Interleukin-6 Is a Crucial Factor in Shaping the Inflammatory Tumor Microenvironment in Ovarian Cancer and Determining Its Hot or Cold Nature with Diagnostic and Prognostic Utilities
Simple Summary
Abstract
1. Introduction
2. Ovarian Cancer Immunogenicity: “Hot” or “Cold”
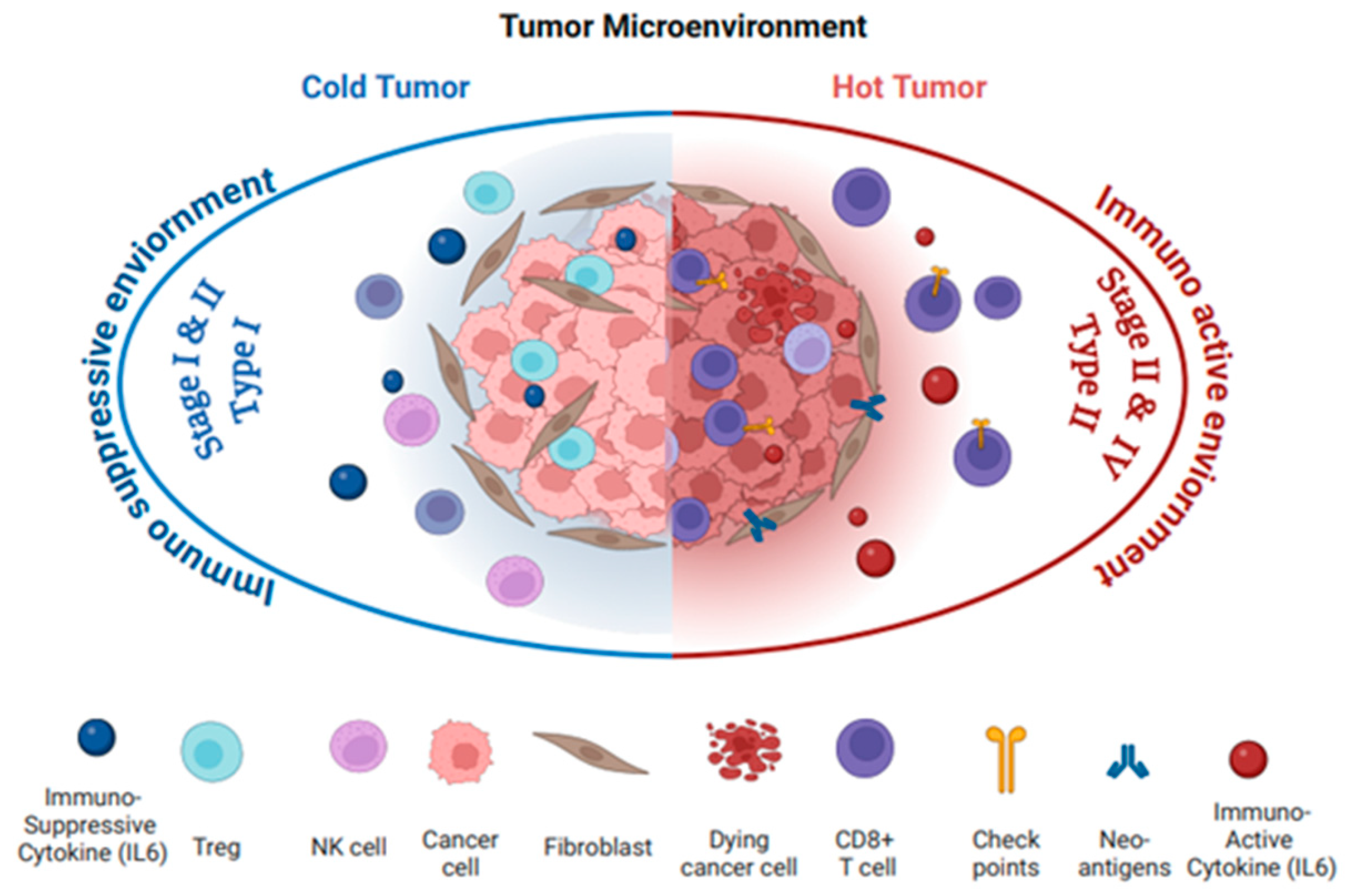
2.1. Tumor Microenvironment: “Hot” or “Cold”
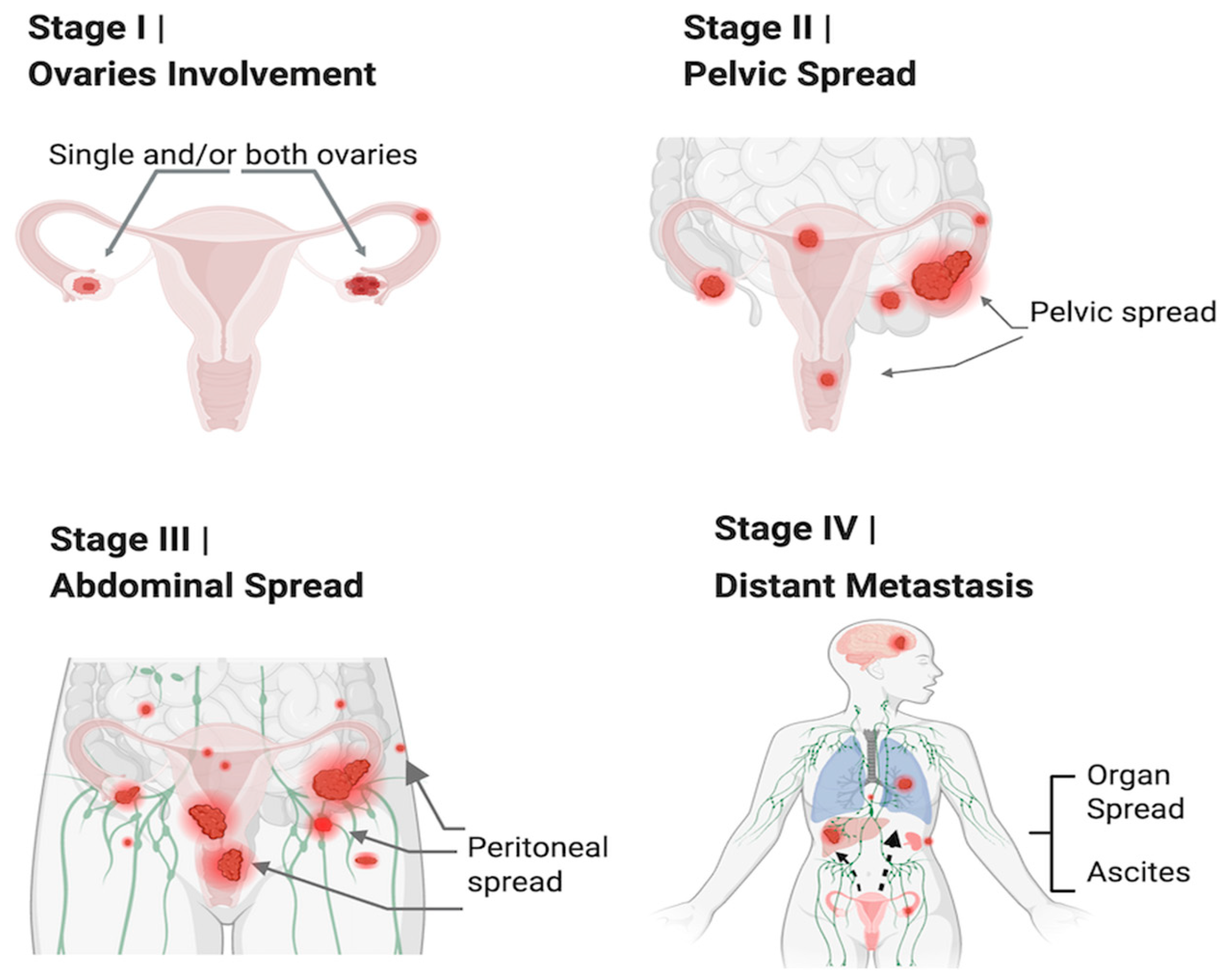
2.2. OC Immunophenotype with Cancer Stages and Histological and Molecular Types
| Ovarian Cancer Subtype | Immune Phenotype and Hot/Cold Tumor | OC Stages | Genetic Mutations | Gene Expression | Epigenetic Changes | Signaling Pathways | Effect and Role of IL6 |
|---|---|---|---|---|---|---|---|
| Endometrioid Carcinoma [21,22] | Cold Tumor: Low T cell and NK cell infiltration, poor immune response | I, II, III | PTEN, CTNNB1 (β-catenin), ARID1A | Estrogen receptor (ER) upregulation, β-catenin expression | Loss of ARID1A, DNA methylation changes | TGFβ pathway disruption, Wnt/β-catenin pathway, PI3K/AKT pathway | IL6 promotes tumor growth by activating JAK/STAT3 signaling, enhancing inflammation, survival, and resistance to apoptosis. It contributes to immune suppression and tumor progression. |
| Clear Cell Carcinoma [23,24] | ARID1A, PIK3CA | HIF1α overexpression, VEGF upregulation | DNA methylation, histone modifications | VEGF signaling, PI3K/AKT, HIF1α driven pathways, ARID1A-related chromatin remodeling | IL6 activates STAT3 and MAPK, promoting chemotherapy resistance, tumor progression, and immune evasion. It contributes to immune suppression and promotes angiogenesis. | ||
| Mucinous Carcinoma [25,26] | KRAS, PIK3CA | MUC1 overexpression | DNA methylation, histone modifications | EGFR pathway activation, MAPK pathway, PI3K/AKT pathway | IL6 activates the JAK/STAT3 pathway, promoting cell proliferation, survival, and migration. It enhances metastatic potential and contributes to immune evasion. | ||
| Low-Grade Serous Carcinoma [27] | BRAF, KRAS | Anti-apoptotic proteins (e.g., Bcl-2) upregulation | Epigenetic alterations in cell cycle regulation | MAPK/ERK pathway, PI3K/AKT pathway, BRAF/KRAS-related signaling | IL6 activates STAT3 signaling, contributing to tumor progression, chemotherapy resistance, and immune evasion. It enhances survival and promotes cell migration. |
| Ovarian Cancer Subtype | Immune Phenotype and Hot/Cold Tumor | OC Stages | Genetic Mutations | Gene Expression | Epigenetic Changes | Signaling Pathways | Effect and Role of IL6 |
|---|---|---|---|---|---|---|---|
| High-Grade Serous Carcinoma (HGSC) [28,29] | Hot Tumor: PDL1 expression, increased T cell infiltration, higher cytokine levels (e.g., IL6, IL2, IFNγ) | III, IV | TP53, BRCA1/2, MYC over- expression | Cyclins and CDKs upregulated, MYC overexpression | DNA methylation in tumor suppressor genes (e.g., BRCA1) | PI3K/AKT/mTOR activation, Wnt/β-catenin signaling, MAPK pathway, p53-related pathways | IL6 activates STAT3 signaling, promoting cell proliferation, survival, metastasis, and immune evasion. It enhances angiogenesis and contributes to chemotherapy resistance. |
| High-Grade Endometrioid Carcinoma [30,31,32] | I, II, III | PTEN, CTNNB1 (β-catenin), ARID1A | Estrogen receptor (ER) upregulation, β-catenin expression | Loss of ARID1A, DNA methylation changes | Wnt/β-catenin pathway, PI3K/AKT pathway, TGFβ pathway disruption | IL6 promotes tumor growth by activating JAK/STAT3 signaling, enhancing inflammation, survival, and resistance to apoptosis. It can suppress the immune response and contribute to immune evasion. | |
| BRCA1/2-Related Tumors [33,34] | Cold Tumor: Low T cell and NK cell infiltration, poor immune response | III, IV | BRCA1/2 mutations (loss of function) | RAD51 upregulation, compensatory DNA repair mechanism | Dysfunction in homologous recombination repair pathways | Fanconi anemia pathway disruption, sensitivity to PARP inhibitors and platinum chemotherapy | IL6 enhances tumor survival by activating JAK/STAT3, contributing to chemotherapy resistance, immune suppression, and tumor progression. It reduces immune cell infiltration. |
