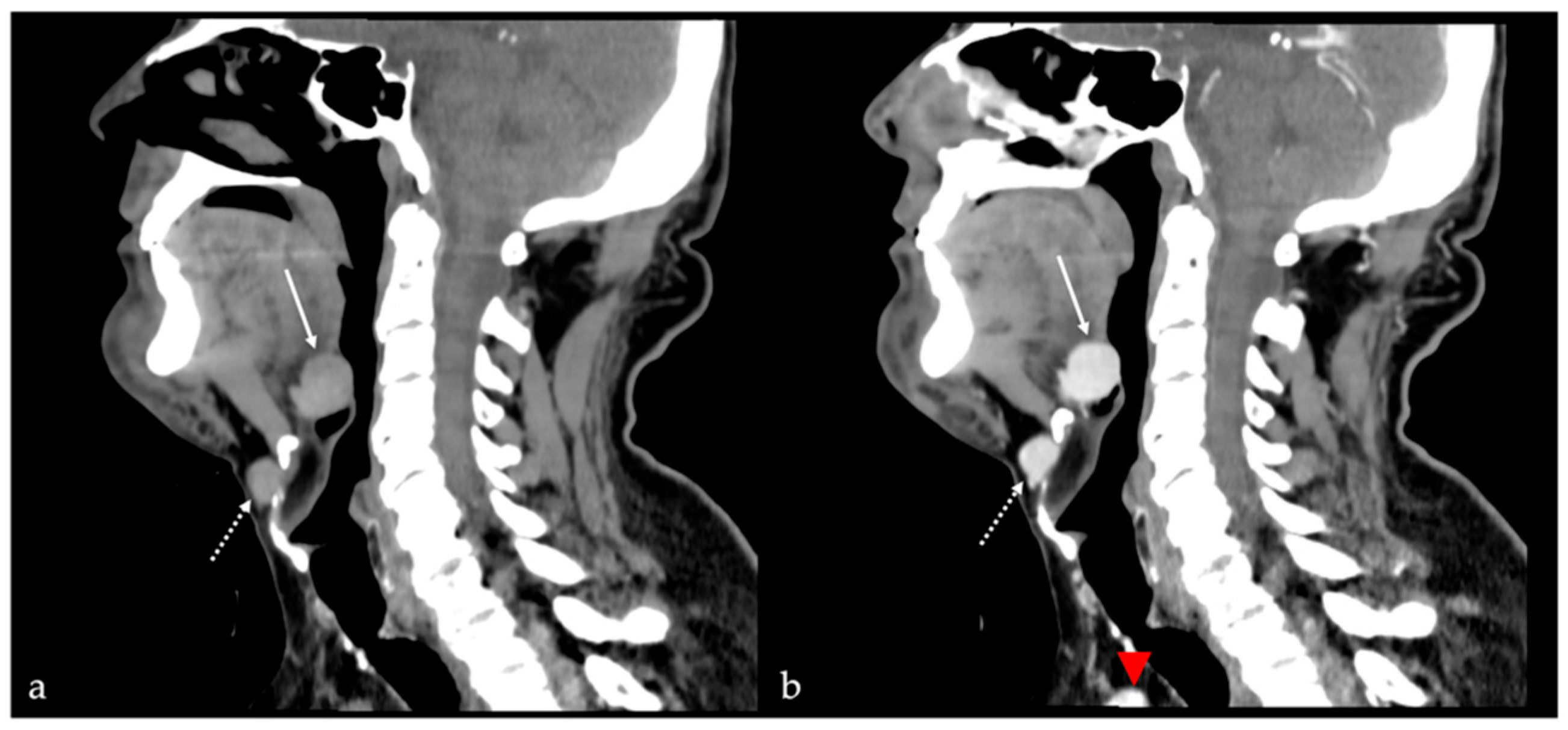
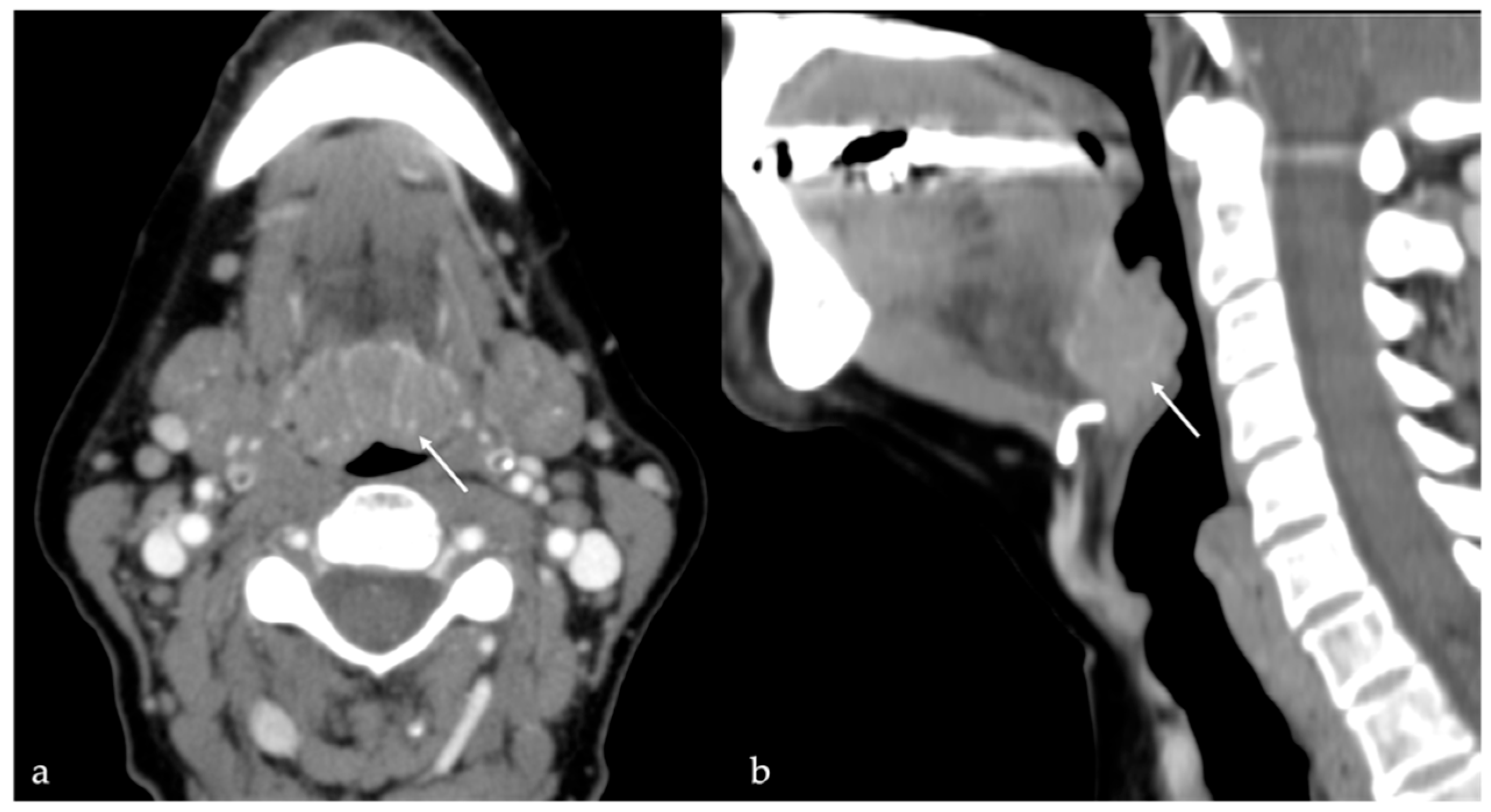
7. Thyroglossal Duct Remnants
7.1. Thyroglossal Duct Cyst
7.2. Ectopic Thyroid Tissue
8. Lingual Abscess
9. Lingual Tonsillitis as Manifestation of Infectious Mononucleosis
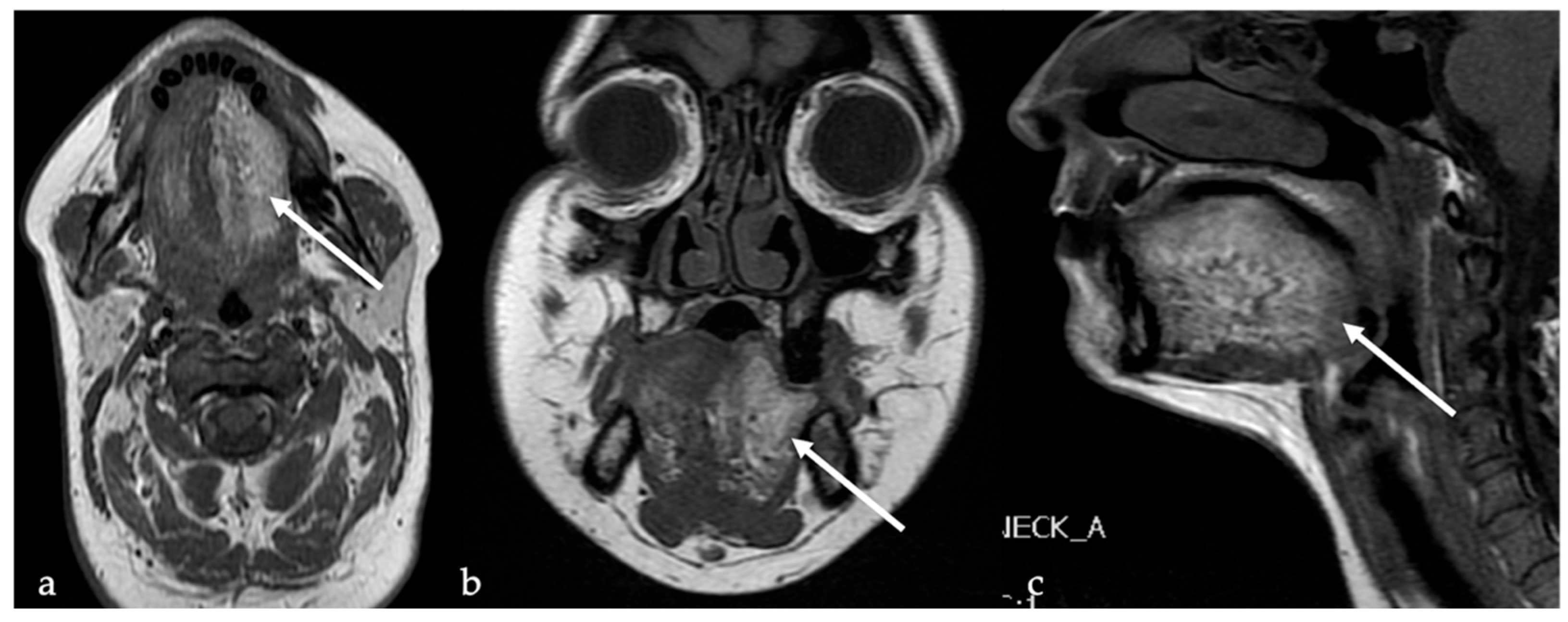
10. Fatty Atrophy of the Tongue (Chronic Hemilingual Denervation)
11. CT X-Ray and MRI Scans Impact on Human Head/Thorax Portion
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, L.; Li, Y.; Zi, Y.; Rong, D.; Li, Y.; Li, X.; Xu, F.; Wu, H. MRI findings of benign tumors and tumor-like diseases of the tongue with radiologic—pathologic correlation. Jpn. J. Radiol. 2023, 41, 19–26. [Google Scholar] [CrossRef]
- Stephen, W.; Moore, M. Griffith’s Instructions for Patients, 8th ed.; Stephen, W., Moore, M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 254–276. ISBN 9781437709094. [Google Scholar] [CrossRef]
- Maraghelli, D.; Pietragalla, M.; Cordopatri, C.; Nardi, C.; Peired, A.J.; Maggiore, G.; Colagrande, S. Magnetic resonance imaging of salivary gland tumours: Key findings for imaging characterisation. Eur. J. Radiol. 2021, 139, 109716. [Google Scholar] [CrossRef]
- Maraghelli, D.; Pietragalla, M.; Calistri, L.; Barbato, L.; Locatello, L.G.; Orlandi, M.; Landini, N.; Lo Casto, A.; Nardi, C. Techniques, Tricks, and Stratagems of Oral Cavity Computed Tomography and Magnetic Resonance Imaging. Appl. Sci. 2022, 12, 1473. [Google Scholar] [CrossRef]
- Ai, S.; Zhu, W.; Liu, Y.; Wang, P.; Yu, Q.; Dai, K. Combined DCE- and DW-MRI in diagnosis of benign and malignant tumors of the tongue. Front. Biosci. 2013, 18, 1098–1111. [Google Scholar] [CrossRef]
- Badar, Z.; Farooq, Z.; Zaccarini, D.; Ezhapilli, S.R. Tongue base schwannoma: Differential diagnosis and imaging features with a case presentation. Radiol. Case Rep. 2016, 11, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, T.; Fisher, S.; Karri, S.; Ramzi, A.; Sharma, R.; Chhabra, A. Advanced MR imaging of peripheral nerve sheath tumors including diffusion imaging. Semin. Musculoskelet. Radiol. 2015, 19, 179–190. [Google Scholar] [CrossRef]
- Yoon, Y.A.; Kwon, Y.E.; Choi, S.Y.; Choi, K.S.; An, C.H.; An, S.Y. Giant lipoma of the tongue: A case report and review of the literature. Imaging Sci. Dent. 2022, 52, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Lo Casto, A.; Salerno, S.; Cannizzaro, F.; Caronia, A.; Bencivinni, F.; Barbiera, F.; Rossello, M.; La Tona, G. MRI findings in lingual venous malformations. Dentomaxillofacial Radiol. 2003, 32, 333–336. [Google Scholar] [CrossRef]