| Undifferentiated High-Grade Carcinoma [35] | III, IV | TP53 mutations, often no clear histologic differentiation | High cyclin D1 and CDK4 expression, varied gene expression | DNA methylation, chromatin remodeling, TP53 mutation- associated alterations | Activation of PI3K/AKT, MAPK, and aberrant cell cycle control pathways | IL6 activates STAT3 and MAPK, promoting chemotherapy resistance, tumor progression, and immune evasion. It contributes to immune suppression and supports metastasis. |
2.3. IL6 Role in “OC Immunogenicity”
3. IL6 in Inflammation and Cancer
3.1. IL6 Signaling in Inflammation
3.2. IL6 Signaling in Cancer
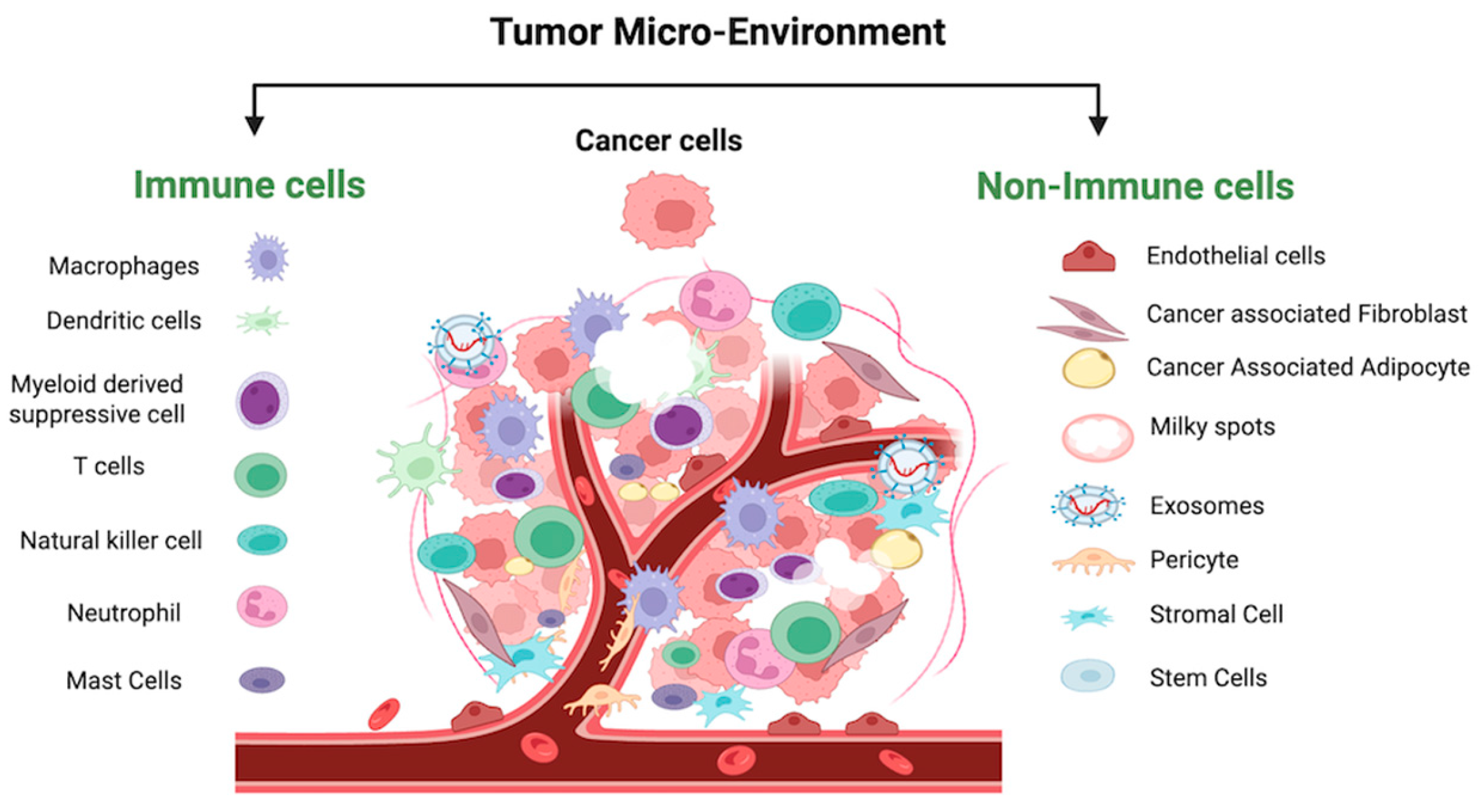
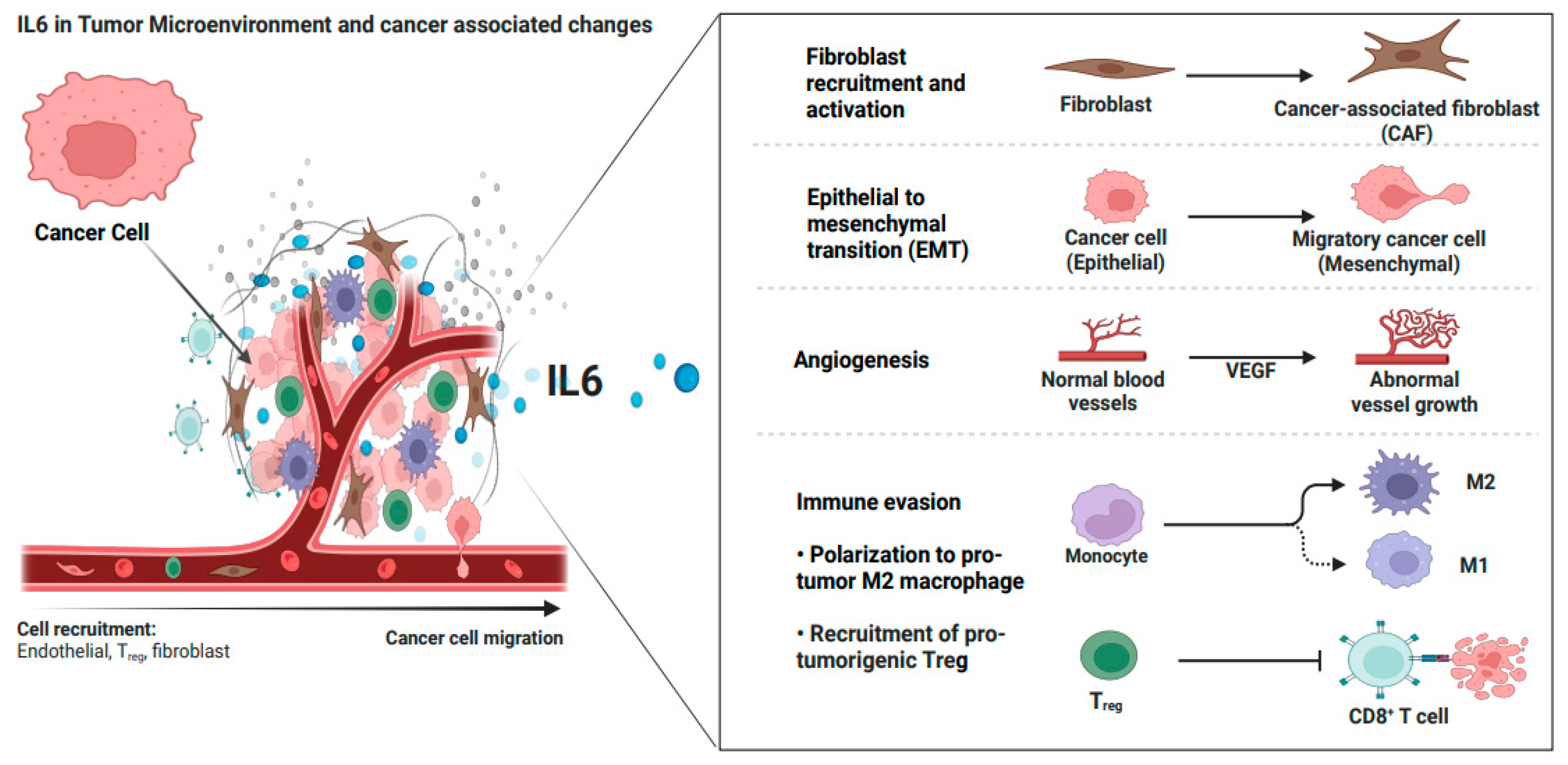
4. IL6 in the Tumor Microenvironment of Ovarian Cancer
4.1. IL6 Crosstalk with Non-Immune Cells in OC-TME
4.2. IL6 Crosstalk with Immune Cells in OC-TME
4.2.1. IL6 and Innate Immune Cells
4.2.2. IL6 and Adaptive Immune Cells—T Cells
5. Orchestrated Role of IL6 in Ovarian Cancer Development, Metastasis, and Recurrence
5.1. IL6 in OC Development and Progression
5.2. IL6 Promotes OC Invasiveness and Formation of Ascitic Fluid
5.3. IL6 Induces Treatment Resistance and Recurrence
5.4. IL6 Worsens the Metabolic Status of OC Patients
5.5. IL6 Effect on PGCCs in OC
6. IL6 and Current OC Management
6.1. OC Management with Current and Targeted Therapies
6.2. Low-Dose Cyclophosphamide Improves OC Recurrence and Relapses
6.3. Immunotherapies for OC
6.4. Combined Targeted and Immunotherapies in OC Potentiate Therapeutic Outcomes
6.5. Targeting IL6 in Combination with Immunotherapies
7. IL6 in Liquid Biopsy: Screening, Diagnostic, Predictive, and Prognostic
| Category | Application | Details | Examples/Methods |
|---|---|---|---|
| Screening [222] | Early Detection | AI analyzes longitudinal IL6 trends to flag high-risk asymptomatic patients. | Time series analysis, anomaly detection models. |
| Risk Stratification | Combines IL6 with other biomarkers (e.g., CA-125, HE4, miRNAs) for better screening accuracy. | Multimodal data fusion, feature selection. | |
| Diagnosis [223] | Disease Diagnosis | Differentiates malignant ovarian cancer from benign ovarian conditions using IL6 and additional biomarkers. | Classification models like Random Forest or Support Vector Machines (SVM). |
| Stages Detection | Integrates IL6 with markers like CRP, miRNAs, and imaging data to enhance diagnostic accuracy of specific stages or types. | Neural networks, ensemble learning. | |
| Prognosis [224,226] | Survival Prediction | Uses IL6 levels and trends with other markers to predict overall survival (OS), progression-free survival (PFS), and disease-free progression (DFP). | Survival analysis with Cox proportional hazards models augmented by ML. |
| Risk Stratification | Identifies high-risk patients based on IL6 levels, tumor aggressiveness, and likelihood of recurrence. | Decision trees, clustering algorithms for subgroup analysis. | |
| Monitoring Disease Evolution | Tracks IL6 and related biomarkers over time to predict metastasis, ascites development, or resistance. | Real-time monitoring with recurrent neural networks (RNNs) or Long Short-Term Memory (LSTM) networks. | |
| Predictive [224,225] | Therapy Response Prediction | Predicts patient responses to chemotherapy, immunotherapy, or IL6/IL6R blockers by analyzing signaling pathways and biomarker profiles. | Predictive analytics using gradient boosting or deep learning. |
| Personalized Treatment Planning | Identifies patients most likely to benefit from targeted therapies by integrating IL6 data with genomic and transcriptomic profiles. | AI-driven precision medicine platforms, supervised learning models. | |
| Clinical Trial Optimization | Selects ideal candidates for trials of novel therapies targeting IL6-mediated pathways. | AI-based cohort selection and predictive modeling. |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zamwar, U.M.; Anjankar, A.P.; Anjankar, A. Aetiology, epidemiology, histopathology, classification, detailed evaluation, and treatment of ovarian cancer. Cureus 2022, 14, e30561. [Google Scholar] [CrossRef] [PubMed]
- Australian-Government. Ovarian Cancer Statistics in Australia. 2025. Available online: https://www.canceraustralia.gov.au/cancer-types/ovarian-cancer/ovarian-cancer-statistics-australia (accessed on 4 April 2025).