- Petkova, M.; Ferby, I.; Mäkinen, T. Lymphatic malformations: Mechanistic insights and evolving therapeutic frontiers. J. Clin. Investig. 2024, 134, e172844. [Google Scholar] [CrossRef]
- Elluru, R.G.; Balakrishnan, K.; Padua, H.M. Lymphatic malformations: Diagnosis and management. Semin. Pediatr. Surg. 2014, 23, 178–185. [Google Scholar] [CrossRef]
- Hendriks, T.; Pollaers, K.; Phillips, T.; Kuthubutheen, J. Tongue arteriovenous malformation with oral haemorrhage treated by embolisation. BMJ Case Rep. 2020, 13, e235366. [Google Scholar] [CrossRef]
- Giarraputo, L.; Savastano, S.; D’Amore, E.; Baciliero, U. Dermoid Cyst of the Floor of the Mouth: Diagnostic Imaging Findings. Cureus 2018, 10, e2403. [Google Scholar] [CrossRef] [PubMed]
- Basla, N.; Sfondrini, D.; Achilli, M.F.; Catalano, M.; Sanvito, F.; Sala, M.G.; Marelli, S.; Benazzo, M.; Preda, L. Imaging features of epidermoid cyst located in the floor of the mouth: Case report and narrative review of literature. Acta Otorhinolaryngol. Ital. 2023, 43, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Glastonbury, C.M.; Davidson, H.C.; Haller, J.R.; Harnsberger, H.R. The CT and MR imaging features of carcinoma arising in thyroglossal duct remnants. AJNR Am. J. Neuroradiol. 2000, 21, 770–774. [Google Scholar] [PubMed] [PubMed Central]
- Sturniolo, G.; Vermiglio, F.; Moleti, M. Thyroid cancer in lingual thyroid and thyroglossal duct cyst. Endocrinol. Diabetes Nutr. 2017, 64, 40–43, English, Spanish. [Google Scholar] [CrossRef]
- Ozturk, M.; Mavili, E.; Erdogan, N.; Cagli, S.; Guney, E. Tongue abscesses: MR imaging findings. AJNR Am. J. Neuroradiol. 2006, 27, 1300–1303. [Google Scholar] [PubMed] [PubMed Central]
- Surov, A.; Ryl, I.; Bartel-Friedrich, S.; Wienke, A.; Kösling, S. MRI of nasopharyngeal adenoid hypertrophy. Neuroradiol. J. 2016, 29, 408–412. [Google Scholar] [CrossRef]
- Gorolay, V.V.; Tran, N.A.; Tade, R.; Baugnon, K.; Aiken, A.; Wu, X. The ptotic tongue—Imaging appearance and pathology localization along the course of the hypoglossal nerve. Neuroradiology 2023, 65, 1425–1438. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, M.H.; Park, S.W.; Chang, K.H. Radiologic-Pathologic Correlation of Unusual Lingual Masses: Part II: Benign and Malignant Tumors. Korean J. Radiol. 2001, 2, 42–51. [Google Scholar] [CrossRef]
- Lo Casto, A.; Cannella, R.; Taravella, R.; Cordova, A.; Matta, D.; Campisi, G.; Attanasio, M.; Rinaldi, G.; Rodolico, V. Diagnostic and prognostic value of magnetic resonance imaging in the detection of tumor depth of invasion and bone invasion in patients with oral cavity cancer. Radiol. Med. 2022, 127, 1364–1372. [Google Scholar] [CrossRef]
- Ong, C.K.; Chong, V.F. Imaging of tongue carcinoma. Cancer Imaging 2006, 6, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Lira, R.B.; Gonçalves Filho, J.; Carvalho, G.B.; Pinto, C.A.; Kowalski, L.P. Lingual schwannoma: Case report and review of the literature. Acta Otorhinolaryngol. Ital. 2013, 33, 137–140. [Google Scholar] [PubMed] [PubMed Central]
- Abreu, I.; Roriz, D.; Rodrigues, P.; Moreira, Â.; Marques, C.; Alves, F.C. Schwannoma of the tongue—A common tumour in a rare location: A case report. Eur. J. Radiol. Open 2017, 4, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.K.; Sahoo, P.K.; Mohapatra, D.; Subudhi, S. Two Concurrent Large Epidermoid Cysts in Sublingual and Submental Region Resembling Plunging Ranula: Report of a Rare Case. Ann. Maxillofac. Surg. 2017, 7, 155–158. [Google Scholar] [CrossRef]
- Koizumi, H.; Ishihama, K.; Enomoto, A.; Kogo, M. Angiomyolipoma of the tongue. Br. J. Oral Maxillofac. Surg. 2008, 46, e3–e4. [Google Scholar] [CrossRef]
- Baonerkar, H.A.; Vora, M.; Sorathia, R.; Shinde, S. The lipoma of tongue—A rare site for a tumor: Case report and review of the literature. Indian. J. Dent. 2015, 6, 207–210. [Google Scholar] [CrossRef]
- Sarfi, D.; Konaté, M.; Adnane, S.; Elbouhairi, M.; Ben Yahya, I. Intra oral lipoma: Report of 3 histologically different cases. Adv. Oral Maxillofac. Surg. 2021, 4, 100182. [Google Scholar] [CrossRef]
- Naruse, T.; Yanamoto, S.; Yamada, S.; Rokutanda, S.; Kawakita, A.; Takahashi, H.; Matsushita, Y.; Hayashida, S.; Imayama, N.; Morishita, K.; et al. Lipomas of the oral cavity: Clinicopathological and immunohistochemical study of 24 cases and review of the literature. Indian J. Otolaryngol. Head Neck Surg. 2015, 67, 67–73. [Google Scholar] [CrossRef]
- Jelinek, J.S.; Wu, A.; Wallace, M.; Kumar, D.; Henshaw, R.M.; Murphey, M.J.; Van Horn, A.; Aboulafia, A.J. Imaging of spindle cell lipoma. Clin. Radiol. 2020, 75, 396.e15–396.e21. [Google Scholar] [CrossRef]
- Raj, J.V.; Vigneshwaran, B.; Subbiah, Y.; Subbiah, Y.; Padmanaban, E.; Amirthalingam, U.; Balavaitheeswar, R.L. Unveiling spindle cell lipoma: A radiological case report. Egypt J. Radiol. Nucl. Med. 2024, 55, 182. [Google Scholar] [CrossRef]