- Vargas, A.N. Natural history of ovarian cancer. Ecancermedicalscience 2014, 8, 456. [Google Scholar]
- Khosravi, G.R.; Mostafavi, S.; Bastan, S.; Ebrahimi, N.; Gharibvand, R.S.; Eskandari, N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun. 2024, 44, 521–553. [Google Scholar] [CrossRef]
- Wang, T.; Luo, Y.; Zhang, Q.; Shen, Y.; Peng, M.; Huang, P.; Zhou, Z.; Wu, X.; Chen, K. COX-2-related tumor immune microenvironment in non-small cell lung cancer: A novel signature to predict hot and cold tumor. J. Thorac. Dis. 2022, 14, 729–740. [Google Scholar] [CrossRef]
- Wang, L.; Geng, H.; Liu, Y.; Liu, L.; Chen, Y.; Wu, F.; Liu, Z.; Ling, S.; Wang, Y.; Zhou, L. Hot and cold tumors: Immunological features and the therapeutic strategies. MedComm 2023, 4, e343. [Google Scholar] [CrossRef]
- Albertí-Valls, M.; Olave, S.; Olomí, A.; Macià, A.; Eritja, N. Advances in Immunotherapy for Endometrial Cancer: Insights into MMR Status and Tumor Microenvironment. Cancers 2024, 16, 3918. [Google Scholar] [CrossRef]
- Liu, Y.T.; Sun, Z.J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Ni, J.-J.; Zhang, Z.-Z.; Ge, M.-J.; Chen, J.-Y.; Zhuo, W. Immune-based combination therapy to convert immunologically cold tumors into hot tumors: An update and new insights. Acta Pharmacol. Sin. 2023, 44, 288–307. [Google Scholar] [CrossRef]
- Pu, W.; Ma, C.; Wang, B.; Zhu, W.; Chen, H. The “Heater” of “Cold” Tumors–Blocking IL-6. Adv. Biol. 2024, 8, 2300587. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, A.E.; Prakash, M.D.; Cox, M.; Wilson, K.; Boer, J.C.; Cauchi, J.A.; Plebanski, M. Therapeutic Cancer Vaccines—T Cell Responses and Epigenetic Modulation. Front. Immunol. 2019, 9, 3109. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, N.G. miR-146a: Overcoming coldness in ovarian cancer. Mol. Ther.-Oncolytics 2023, 31, 100753. [Google Scholar] [CrossRef]
- Huang, R.Y.J.; Huang, K.J.; Chen, K.C.; Hsiao, S.M.; Tan, T.Z.; Wu, C.J.; Hsu, C.; Chang, W.C.; Pan, C.Y.; Sheu, B.C. Immune-Hot tumor features associated with recurrence in early-stage ovarian clear cell carcinoma. Int. J. Cancer 2023, 152, 2174–2185. [Google Scholar] [CrossRef]
- Saida, T.; Tanaka, Y.O.; Matsumoto, K.; Satoh, T.; Yoshikawa, H.; Minami, M. Revised FIGO staging system for cancer of the ovary, fallopian tube, and peritoneum: Important implications for radiologists. Jpn. J. Radiol. 2016, 34, 117–124. [Google Scholar] [CrossRef]
- Ravindran, F.; Choudhary, B. Ovarian cancer: Molecular classification and targeted therapy. In Ovarian Cancer-Updates in Tumour Biology and Therapeutics; IntechOpen: London, UK, 2021. [Google Scholar]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An integrated review. In Seminars in Oncology Nursing; WB Saunders: Philadelphia, PA, USA, 2019; pp. 151–156. [Google Scholar] [CrossRef]
- Lisio, M.-A.; Fu, L.; Goyeneche, A.; Gao, Z.-H.; Telleria, C. High-grade serous ovarian cancer: Basic sciences, clinical and therapeutic standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef]
- Rojas, V.; Hirshfield, K.M.; Ganesan, S.; Rodriguez-Rodriguez, L. Molecular characterization of epithelial ovarian cancer: Implications for diagnosis and treatment. Int. J. Mol. Sci. 2016, 17, 2113. [Google Scholar] [CrossRef]
- De Leo, A.; de Biase, D.; Lenzi, J.; Barbero, G.; Turchetti, D.; Grillini, M.; Ravegnini, G.; Angelini, S.; Zamagni, C.; Coluccelli, S. ARID1A and CTNNB1/β-catenin molecular status affects the clinicopathologic features and prognosis of endometrial carcinoma: Implications for an improved surrogate molecular classification. Cancers 2021, 13, 950. [Google Scholar] [CrossRef]
- Chiu, H.-C.; Li, C.-J.; Yiang, G.-T.; Tsai, A.P.-Y.; Wu, M.-Y. Epithelial to mesenchymal transition and cell biology of molecular regulation in endometrial carcinogenesis. J. Clin. Med. 2019, 8, 439. [Google Scholar] [CrossRef]
- Tan, D.; Miller, R.; Kaye, S. New perspectives on molecular targeted therapy in ovarian clear cell carcinoma. Br. J. Cancer 2013, 108, 1553–1559. [Google Scholar] [CrossRef]
- Tong, A.; Di, X.; Zhao, X.; Liang, X. Review the progression of ovarian clear cell carcinoma from the perspective of genomics and epigenomics. Front. Genet. 2023, 14, 952379. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-C.; Chen, S.-J.; Chen, H.-C.; Tan, K.T.; Jung, S.-M.; Lin, C.-Y.; Chao, A.-S.; Huang, K.-G.; Chou, H.-H.; Chang, T.-C. Comprehensive genomic profiling reveals ubiquitous KRAS mutations and frequent PIK3CA mutations in ovarian seromucinous borderline tumor. Mod. Pathol. 2020, 33, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.; Kommoss, S.; Winterhoff, B.J.; Kipp, B.R.; Garcia, J.J.; Voss, J.; Halling, K.; Karnezis, A.; Senz, J.; Yang, W. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer 2015, 15, 415. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nakayama, K.; Ishibashi, T.; Ishikawa, N.; Ishikawa, M.; Katagiri, H.; Minamoto, T.; Sato, E.; Sanuki, K.; Yamashita, H. KRAS/BRAF analysis in ovarian low-grade serous carcinoma having synchronous all pathological precursor regions. Int. J. Mol. Sci. 2016, 17, 625. [Google Scholar] [CrossRef]
- Wang, Y.; Duval, A.J.; Adli, M.; Matei, D. Biology-driven therapy advances in high-grade serous ovarian cancer. J. Clin. Investig. 2024, 134, e174013. [Google Scholar] [CrossRef]
- Cole, A.J.; Dwight, T.; Gill, A.J.; Dickson, K.-A.; Zhu, Y.; Clarkson, A.; Gard, G.B.; Maidens, J.; Valmadre, S.; Clifton-Bligh, R. Assessing mutant p53 in primary high-grade serous ovarian cancer using immunohistochemistry and massively parallel sequencing. Sci. Rep. 2016, 6, 26191. [Google Scholar] [CrossRef]
- Toumpeki, C.; Liberis, A.; Tsirkas, I.; Tsirka, T.; Kalagasidou, S.; Inagamova, L.; Anthoulaki, X.; Tsatsaris, G.; Kontomanolis, E.N. The role of ARID1A in endometrial cancer and the molecular pathways associated with pathogenesis and cancer progression. In Vivo 2019, 33, 659–667. [Google Scholar] [CrossRef]
- Che, Q.; Xiao, X.; Liu, M.; Lu, Y.; Dong, X.; Liu, S. IL-6 promotes endometrial cancer cells invasion and migration through signal transducers and activators of transcription 3 signaling pathway. Pathol.-Res. Pract. 2019, 215, 152392. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Pei, X.; Zhou, Y.; Wei, Z. CCDC68 upregulation by IL-6 promotes endometrial carcinoma progression. J. Interferon Cytokine Res. 2021, 41, 12–19. [Google Scholar] [CrossRef]
- Pothuri, B. BRCA1-and BRCA2-related mutations: Therapeutic implications in ovarian cancer. Ann. Oncol. 2013, 24, viii22–viii27. [Google Scholar] [CrossRef]
- Matos-Rodrigues, G.; Guirouilh-Barbat, J.; Martini, E.; Lopez, B.S. Homologous recombination, cancer and the ‘RAD51 paradox’. NAR Cancer 2021, 3, zcab016. [Google Scholar] [CrossRef]
- Tessier-Cloutier, B.; Kommoss, F.K.F.; Kolin, D.L.; Němejcová, K.; Smith, D.; Pors, J.; Stewart, C.J.R.; McCluggage, W.G.; Foulkes, W.D.; von Deimling, A.; et al. Dedifferentiated and Undifferentiated Ovarian Carcinoma: An Aggressive and Molecularly Distinct Ovarian Tumor Characterized by Frequent SWI/SNF Complex Inactivation. Mod. Pathol. 2024, 37, 100374. [Google Scholar] [CrossRef]
- Amer, H.; Kampan, N.C.; Itsiopoulos, C.; Flanagan, K.L.; Scott, C.L.; Kartikasari, A.E.; Plebanski, M. Interleukin-6 Modulation in Ovarian Cancer Necessitates a Targeted Strategy: From the Approved to Emerging Therapies. Cancers 2024, 16, 4187. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.; Assi, M.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.; Hezmee, M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Huseni, M.A.; Wang, L.; Klementowicz, J.E.; Yuen, K.; Breart, B.; Orr, C.; Liu, L.F.; Li, Y.; Gupta, V.; Li, C.; et al. CD8+ T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep. Med. 2023, 4, 100878. [Google Scholar] [CrossRef]