- Kunimoto, K.; Yamamoto, Y.; Jinnin, M. ISSVA Classification of Vascular Anomalies and Molecular Biology. Int. J. Mol. Sci. 2022, 23, 2358. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.T.; Friedman, A.B. Hemangiomas and vascular malformations: Current theory and management. Int. J. Pediatr. 2012, 2012, 645678. [Google Scholar] [CrossRef]
- Pourbagher, A.; Pourbagher, M.A.; Karan, B.; Ozkoc, G. MRI manifestations of soft-tissue haemangiomas and accompanying reactive bone changes. Br. J. Radiol. 2011, 84, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.B. Venous malformation and haemangioma: Differential diagnosis, diagnosis, natural history and consequences. Phlebol. J. Venous Dis. 2013, 28 (Suppl. S1), 176–187. [Google Scholar] [CrossRef]
- Baker, L.L.; Dillon, W.P.; Hieshima, G.B.; Dowd, C.F.; Frieden, I.J. Hemangiomas and vascular malformations of the head and neck: MR characterization. AJNR Am. J. Neuroradiol. 1993, 14, 307–314. [Google Scholar] [PubMed] [PubMed Central]
- Kollipara, R.; Dinneen, L.; Rentas, K.E.; Saettele, M.R.; Patel, S.A.; Rivard, D.C.; Lowe, L.H. Current classification and terminology of pediatric vascular anomalies. AJR Am. J. Roentgenol. 2013, 201, 1124–1135. [Google Scholar] [CrossRef]
- Legiehn, G.M.; Heran, M.K. Venous Malformations: Classification, Development, Diagnosis, and Interventional Radiologic Management. Radiol. Clin. N. Am. 2008, 46, 545–597. [Google Scholar] [CrossRef]
- Wiegand, S.; Tiburtius, J.; Zimmermann, A.P.; Güldner, C.; Eivazi, B.; Werner, J.A. Localization and treatment of lingual venous and arteriovenous malformations. Vasc. Med. 2014, 19, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Cappabianca, S.; Del Vecchio, W.; Giudice, A.; Colella, G. Vascular malformations of the tongue: MRI findings on three cases. Dentomaxillofacial Radiol. 2006, 35, 205–208. [Google Scholar] [CrossRef]
- Shah, K.M.; Karagir, A.; Adaki, S. Slow-flow-type venous malformation of tongue. BMJ Case Rep. 2013, 2013, bcr2013008945. [Google Scholar] [CrossRef]
- White, C.L.; Olivieri, B.; Restrepo, R.; McKeon, B.; Karakas, S.P.; Lee, E.Y. Low-Flow Vascular Malformation Pitfalls: From Clinical Examination to Practical Imaging Evaluation--Part 1, Lymphatic Malformation Mimickers. AJR Am. J. Roentgenol. 2016, 206, 940–951. [Google Scholar] [CrossRef]