- Kampan, N.C.; Kartikasari, A.; Deceneuux, C.; Madondo, M.T.; McNally, O.M.; Flanagan, K.L.; Aziz, N.A.; Stephens, A.N.; Reynolds, J.; Quinn, M.A. Combining TNFR2-Expressing Tregs and IL-6 as Superior Diagnostic Biomarkers for High-Grade Serous Ovarian Cancer Masses. Cancers 2023, 15, 667. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Johnson, D.H.; Abdel-Wahab, N.; Foo, W.C.; Bentebibel, S.-E.; Daher, M.; Haymaker, C.; Wani, K.; Saberian, C.; Ogata, D. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022, 40, 509–523.e6. [Google Scholar] [CrossRef]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef]
- Soler, M.F.; Abaurrea, A.; Azcoaga, P.; Araujo, A.M.; Caffarel, M.M. New perspectives in cancer immunotherapy: Targeting IL-6 cytokine family. J. Immunother. Cancer 2023, 11, e007530. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Smith, A.S.; Bowers, J.S.; Wyatt, M.M.; Nelson, M.H.; Rangel Rivera, G.O.; Horton, J.D.; Krieg, C.; Armeson, K. IL6 fuels durable memory for Th17 cell–mediated responses to tumors. Cancer Res. 2020, 80, 3920–3932. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Metcalfe, R.D.; Putoczki, T.L.; Griffin, M.D. Structural understanding of interleukin 6 family cytokine signaling and targeted therapies: Focus on interleukin 11. Front. Immunol. 2020, 11, 1424. [Google Scholar] [CrossRef]
- Reeh, H.; Rudolph, N.; Billing, U.; Christen, H.; Streif, S.; Bullinger, E.; Schliemann-Bullinger, M.; Findeisen, R.; Schaper, F.; Huber, H.J. Response to IL-6 trans-and IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: Fusing experimental insights and dynamic modelling. Cell Commun. Signal. 2019, 17, 46. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Seeger, W.; Savai, R. Classical IL-6 signaling: A promising therapeutic target for pulmonary arterial hypertension. J. Clin. Investig. 2018, 128, 1720–1723. [Google Scholar] [CrossRef]
- Scheller, J.; Garbers, C.; Rose-John, S. Interleukin-6: From basic biology to selective blockade of pro-inflammatory activities. Semin. Immunol. 2014, 26, 2–12. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the pro-inflammatory activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1274. [Google Scholar] [CrossRef]
- Matthews, V.; Schuster, B.r.; Schutze, S.; Bussmeyer, I.; Ludwig, A.; Hundhausen, C.; Sadowski, T.; Saftig, P.; Hartmann, D.; Kallen, K.-J. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE). J. Biol. Chem. 2003, 278, 38829–38839. [Google Scholar] [CrossRef]
- Jostock, T.; Müllberg, J.; Özbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef]
- Tamura, T.; Udagawa, N.; Takahashi, N.; Miyaura, C.; Tanaka, S.; Yamada, Y.; Koishihara, Y.; Ohsugi, Y.; Kumaki, K.; Taga, T. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc. Natl. Acad. Sci. USA 1993, 90, 11924–11928. [Google Scholar] [CrossRef]
- Romano, M.; Sironi, M.; Toniatti, C.; Polentarutti, N.; Fruscella, P.; Ghezzi, P.; Faggioni, R.; Luini, W.; van Hinsbergh, V.; Sozzani, S. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 1997, 6, 315–325. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Müller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998, 334, 297–314. [Google Scholar] [CrossRef]
- Muller-Newen, G.; Kuster, A.; Hemmann, U.; Keul, R.; Horsten, U.; Martens, A.; Graeve, L.; Wijdenes, J.; Heinrich, P.C. Soluble IL-6 receptor potentiates the antagonistic activity of soluble gp130 on IL-6 responses. J. Immunol. 1998, 161, 6347–6355. [Google Scholar] [CrossRef]
- Lamertz, L.; Rummel, F.; Polz, R.; Baran, P.; Hansen, S.; Waetzig, G.H.; Moll, J.M.; Floss, D.M.; Scheller, J. Soluble gp130 prevents interleukin-6 and interleukin-11 cluster signaling but not intracellular autocrine responses. Sci. Signal. 2018, 11, eaar7388. [Google Scholar] [CrossRef]
- Rao, D.A. T cells that help B cells in chronically inflamed tissues. Front. Immunol. 2018, 9, 1924. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of inflammation: What controls its onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Quinn, K.M.; Kartikasari, A.E.; Cooke, R.E.; Koldej, R.M.; Ritchie, D.S.; Plebanski, M. Impact of age-, cancer-, and treatment-driven inflammation on T cell function and immunotherapy. J. Leukoc. Biol. 2020, 108, 953–965. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Schaefer, L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014, 289, 35237–35245. [Google Scholar] [CrossRef]
- Hu, A.; Sun, L.; Lin, H.; Liao, Y.; Yang, H.; Mao, Y. Harnessing innate immune pathways for therapeutic advancement in cancer. Signal Transduct. Target. Ther. 2024, 9, 68. [Google Scholar] [CrossRef]
- Zaid, Y.; Dore, E.; Dubuc, I.; Archambault, A.-S.; Flamand, O.; Laviolette, M.; Flamand, N.; Boilard, E.; Flamand, L. Chemokines and eicosanoids fuel the hyperinflammation within the lungs of patients with severe COVID-19. J. Allergy Clin. Immunol. 2021, 148, 368–380.e3. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef]
- Norris, C.A.; He, M.; Kang, L.-I.; Ding, M.Q.; Radder, J.E.; Haynes, M.M.; Yang, Y.; Paranjpe, S.; Bowen, W.C.; Orr, A. Synthesis of IL-6 by hepatocytes is a normal response to common hepatic stimuli. PLoS ONE 2014, 9, e96053. [Google Scholar] [CrossRef]
- Ehlting, C.; Wolf, S.D.; Bode, J.G. Acute-phase protein synthesis: A key feature of innate immune functions of the liver. Biol. Chem. 2021, 402, 1129–1145. [Google Scholar] [CrossRef]
- Mold, C.; Gewurz, H.; Du Clos, T.W. Regulation of complement activation by C-reactive protein. Immunopharmacology 1999, 42, 23–30. [Google Scholar] [CrossRef]
- Williams, N.; Bertoncello, I.; Jackson, H.; Arnold, J.; Kavnoudias, H. The role of interleukin 6 in megakaryocyte formation, megakaryocyte development and platelet production. In Ciba Foundation Symposium; John Wiley & Sons, Ltd.: Chichester, UK, 2007; pp. 160–173. [Google Scholar]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Kartikasari, A.E.; Roelofs, R.; Schaeps, R.M.; Kemna, E.H.; Peters, W.H.; Swinkels, D.W.; Tjalsma, H. Secretion of bioactive hepcidin-25 by liver cells correlates with its gene transcription and points towards synergism between iron and inflammation signaling pathways. Biochim. Biophys. Acta-Proteins Proteom. 2008, 1784, 2029–2037. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef]
- Patchen, M.; MacVittie, T.; Williams, J.; Schwartz, G.; Souza, L. Administration of interleukin-6 stimulates multilineage hematopoiesis and accelerates recovery from radiation-induced hematopoietic depression. Blood 1991, 77, 472–480. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Yang, R.; Masters, A.R.; Fortner, K.A.; Champagne, D.P.; Yanguas-Casás, N.; Silberger, D.J.; Weaver, C.T.; Haynes, L.; Rincon, M. IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21–producing B helper CD8+ T cells. J. Exp. Med. 2016, 213, 2281–2291. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Liu, F.; Wan, L.; Deng, Z. The effect of cytokines on osteoblasts and osteoclasts in bone remodeling in osteoporosis: A review. Front. Immunol. 2023, 14, 1222129. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Yang, H.-Y.; Huang, S.-W.; Ou, G.; Hsu, Y.-F.; Hsu, M.-J. Interleukin-6 induces vascular endothelial growth factor-C expression via Src-FAK-STAT3 signaling in lymphatic endothelial cells. PLoS ONE 2016, 11, e0158839. [Google Scholar] [CrossRef]
- Thompson, D.K.; Huffman, K.M.; Kraus, W.E.; Kraus, V.B. Critical appraisal of four IL-6 immunoassays. PLoS ONE 2012, 7, e30659. [Google Scholar] [CrossRef]
- Lo, C.-W.; Chen, M.-W.; Hsiao, M.; Wang, S.; Chen, C.-A.; Hsiao, S.-M.; Chang, J.-S.; Lai, T.-C.; Rose-John, S.; Kuo, M.-L. IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res. 2011, 71, 424–434. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Oiseth, S.J.; Aziz, M.S. Cancer immunotherapy: A brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 2017, 3, 250–261. [Google Scholar] [CrossRef]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A.D.M. Paget’s “seed and soil” theory of cancer metastasis: An idea whose time has come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef]