- Nagarajan, K.; Prasanth, P.; Elango, S. Two Cases of Lingual Arteriovenous Malformations with Comorbidities Treated by Glue Embolization: A Report with Review of Literature. Indian J. Otolaryngol. Head Neck Surg. 2019, 71 (Suppl. S1), 696–701. [Google Scholar] [CrossRef]
- Hammer, S.; Zeman, F.; Fellner, C.; Wohlgemuth, W.A.; Uller, W. Venous Malformations: Phleboliths Correlate With the Presence of Arteriovenous Microshunts. AJR Am. J. Roentgenol. 2018, 211, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, V.; Palagani, R.; Gharpure, V. Dermoid Cyst of Tongue Masquerading as a Lingual Neurofibroma. J. Indian. Assoc. Pediatr. Surg. 2023, 28, 82–83. [Google Scholar] [CrossRef] [PubMed]
- Shaari, C.M.; Ho, B.T.; Shah, K.; Biller, H.F. Lingual dermoid cyst. Otolaryngol. Head. Neck Surg. 1995, 112, 476–478. [Google Scholar] [CrossRef]
- Lin, H.W.; Silver, A.L.; Cunnane, M.E.; Sadow, P.M.; Keiff, D.A. Lateral dermoid cyst of floor of the mouth: Unusual radiologic and pathologic findings. Auris Nasus Larynx 2011, 38, 650–653. [Google Scholar] [CrossRef]
- Gupta, S.; Singh, S.; Gill, M.; Goyal, R.; Hasija, S.; Sen, R. Teratoma of the tongue. J. Surg. Case Rep. 2012, 2012, 6. [Google Scholar] [CrossRef][Green Version]
- Smirniotopoulos, J.G.; Chiechi, M.V. Teratomas, dermoids, and epidermoids of the head and neck. Radiographics 1995, 15, 1437–1455. [Google Scholar] [CrossRef]
- Turki, M.; Mouaffak-Zidi, Y.; Rajhi, H.; Triki, H.; Naija, S.; Ben Jilani, S.; M’Niff, N.; Adouani, A. A case of large dermoid cyst of the tongue. Egypt. J. Ear Nose Throat Allied Sci. 2011, 12, 171–174. [Google Scholar] [CrossRef][Green Version]
- Patel, S.; Bhatt, A.A. Thyroglossal duct pathology and mimics. Insights Imaging 2019, 10, 12. [Google Scholar] [CrossRef]
- Mondin, V.; Ferlito, A.; Muzzi, E.; Silver, C.E.; Fagan, J.J.; Devaney, K.O.; Rinaldo, A. Thyroglossal duct cyst: Personal experience and literature review. Auris Nasus Larynx 2008, 35, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Noussios, G.; Anagnostis, P.; Goulis, D.G.; Lappas, D.; Natsis, K. Ectopic thyroid tissue: Anatomical, clinical, and surgical implications of a rare entity. Eur. J. Endocrinol. 2011, 165, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.; Cinelli, M.; Mesolella, M.; Tafuri, D.; Rocca, A.; Amato, B.; Rengo, S.; Testa, D. Morphological, diagnostic and surgical features of ectopic thyroid gland: A review of literature. Int. J. Surg. 2014, 12 (Suppl. S1), S3–S11. [Google Scholar] [CrossRef]