- Swann, J.B.; Smyth, M.J. Immune surveillance of tumors. J. Clin. Investig. 2007, 117, 1137–1146. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Amer, H.; Kartikasari, A.E.; Plebanski, M. Elevated Interleukin-6 Levels in the Circulation and Peritoneal Fluid of Patients with Ovarian Cancer as a Potential Diagnostic Biomarker: A Systematic Review and Meta-Analysis. J. Pers. Med. 2021, 11, 1335. [Google Scholar] [CrossRef]
- Ghisoni, E.; Imbimbo, M.; Zimmermann, S.; Valabrega, G. Ovarian cancer immunotherapy: Turning up the heat. Int. J. Mol. Sci. 2019, 20, 2927. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zheng, S.; Zhang, T.; Wu, J.; Sun, Y.; Zhang, J.; Liu, G. Role of exosomes in the immune microenvironment of ovarian cancer. Oncol. Lett. 2021, 21, 377. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, Y.; Yang, J.; Zhao, X.; Wei, X. Tumor microenvironment in ovarian cancer: Function and therapeutic strategy. Front. Cell Dev. Biol. 2020, 8, 758. [Google Scholar] [CrossRef]
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; Carrami, E.M.; Hu, Z. An evolving story of the metastatic voyage of ovarian cancer cells: Cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 2019, 38, 2885–2898. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Z.; Wang, Y.; Zhao, H.; Du, Y. The role of cancer-associated fibroblasts in ovarian cancer. Cancers 2022, 14, 2637. [Google Scholar] [CrossRef]
- Fang, Z.; Meng, Q.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Zhao, Y.; Yu, X. Signaling pathways in cancer-associated fibroblasts: Recent advances and future perspectives. Cancer Commun. 2023, 43, 3–41. [Google Scholar] [CrossRef]
- Nilsson, M.B.; Langley, R.R.; Fidler, I.J. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005, 65, 10794–10800. [Google Scholar] [CrossRef]
- Ribeiro, A.L.; Okamoto, O.K. Combined effects of pericytes in the tumor microenvironment. Stem Cells Int. 2015, 2015, 868475. [Google Scholar] [CrossRef] [PubMed]
- Katoh, H.; Hosono, K.; Ito, Y.; Suzuki, T.; Ogawa, Y.; Kubo, H.; Kamata, H.; Mishima, T.; Tamaki, H.; Sakagami, H. COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am. J. Pathol. 2010, 176, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Song, K.; Di, W. Adipocytes: Active facilitators in epithelial ovarian cancer progression? J. Ovarian Res. 2020, 13, 115. [Google Scholar] [CrossRef]
- Deng, W.; Chen, H.; Su, H.; Wu, X.; Xie, Z.; Wu, Y.; Shen, H. IL6 receptor facilitates adipogenesis differentiation of human mesenchymal stem cells through activating P38 pathway. Int. J. Stem Cells 2020, 13, 142–150. [Google Scholar] [CrossRef]
- Gong, X.; Chi, H.; Strohmer, D.F.; Teichmann, A.T.; Xia, Z.; Wang, Q. Exosomes: A potential tool for immunotherapy of ovarian cancer. Front. Immunol. 2022, 13, 1089410. [Google Scholar] [CrossRef]
- Feng, W.; Dean, D.C.; Hornicek, F.J.; Shi, H.; Duan, Z. Exosomes promote pre-metastatic niche formation in ovarian cancer. Mol. Cancer 2019, 18, 124. [Google Scholar] [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef]
- Weber, R.; Groth, C.; Lasser, S.; Arkhypov, I.; Petrova, V.; Altevogt, P.; Utikal, J.; Umansky, V. IL-6 as a major regulator of MDSC activity and possible target for cancer immunotherapy. Cell. Immunol. 2021, 359, 104254. [Google Scholar] [CrossRef]
- Wouters, M.; Dijkgraaf, E.; Kuijjer, M.; Jordanova, E.; Hollema, H.; Welters, M.; van der Hoeven, J.; Daemen, T.; Kroep, J.; Nijman, H. Interleukin-6 receptor and its ligand interleukin-6 are opposite markers for survival and infiltration with mature myeloid cells in ovarian cancer. Oncoimmunology 2014, 3, e962397. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, S.; Li, A.; Xu, H.; Han, X.; Wu, K. Targeting interlukin-6 to relieve immunosuppression in tumor microenvironment. Tumor Biol. 2017, 39, 1010428317712445. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-D.; Cheng, M.; Shang, P.-P.; Yang, Y.-Q. Role of IL-6 in dendritic cell functions. J. Leukoc. Biol. 2022, 111, 695–709. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the tumor microenvironment on NK cell function in solid tumors. Front. Immunol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Nersesian, S.; Carter, E.B.; Lee, S.N.; Westhaver, L.P.; Boudreau, J.E. Killer instincts: Natural killer cells as multifactorial cancer immunotherapy. Front. Immunol. 2023, 14, 1269614. [Google Scholar] [CrossRef]
- Belisle, J.A.; Gubbels, J.A.; Raphael, C.A.; Migneault, M.; Rancourt, C.; Connor, J.P.; Patankar, M.S. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125). Immunology 2007, 122, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-J.; Jeung, I.C.; Park, A.; Park, Y.-J.; Jung, H.; Kim, T.-D.; Lee, H.G.; Choi, I.; Yoon, S.R. An increased level of IL-6 suppresses NK cell activity in peritoneal fluid of patients with endometriosis via regulation of SHP-2 expression. Hum. Reprod. 2014, 29, 2176–2189. [Google Scholar] [CrossRef]
- Maas, R.J.; Hoogstad-van Evert, J.S.; Hagemans, I.M.; Brummelman, J.; Van Ens, D.; de Jonge, P.K.; Hooijmaijers, L.; Mahajan, S.; Van Der Waart, A.B.; Hermans, C.K. Increased peritoneal TGF-β1 is associated with ascites-induced NK-cell dysfunction and reduced survival in high-grade epithelial ovarian cancer. Front. Immunol. 2024, 15, 1448041. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Fielding, C.A.; McLoughlin, R.M.; McLeod, L.; Colmont, C.S.; Najdovska, M.; Grail, D.; Ernst, M.; Jones, S.A.; Topley, N.; Jenkins, B.J. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J. Immunol. 2008, 181, 2189–2195. [Google Scholar] [CrossRef]
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxid. Med. Cell. Longev. 2016, 2016, 1580967. [Google Scholar] [CrossRef]
- Yin, X.; Wu, L.; Yang, H.; Yang, H. Prognostic significance of neutrophil–lymphocyte ratio (NLR) in patients with ovarian cancer: A systematic review and meta-analysis. Medicine 2019, 98, e17475. [Google Scholar] [CrossRef] [PubMed]
- Waring, P.; Müllbacher, A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 1999, 77, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, M.; Hasenburg, A.; Riener, M.; Jütting, U.; Wang, C.; Shen, Y.; Orlowska-Volk, M.; Fisch, P.; Wang, Z.; Gitsch, G. Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: Relevance of clonal selection of T lymphocytes. Br. J. Cancer 2009, 101, 1513–1521. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef]
- Tomšová, M.; Melichar, B.; Sedláková, I.; Šteiner, I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecol. Oncol. 2008, 108, 415–420. [Google Scholar] [CrossRef]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef]
- Leffers, N.; Gooden, M.J.; de Jong, R.A.; Hoogeboom, B.-N.; ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; van der Zee, A.G.; Daemen, T.; Nijman, H.W. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 2009, 58, 449–459. [Google Scholar] [CrossRef]
- Cassar, E.; Kartikasari, A.E.; Plebanski, M. Regulatory T cells in ovarian carcinogenesis and future therapeutic opportunities. Cancers 2022, 14, 5488. [Google Scholar] [CrossRef]
- Gao, R.; Shi, G.-P.; Wang, J. Functional diversities of regulatory T cells in the context of cancer immunotherapy. Front. Immunol. 2022, 13, 833667. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020, 490, 174–185. [Google Scholar] [CrossRef]
- Whiteside, T.L. The role of regulatory T cells in cancer immunology. ImmunoTargets Ther. 2015, 4, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Siu, M.K.-Y.; Jiang, Y.-X.; Chan, D.W.; Cheung, A.N.-Y.; Ngan, H.Y.-S.; Chan, K.K.-L. Infiltration of T cells promotes the metastasis of ovarian cancer cells via the modulation of metastasis-related genes and PD-L1 expression. Cancer Immunol. Immunother. 2020, 69, 2275–2289. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.C.; Maurer, M.J.; Oberg, A.L.; Visscher, D.W.; Kalli, K.R.; Hartmann, L.C.; Goode, E.L.; Knutson, K.L. The ratios of CD8+ T cells to CD4+ CD25+ FOXP3+ and FOXP3-T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS ONE 2013, 8, e80063. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Fujieda, K.; Senju, S.; Ikeda, T.; Oshiumi, H.; Nishimura, Y. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci. 2018, 109, 523–530. [Google Scholar] [CrossRef]
- Duluc, D.; Delneste, Y.; Tan, F.; Moles, M.-P.; Grimaud, L.; Lenoir, J.; Preisser, L.; Anegon, I.; Catala, L.; Ifrah, N. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood J. Am. Soc. Hematol. 2007, 110, 4319–4330. [Google Scholar] [CrossRef]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef]
- Li, B.; Jones, L.L.; Geiger, T.L. IL-6 promotes T cell proliferation and expansion under inflammatory conditions in association with low-level RORγt expression. J. Immunol. 2018, 201, 2934–2946. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Interleukin 6 present in inflammatory ascites from advanced epithelial ovarian cancer patients promotes tumor necrosis factor receptor 2-expressing regulatory T cells. Front. Immunol. 2017, 8, 1482. [Google Scholar] [CrossRef]
- Lane, D.; Matte, I.; Rancourt, C.; Piché, A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer 2011, 11, 210. [Google Scholar] [CrossRef]
- Garcia-Tuñón, I.; Ricote, M.; Ruiz, A.; Fraile, B.; Paniagua, R.; Royuela, M. IL-6, its receptors and its relationship with bcl-2 and bax proteins in infiltrating and in situ human breast carcinoma. Histopathology 2005, 47, 82–89. [Google Scholar] [CrossRef]
- Jourdan, M.; Veyrune, J.-L.; Vos, J.D.; Redal, N.; Couderc, G.; Klein, B. A major role for Mcl-1 antiapoptotic protein in the IL-6-induced survival of human myeloma cells. Oncogene 2003, 22, 2950–2959. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Duan, Z.; Yu, G.; Wang, S. Bcl-2 inhibitors as sensitizing agents for cancer chemotherapy. In Protein Kinase Inhibitors as Sensitizing Agents for Chemotherapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 151–168. [Google Scholar]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Pop, C.; Salvesen, G.S. Human caspases: Activation, specificity, and regulation. J. Biol. Chem. 2009, 284, 21777–21781. [Google Scholar] [CrossRef] [PubMed]
- Martincuks, A.; Li, P.-C.; Zhao, Q.; Zhang, C.; Li, Y.-J.; Yu, H.; Rodriguez-Rodriguez, L. CD44 in ovarian cancer progression and therapy resistance—A critical role for STAT3. Front. Oncol. 2020, 10, 589601. [Google Scholar] [CrossRef]
- Meraviglia-Crivelli, D.; Villanueva, H.; Zheleva, A.; Villalba-Esparza, M.; Moreno, B.; Menon, A.P.; Calvo, A.; Cebollero, J.; Barainka, M.; de Los Mozos, I.R. IL-6/STAT3 signaling in tumor cells restricts the expression of frameshift-derived neoantigens by SMG1 induction. Mol. Cancer 2022, 21, 211. [Google Scholar] [CrossRef]
- Popp, M.W.; Maquat, L.E. Nonsense-mediated mRNA decay and cancer. Curr. Opin. Genet. Dev. 2018, 48, 44–50. [Google Scholar] [CrossRef]
- Mei, C.; Gong, W.; Wang, X.; Lv, Y.; Zhang, Y.; Wu, S.; Zhu, C. Anti-angiogenic therapy in ovarian cancer: Current understandings and prospects of precision medicine. Front. Pharmacol. 2023, 14, 1147717. [Google Scholar] [CrossRef]
- Xu, S.; Yu, C.; Ma, X.; Li, Y.; Shen, Y.; Chen, Y.; Huang, S.; Zhang, T.; Deng, W.; Wang, Y. IL-6 promotes nuclear translocation of HIF-1α to aggravate chemoresistance of ovarian cancer cells. Eur. J. Pharmacol. 2021, 894, 173817. [Google Scholar] [CrossRef]
- Fields, G.B. Mechanisms of action of novel drugs targeting angiogenesis-promoting matrix metalloproteinases. Front. Immunol. 2019, 10, 1278. [Google Scholar] [CrossRef]
- Franke, F.E.; Von Georgi, R.; Zygmunt, M.; Münstedt, K. Association between fibronectin expression and prognosis in ovarian carcinoma. Anticancer Res. 2003, 23, 4261–4267. [Google Scholar]
- Rosso, M.; Majem, B.; Devis, L.; Lapyckyj, L.; Besso, M.J.; Llauradó, M.; Abascal, M.F.; Matos, M.L.; Lanau, L.; Castellví, J. E-cadherin: A determinant molecule associated with ovarian cancer progression, dissemination and aggressiveness. PLoS ONE 2017, 12, e0184439. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, D.; Zhang, W.; Zhou, J.; Tang, B.; Li, L. Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch. Gynecol. Obstet. 2012, 286, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yu, Y.; Zhang, T.; Zhou, X.; Zhou, J.; Jia, L.; Wu, Y.; Zhou, B.P.; Feng, Y. Snail is critical for tumor growth and metastasis of ovarian carcinoma. Int. J. Cancer 2010, 126, 2102–2111. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, J.; Zhang, S.; Yu, D.; Chen, Y.; Liu, Q.; Shi, M.; Ni, C.; Zhu, M. Diagnostic and biological significance of microRNA-192 in pancreatic ductal adenocarcinoma. Oncol. Rep. 2013, 30, 276–284. [Google Scholar] [CrossRef]
- Kujawa, K.A.; Zembala-Nożyńska, E.; Cortez, A.J.; Kujawa, T.; Kupryjańczyk, J.; Lisowska, K.M. Fibronectin and periostin as prognostic markers in ovarian cancer. Cells 2020, 9, 149. [Google Scholar] [CrossRef]
- Kenny, H.A.; Kaur, S.; Coussens, L.M.; Lengyel, E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J. Clin. Investig. 2008, 118, 1367–1379. [Google Scholar] [CrossRef]
- Patel, I.S.; Madan, P.; Getsios, S.; Bertrand, M.A.; MacCalman, C.D. Cadherin switching in ovarian cancer progression. Int. J. Cancer 2003, 106, 172–177. [Google Scholar] [CrossRef]
- Assidi, M. High N-cadherin protein expression in ovarian cancer predicts poor survival and triggers cell invasion. Front. Oncol. 2022, 12, 870820. [Google Scholar] [CrossRef]
- Reddy, P.; Liu, L.; Ren, C.; Lindgren, P.; Boman, K.; Shen, Y.; Lundin, E.; Ottander, U.; Rytinki, M.; Liu, K. Formation of E-cadherin-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phosphatidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol. Endocrinol. 2005, 19, 2564–2578. [Google Scholar] [CrossRef]
- Chuang, K.-T.; Chiou, S.-S.; Hsu, S.-H. Recent Advances in Transcription Factors Biomarkers and Targeted Therapies Focusing on Epithelial–Mesenchymal Transition. Cancers 2023, 15, 3338. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Malissen, B.; Mantovani, A.; De Baetselier, P.; Van Ginderachter, J.A. Regulation and function of the E-cadherin/catenin complex in cells of the monocyte-macrophage lineage and DCs. Blood J. Am. Soc. Hematol. 2012, 119, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Mitra, A.K.; Radjabi, A.R.; Bhaskar, V.; Kistner, E.O.; Tretiakova, M.; Jagadeeswaran, S.; Montag, A.; Becker, A.; Kenny, H.A. Loss of E-cadherin promotes ovarian cancer metastasis via α5-integrin, which is a therapeutic target. Cancer Res. 2008, 68, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K. Ovarian Cancer Metastasis: A Unique Mechanism of Dissemination; IntechOpen: London, UK, 2016. [Google Scholar]
- Mei, S.; Chen, X.; Wang, K.; Chen, Y. Tumor microenvironment in ovarian cancer peritoneal metastasis. Cancer Cell Int. 2023, 23, 11. [Google Scholar] [CrossRef]
- Rickard, B.P.; Conrad, C.; Sorrin, A.J.; Ruhi, M.K.; Reader, J.C.; Huang, S.A.; Franco, W.; Scarcelli, G.; Polacheck, W.J.; Roque, D.M. Malignant ascites in ovarian cancer: Cellular, acellular, and biophysical determinants of molecular characteristics and therapy response. Cancers 2021, 13, 4318. [Google Scholar] [CrossRef]
- Kolomeyevskaya, N.; Eng, K.H.; Khan, A.N.H.; Grzankowski, K.S.; Singel, K.L.; Moysich, K.; Segal, B.H. Cytokine profiling of ascites at primary surgery identifies an interaction of tumor necrosis factor-α and interleukin-6 in predicting reduced progression-free survival in epithelial ovarian cancer. Gynecol. Oncol. 2015, 138, 352–357. [Google Scholar] [CrossRef]