- Little, C.C.; Filimonov, A.; Schwam, Z.G. Lingual abscess: A case report of a rare clinical entity. Otolaryngol. Case Rep. 2022, 23, 100411. [Google Scholar] [CrossRef]
- Rampi, A.; Tettamanti, A.; Bertotto, I.; Comini, L.V.; Howardson, B.O.; Luparello, P.; Di Santo, D.; Bondi, S. Atypical Tongue Abscesses Mimicking Submucosal Malignancies: A Review of the Literature Focusing on Diagnostic Challenges. Cancers 2023, 15, 5871. [Google Scholar] [CrossRef]
- Kuroyanagi, N. Lingual Abscess Induced By Tongue Cancer: A Rare Case Report. J. Oral Maxillofac. Surg. 2017, 75, e378–e379. [Google Scholar] [CrossRef]
- Balatsouras, D.G.; Eliopoulos, P.N.; Kaberos, A.C. Lingual abscess: Diagnosis and treatment. Head Neck 2004, 26, 550–554. [Google Scholar] [CrossRef]
- Mulry, E.; Husain, S.; Gigliotti, A.; Leahy, K.; Shanti, R.; Rajasekaran, K. Submucosal squamous cell carcinoma of the oral tongue presenting as lingual abscess. Otorhinolaryngol. Head Neck Surg. 2020, 5, 1–6. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Lam, J.M.; Barankin, B. Infectious Mononucleosis: An Updated Review. Curr. Pediatr. Rev. 2024, 20, 305–322. [Google Scholar] [CrossRef]
- Horwitz, C.A.; Henle, W.; Henle, G.; Schapiro, R.; Borken, S.; Bundtzen, R. Infectious mononucleosis in patients aged 40 to 72 years: Report of 27 cases, including 3 without heterophil-antibody responses. Medicine 1983, 62, 256–262. [Google Scholar] [CrossRef]
- Har-El, G.; Josephson, J.S. Infectious mononucleosis complicated by lingual tonsillitis. J. Laryngol. Otol. 1990, 104, 651–653. [Google Scholar] [CrossRef]
- Roberge, R.J.; Simon, M.; Russell, M.; Decker, M. Lingual tonsillitis: An unusual presentation of mononucleosis. Am. J. Emerg. Med. 2001, 19, 173–175. [Google Scholar] [CrossRef]
- King, A.D.; Wong, L.Y.S.; Law, B.K.H.; Bhatia, K.S.; Woo, J.K.S.; Ai, Q.Y.; Tan, T.Y.; Goh, J.; Chuah, K.L.; Mo, F.K.F.; et al. MR Imaging Criteria for the Detection of Nasopharyngeal Carcinoma: Discrimination of Early-Stage Primary Tumors from Benign Hyperplasia. AJNR Am. J. Neuroradiol. 2018, 39, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Lo Casto, A.; Spataro, R.; Purpura, P.; La Bella, V. Unilateral laryngeal and hypoglossal paralysis (Tapia’s syndrome) in a patient with an inflammatory pseudotumor of the neck. Clin. Neurol. Neurosurg. 2013, 115, 1499–1501. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Chu, P.W.; Azman Firdaus, H.; Stewart, C.; Malekhedayat, M.; Alber, S.; Bolch, W.E.; Mahendra, M.; Berrington de González, A.; Miglioretti, D.L. Projected Lifetime Cancer Risks From Current Computed Tomography Imaging. JAMA Intern. Med. 2025, e250505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allison, J.; Yanasak, N. What MRI Sequences Produce the Highest Specific Absorption Rate (SAR), and Is There Something We Should Be Doing to Reduce the SAR During Standard Examinations? AJR Am. J. Roentgenol. 2015, 205, W140. [Google Scholar] [CrossRef] [PubMed]
- Popp, F.A. Properties of biophotons and their theoretical implications. Indian J. Exp. Biol. 2003, 41, 391–402. [Google Scholar] [PubMed]
- Takeda, M.; Kobayashi, M.; Takayama, M.; Suzuki, S.; Ishida, T.; Ohnuki, K.; Moriya, T.; Ohuchi, N. Biophoton detection as a novel technique for cancer imaging. Cancer Sci. 2004, 95, 656–661. [Google Scholar] [CrossRef]

| Histologic Type | Tumor/Tumor-like Lesion | Relative Frequency | Typical Tongue Location |
|---|---|---|---|
| Epithelial tumors | Papilloma | Common | Tongue base surface; mucosal |
| Soft tissue and neural tumors/lesions | Hemangioma Schwannoma Neurofibroma Rhabdomyoma | Common Uncommon Rare Rare | Dorsal surface; submucosal Tongue base; submucosal Unknown/none; submucosal Tongue base; submucosal |
| Salivary gland tumors | Pleomorphic adenoma | Rare | Unknown/none |
| “Dermoid” cysts | Epidermoid cyst Dermoid cyst Teratoma | Rare Rare Rare | Tongue base, submucosal Tongue base, submucosal Tongue base, submucosal |
| Thyroglossal duct remnants | Thyroglossal duct cyst Ectopic thyroid tissue | Common Rare | Tongue base, midline Tongue base, midline |
| Others (a) | Lipoma Angiomyolipoma Vascular malformation Abscess Mononucleosis | Rare Rare Uncommon Very rare Very rare | Lateral tongue edge, submucosal Lateral tongue edge, submucosal Ventral tongue; submucosal Mobile tongue; submucosal Lingual tonsils |
| Benign Tumor/Tumor-like Lesion | Radiological Features | Differential Diagnosis |
|---|---|---|
| Schwannoma [6,7] | CT: hypodense MRI: high T2 SI, split fat, target and fascicular signs | Venous malformation, dermoid cysts, lipoma |
| Lipoma [8] Angiomyolipoma | CT: −83 to −134 HU (like subcutaneous fat tissue) MRI: high T1 and T2 SI, fat saturation signal | Chronic hemilingual denervation |
| Venous malformation [1,9] | CT: phleboliths MRI: high T2 SI, +CE | Other vascular malformations, schwannoma, dermoid cysts |