- Penet, M.-F.; Krishnamachary, B.; Wildes, F.B.; Mironchik, Y.; Hung, C.-F.; Wu, T.; Bhujwalla, Z.M. Ascites volumes and the ovarian cancer microenvironment. Front. Oncol. 2018, 8, 595. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Nie, J.; Huang, J.-P.; Zheng, G.-J.; Feng, B. Targeting STAT3 inhibition to reverse cisplatin resistance. Biomed. Pharmacother. 2019, 117, 109135. [Google Scholar] [CrossRef]
- Jin, P.; Liu, Y.; Wang, R. STAT3 regulated miR-216a promotes ovarian cancer proliferation and cisplatin resistance. Biosci. Rep. 2018, 38, BSR20180547. [Google Scholar] [CrossRef]
- Abildgaard, C.; Do Canto, L.M.; Steffensen, K.D.; Rogatto, S.R. Long non-coding RNAs involved in resistance to chemotherapy in ovarian cancer. Front. Oncol. 2020, 9, 1549. [Google Scholar] [CrossRef]
- Wang, T.; Chan, Y.-H.; Chen, C.-W.; Kung, W.; Lee, Y.; Wang, S.; Chang, T.; Wang, H. Paclitaxel (Taxol) upregulates expression of functional interleukin-6 in human ovarian cancer cells through multiple signaling pathways. Oncogene 2006, 25, 4857–4866. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Zhang, F.; Cui, J.-Y.; Chen, L.; Chen, Y.-T.; Liu, B.-W. CAFs enhance paclitaxel resistance by inducing EMT through the IL-6/JAK2/STAT3 pathway. Oncol. Rep. 2018, 39, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Momeny, M.; Ghaffari, S.H.; Parsanejad, N.; Poursheikhani, A.; Javadikooshesh, S.; Zarrinrad, G.; Esmaeili, F.; Alishahi, Z.; Sabourinejad, Z. IL-6/IL-6R pathway is a therapeutic target in chemoresistant ovarian cancer. Tumori J. 2019, 105, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Giuntoli, R.L.; Webb, T.J.; Zoso, A.; Rogers, O.; Diaz-Montes, T.P.; Bristow, R.E.; Oelke, M. Ovarian cancer-associated ascites demonstrates altered immune environment: Implications for antitumor immunity. Anticancer Res. 2009, 29, 2875–2884. [Google Scholar] [PubMed]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Siu, M.K.; Jiang, Y.-X.; Wang, J.-J.; Leung, T.H.; Han, C.Y.; Tsang, B.K.; Cheung, A.N.; Ngan, H.Y.; Chan, K.K. Hexokinase 2 regulates ovarian cancer cell migration, invasion and stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 signaling cascades. Cancers 2019, 11, 813. [Google Scholar] [CrossRef]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Kemna, E.H.; Kartikasari, A.E.; van Tits, L.J.; Pickkers, P.; Tjalsma, H.; Swinkels, D.W. Regulation of hepcidin: Insights from biochemical analyses on human serum samples. Blood Cells Mol. Dis. 2008, 40, 339–346. [Google Scholar] [CrossRef]
- Basuli, D.; Tesfay, L.; Deng, Z.; Paul, B.; Yamamoto, Y.; Ning, G.; Xian, W.; McKeon, F.; Lynch, M.; Crum, C.P. Iron addiction: A novel therapeutic target in ovarian cancer. Oncogene 2017, 36, 4089–4099. [Google Scholar] [CrossRef]
- Maccio, A.; Madeddu, C.; Massa, D.; Mudu, M.C.; Lusso, M.R.; Gramignano, G.; Serpe, R.; Melis, G.B.; Mantovani, G. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: Role of inflammation in cancer-related anemia. Blood 2005, 106, 362–367. [Google Scholar] [CrossRef]
- Orange, S.T.; Leslie, J.; Ross, M.; Mann, D.A.; Wackerhage, H. The exercise IL-6 enigma in cancer. Trends Endocrinol. Metab 2023, 34, 749–763. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Kanter, N.G.; Cohen-Woods, S.; Balfour, D.; Burt, M.G.; Waterman, A.L.; Koczwara, B. Hypothalamic–Pituitary–Adrenal Axis Dysfunction in People With Cancer: A Systematic Review. Cancer Med. 2024, 13, e70366. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorf, S.K.; Weinrib, A.Z.; Penedo, F.; Russell, D.; DeGeest, K.; Costanzo, E.S.; Henderson, P.J.; Sephton, S.E.; Rohleder, N.; Lucci III, J.A. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J. Clin. Oncol. 2008, 26, 4820–4827. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C.; Massa, D.; Astara, G.; Farci, D.; Melis, G.B.; Mantovani, G. Interleukin-6 and leptin as markers of energy metabolicchanges in advanced ovarian cancer patients. J. Cell. Mol. Med. 2009, 13, 3951–3959. [Google Scholar] [CrossRef]
- Flint, T.R.; Janowitz, T.; Connell, C.M.; Roberts, E.W.; Denton, A.E.; Coll, A.P.; Jodrell, D.I.; Fearon, D.T. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016, 24, 672–684. [Google Scholar] [CrossRef]
- Liu, P.; Wang, L.; Yu, H. Polyploid giant cancer cells: Origin, possible pathways of formation, characteristics, and mechanisms of regulation. Front. Cell Dev. Biol. 2024, 12, 1410637. [Google Scholar] [CrossRef]
- Richards, J.S.; Candelaria, N.R.; Lanz, R.B. Polyploid giant cancer cells and ovarian cancer: New insights into mitotic regulators and polyploidy†. Biol. Reprod. 2021, 105, 305–316. [Google Scholar] [CrossRef]
- Rajaraman, R.; Rajaraman, M.M.; Rajaraman, S.R.; Guernsey, D.L. Neosis—A paradigm of self-renewal in cancer. Cell Biol. Int. 2005, 29, 1084–1097. [Google Scholar] [CrossRef]
- Niu, N.; Yao, J.; Bast, R.C.; Sood, A.K.; Liu, J. IL-6 promotes drug resistance through formation of polyploid giant cancer cells and stromal fibroblast reprogramming. Oncogenesis 2021, 10, 65. [Google Scholar] [CrossRef]
- Nunes, M.; Bartosch, C.; Abreu, M.H.; Richardson, A.; Almeida, R.; Ricardo, S. Deciphering the Molecular Mechanisms behind Drug Resistance in Ovarian Cancer to Unlock Efficient Treatment Options. Cells 2024, 13, 786. [Google Scholar] [CrossRef]
- Lu, M. Do Bilateral Oophorectomy with Hysterectomy and Omentectomy Improve Epithelial Ovarian Cancer Survival Rate Compared with Bilateral Oophorectomy Only? Ph.D. Thesis, UC Irvine, Irvine, CA, USA, 2018. [Google Scholar]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef] [PubMed]
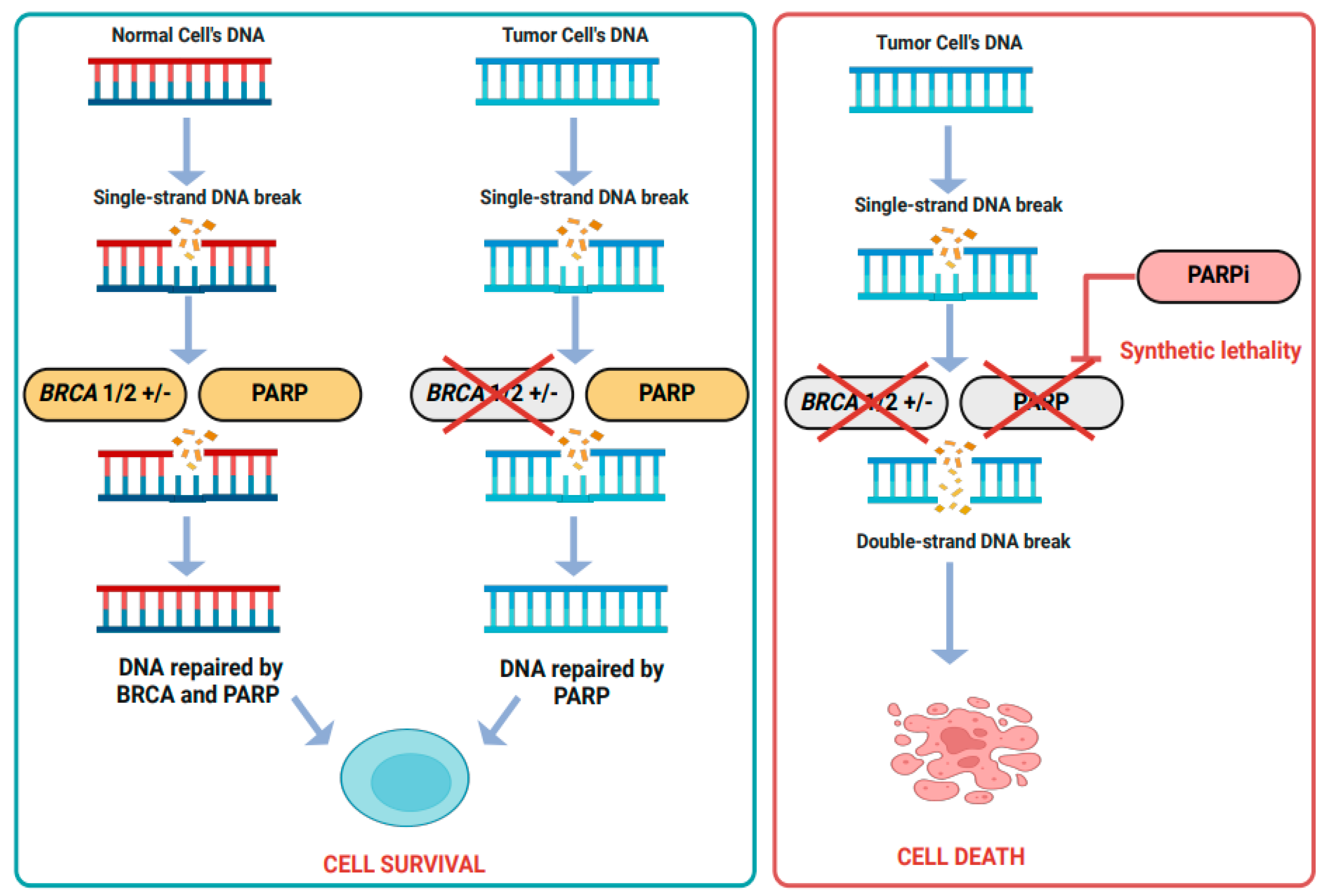
- Bound, N.T.; Vandenberg, C.J.; Kartikasari, A.E.; Plebanski, M.; Scott, C.L. Improving PARP inhibitor efficacy in high-grade serous ovarian carcinoma: A focus on the immune system. Front. Genet. 2022, 13, 886170. [Google Scholar] [CrossRef] [PubMed]
- Sznurkowski, J.J. To Bev or Not to Bev during Ovarian Cancer Maintenance Therapy? Cancers 2023, 15, 2980. [Google Scholar] [CrossRef]
- Byrne, A.T.; Ross, L.; Holash, J.; Nakanishi, M.; Hu, L.; Hofmann, J.I.; Yancopoulos, G.D.; Jaffe, R.B. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin. Cancer Res. 2003, 9, 5721–5728. [Google Scholar]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: The SOLO1/GOG 3004 trial. J. Clin. Oncol. 2023, 41, 609–617. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Leary, A.; Pignata, S.; Cropet, C.; González-Martín, A.; Marth, C.; Nagao, S.; Vergote, I.; Colombo, N.; Mäenpää, J. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: Final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann. Oncol. 2023, 34, 681–692. [Google Scholar] [CrossRef]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef]
- Handolias, D.; Quinn, M.; Foo, S.; Mileshkin, L.; Grant, P.; Dutu, G.; Rischin, D. Oral cyclophosphamide in recurrent ovarian cancer. Asia-Pac. J. Clin. Oncol. 2016, 12, e154–e160. [Google Scholar] [CrossRef]
- Le, D.T.; Jaffee, E.M. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: A current perspective. Cancer Res. 2012, 72, 3439–3444. [Google Scholar] [CrossRef]
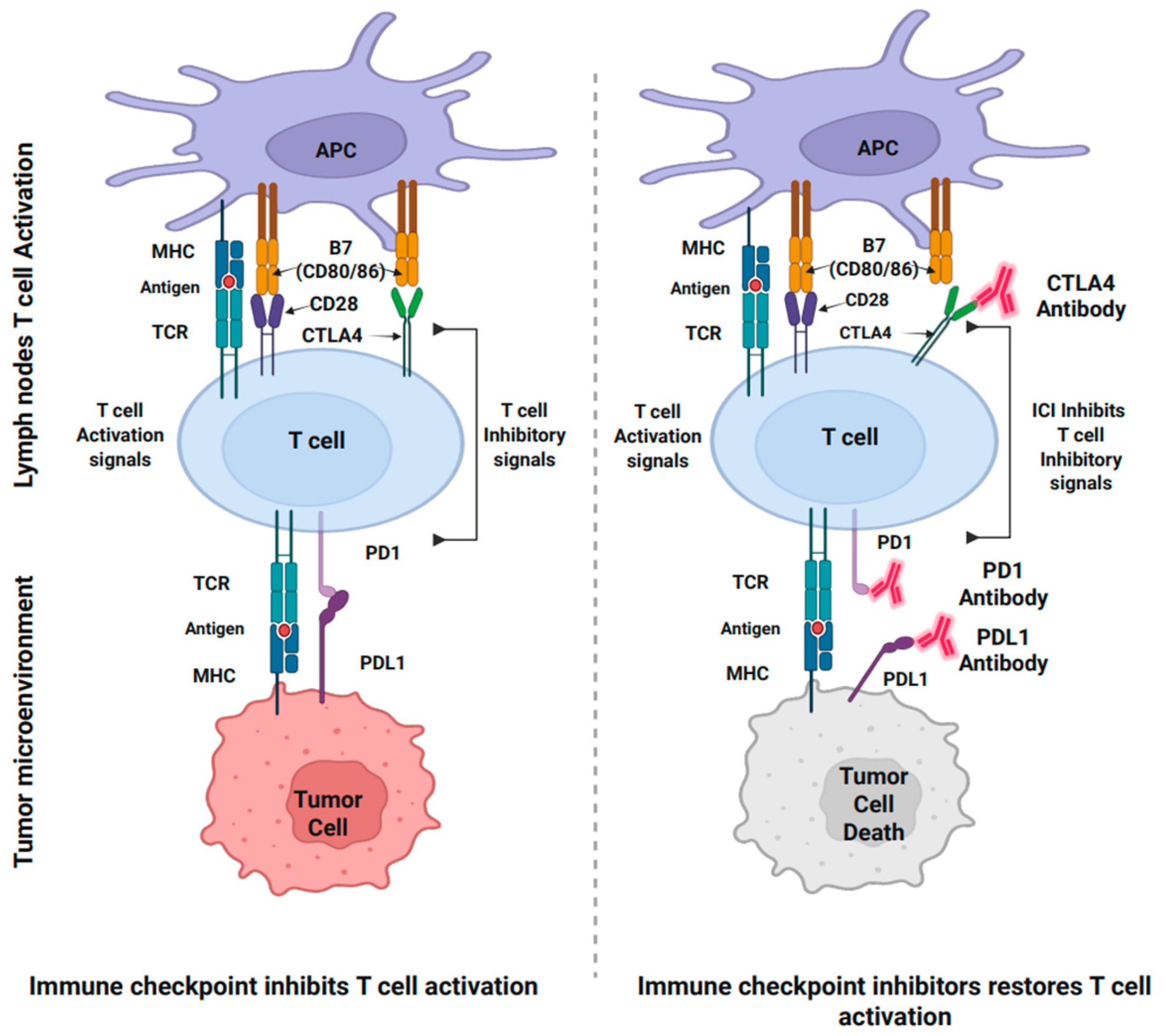
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Hodi, F.S.; Butler, M.; Oble, D.A.; Seiden, M.V.; Haluska, F.G.; Kruse, A.; MacRae, S.; Nelson, M.; Canning, C.; Lowy, I. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc. Natl. Acad. Sci. USA 2008, 105, 3005–3010. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Burger, R.A.; Sill, M.W.; Powell, D.J., Jr.; Lankes, H.A.; Feldman, M.D.; Zivanovic, O.; Gunderson, C.; Ko, E.; Mathews, C. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: An NRG oncology study. J. Clin. Oncol. 2020, 38, 1814–1823. [Google Scholar] [CrossRef]
- Liu, J.F.; Herold, C.; Gray, K.P.; Penson, R.T.; Horowitz, N.; Konstantinopoulos, P.A.; Castro, C.M.; Hill, S.J.; Curtis, J.; Luo, W. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: A phase 2 clinical trial. JAMA Oncol. 2019, 5, 1731–1738. [Google Scholar] [CrossRef]
- Vikas, P.; Borcherding, N.; Chennamadhavuni, A.; Garje, R. Therapeutic potential of combining PARP inhibitor and immunotherapy in solid tumors. Front. Oncol. 2020, 10, 570. [Google Scholar] [CrossRef]
- Drew, Y.; de Jonge, M.; Hong, S.-H.; Park, Y.H.; Wolfer, A.; Brown, J.; Ferguson, M.; Gore, M.E.; Alvarez, R.H.; Gresty, C. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): Results in germline BRCA-mutated (gBRCAm) platinum-sensitive relapsed (PSR) ovarian cancer (OC). Gynecol. Oncol. 2018, 149, 246–247. [Google Scholar] [CrossRef]
- Zsiros, E.; Lynam, S.; Attwood, K.M.; Wang, C.; Chilakapati, S.; Gomez, E.C.; Liu, S.; Akers, S.; Lele, S.; Frederick, P.J. Efficacy and safety of pembrolizumab in combination with bevacizumab and oral metronomic cyclophosphamide in the treatment of recurrent ovarian cancer: A phase 2 nonrandomized clinical trial. JAMA Oncol. 2021, 7, 78–85. [Google Scholar] [CrossRef]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef]
- Anglesio, M.S.; George, J.; Kulbe, H.; Friedlander, M.; Rischin, D.; Lemech, C.; Power, J.; Coward, J.; Cowin, P.A.; House, C.M. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin. Cancer Res. 2011, 17, 2538–2548. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K. Stress, food, and inflammation: Psychoneuroimmunology and nutrition at the cutting edge. Psychosom. Med. 2010, 72, 365–369. [Google Scholar] [CrossRef]
- Berek, J.S.; Chung, C.; Kaldi, K.; Watson, J.M.; Knox, R.M.; Martínez-Maza, O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am. J. Obstet. Gynecol. 1991, 164, 1038–1043. [Google Scholar] [CrossRef]
- Kryczek, I.; Gryboś, M.; Karabon, L.; Klimczak, A.; Lange, A. IL-6 production in ovarian carcinoma is associated with histiotype and biological characteristics of the tumour and influences local immunity. Br. J. Cancer 2000, 82, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Kartikasari, A.E.R.; Michel-Lara, P.; Exton, H.; Tekin-Sari, K.; Alnefai, E.M.M.; Mitchell, A.; Sanchez-Huertas, C.; Plebanski, M. Circulating microRNAs as Diagnostic Biomarkers to Detect Specific Stages of Ovarian Cancer: A Comprehensive Meta-Analysis. Cancers 2024, 16, 4190. [Google Scholar] [CrossRef] [PubMed]
- Daoud, E.; Bodor, G. CA-125 concentrations in malignant and nonmalignant disease. Clin. Chem. 1991, 37, 1968–1974. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; Reynolds, J.; Hallo, J.; McNally, O.M.; Jobling, T.W.; Stephens, A.N.; Quinn, M.A.; Plebanski, M. Pre-operative sera interleukin-6 in the diagnosis of high-grade serous ovarian cancer. Sci. Rep. 2020, 10, 2213. [Google Scholar] [CrossRef]
- Wang, Y.; Zong, X.; Mitra, S.; Mitra, A.K.; Matei, D.; Nephew, K.P. IL-6 mediates platinum-induced enrichment of ovarian cancer stem cells. JCI Insight 2018, 3, e122360. [Google Scholar] [CrossRef]
- Bautista-Sánchez, D.; Arriaga-Canon, C.; Pedroza-Torres, A.; De La Rosa-Velázquez, I.A.; González-Barrios, R.; Contreras-Espinosa, L.; Montiel-Manríquez, R.; Castro-Hernández, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M. The promising role of miR-21 as a cancer biomarker and its importance in RNA-based therapeutics. Mol. Ther. Nucleic Acids 2020, 20, 409–420. [Google Scholar] [CrossRef]
- Gastl, G.; Plante, M.; Finstad, C.; Wong, G.; Federici, M.; Bander, N.; Rubin, S. High IL-6 levels in ascitic fluid correlate with reactive thrombocytosis in patients with epithelial ovarian cancer. Br. J. Haematol. 1993, 83, 433–441. [Google Scholar] [CrossRef]
- Van Der Zee, A.G.; De Bruijn, H.W.; Krans, M.; De Cuyper, E.M.; Hollema, H.; Limburg, P.C.; Bijzet, J.; De Vries, E.G. Higher levels of interleukin-6 in cystic fluids from patients with malignant versus benign ovarian tumors correlate with decreased hemoglobin levels and increased platelet counts. Cancer 1995, 75, 1004–1009. [Google Scholar] [CrossRef]
- Hufnagel, D.H.; Cozzi, G.D.; Crispens, M.A.; Beeghly-Fadiel, A. Platelets, thrombocytosis, and ovarian cancer prognosis: Surveying the landscape of the literature. Int. J. Mol. Sci. 2020, 21, 8169. [Google Scholar] [CrossRef]
- Jordan, K.R.; Sikora, M.J.; Slansky, J.E.; Minic, A.; Richer, J.K.; Moroney, M.R.; Hu, J.; Wolsky, R.J.; Watson, Z.L.; Yamamoto, T.M. The capacity of the ovarian cancer tumor microenvironment to integrate inflammation signaling conveys a shorter disease-free interval. Clin. Cancer Res. 2020, 26, 6362–6373. [Google Scholar] [CrossRef]
- Alvarez Secord, A.; Bell Burdett, K.; Owzar, K.; Tritchler, D.; Sibley, A.B.; Liu, Y.; Starr, M.D.; Brady, J.C.; Lankes, H.A.; Hurwitz, H.I. Predictive blood-based biomarkers in patients with epithelial ovarian cancer treated with carboplatin and paclitaxel with or without bevacizumab: Results from GOG-0218. Clin. Cancer Res. 2020, 26, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Connor, A.; Lyons, P.; Kilgallon, A.; Simpson, J.; Perry, A.; Lysaght, J. Examining the evidence for immune checkpoint therapy in high-grade serous ovarian cancer. Heliyon 2024, 10, e38888. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef]
- Kartikasari, A.E.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Nopour, R. Screening ovarian cancer by using risk factors: Machine learning assists. Biomed. Eng. Online 2024, 23, 18. [Google Scholar] [CrossRef]
- Boehm, K.M.; Aherne, E.A.; Ellenson, L.; Nikolovski, I.; Alghamdi, M.; Vázquez-García, I.; Zamarin, D.; Long Roche, K.; Liu, Y.; Patel, D. Multimodal data integration using machine learning improves risk stratification of high-grade serous ovarian cancer. Nat. Cancer 2022, 3, 723–733. [Google Scholar] [CrossRef]
- Kourou, K.; Exarchos, T.P.; Exarchos, K.P.; Karamouzis, M.V.; Fotiadis, D.I. Machine learning applications in cancer prognosis and prediction. Comput. Struct. Biotechnol. J. 2015, 13, 8–17. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision medicine, AI, and the future of personalized health care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Lu, T.-P.; Kuo, K.-T.; Chen, C.-H.; Chang, M.-C.; Lin, H.-P.; Hu, Y.-H.; Chiang, Y.-C.; Cheng, W.-F.; Chen, C.-A. Developing a prognostic gene panel of epithelial ovarian cancer patients by a machine learning model. Cancers 2019, 11, 270. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, H.; Flanagan, K.L.; Kampan, N.C.; Itsiopoulos, C.; Scott, C.L.; Kartikasari, A.E.R.; Plebanski, M. Interleukin-6 Is a Crucial Factor in Shaping the Inflammatory Tumor Microenvironment in Ovarian Cancer and Determining Its Hot or Cold Nature with Diagnostic and Prognostic Utilities. Cancers 2025, 17, 1691. https://doi.org/10.3390/cancers17101691
Amer H, Flanagan KL, Kampan NC, Itsiopoulos C, Scott CL, Kartikasari AER, Plebanski M. Interleukin-6 Is a Crucial Factor in Shaping the Inflammatory Tumor Microenvironment in Ovarian Cancer and Determining Its Hot or Cold Nature with Diagnostic and Prognostic Utilities. Cancers. 2025; 17(10):1691. https://doi.org/10.3390/cancers17101691
Chicago/Turabian StyleAmer, Hina, Katie L. Flanagan, Nirmala C. Kampan, Catherine Itsiopoulos, Clare L. Scott, Apriliana E. R. Kartikasari, and Magdalena Plebanski. 2025. "Interleukin-6 Is a Crucial Factor in Shaping the Inflammatory Tumor Microenvironment in Ovarian Cancer and Determining Its Hot or Cold Nature with Diagnostic and Prognostic Utilities" Cancers 17, no. 10: 1691. https://doi.org/10.3390/cancers17101691
APA StyleAmer, H., Flanagan, K. L., Kampan, N. C., Itsiopoulos, C., Scott, C. L., Kartikasari, A. E. R., & Plebanski, M. (2025). Interleukin-6 Is a Crucial Factor in Shaping the Inflammatory Tumor Microenvironment in Ovarian Cancer and Determining Its Hot or Cold Nature with Diagnostic and Prognostic Utilities. Cancers, 17(10), 1691. https://doi.org/10.3390/cancers17101691