| Lymphatic malformation [10,11] | Unilocular or multilocular, microcystic (<1 cm) or macrocystic (>1 cm) CT, MRI: no solid nodule with +CE MRI: high T2 SI, fluid-fluid levels | Other vascular malformations, dermoid cysts |
| Arteriovenous malformation [6,12] | MRA: arterial feeding vessel, nidus, and venous drainage vessels MRI: flow voids | Other vascular malformations |
| Dermoid cyst [1,13,14] | CT: free fat and calcified corpuscles (“sack of marbles” sign) | Vascular malformation, epidermoid cysts, lipoma |
| Epidermoid cyst [14] | MRI: high SI on DWI, and restricted diffusion with low values on ADC map | Vascular malformation, dermoid cysts |
| Thyroglossal duct cyst [15] Ectopic thyroid tissue [16] | Cyst Same features as thyroid tissue | Lingual tonsil mucous retention cyst Squamous cell carcinoma and lymphoma of the tongue base |
| Abscess [17] | CT, MRI: peripheral enhancement MRI: core of the lesion shows high SI on DWI, and restricted diffusion with low values on ADC map | Submucosal malignancy |
| Lingual tonsillitis in infectious mononucleosis [18] | CT, MRI: symmetric tonsil enlargement, retention cysts, and “linear or columnar pattern” of enhancement (features of benign tonsillar hypertrophy) | Lingual tonsil lymphoma |
| Chronic hemilingual denervation [19] | Fatty atrophy of the affected hemilingual muscles with volume loss and lingual deviation, without mass-like lesion CT: −83 to −134 HU (like subcutaneous fat tissue) MRI: high T1 and T2 SI, fat saturation signal | Lipoma, angiomyolipoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietragalla, M.; Gattuso, E.; Nardi, C.; Lo Casto, A. CT and MRI Key Features of Benign Tumors and Tumor-like Lesions of the Tongue: A Pictorial Review. Cancers 2025, 17, 1695. https://doi.org/10.3390/cancers17101695
Pietragalla M, Gattuso E, Nardi C, Lo Casto A. CT and MRI Key Features of Benign Tumors and Tumor-like Lesions of the Tongue: A Pictorial Review. Cancers. 2025; 17(10):1695. https://doi.org/10.3390/cancers17101695
Chicago/Turabian StylePietragalla, Michele, Emanuele Gattuso, Cosimo Nardi, and Antonio Lo Casto. 2025. "CT and MRI Key Features of Benign Tumors and Tumor-like Lesions of the Tongue: A Pictorial Review" Cancers 17, no. 10: 1695. https://doi.org/10.3390/cancers17101695
APA StylePietragalla, M., Gattuso, E., Nardi, C., & Lo Casto, A. (2025). CT and MRI Key Features of Benign Tumors and Tumor-like Lesions of the Tongue: A Pictorial Review. Cancers, 17(10), 1695. https://doi.org/10.3390/cancers17101695








