A Preoperative Diagnostic Nomogram to Predict Tumor Subclassifications of Intrahepatic Cholangiocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Ethical Statement
2.2. Factors Included in the Nomogram
2.3. Pathological Examination
2.4. Diagnostic Imaging
2.5. Statistical Analyses and Model Development
3. Results
3.1. Differences in Characteristics Between Small and Large Duct-Type ICCs
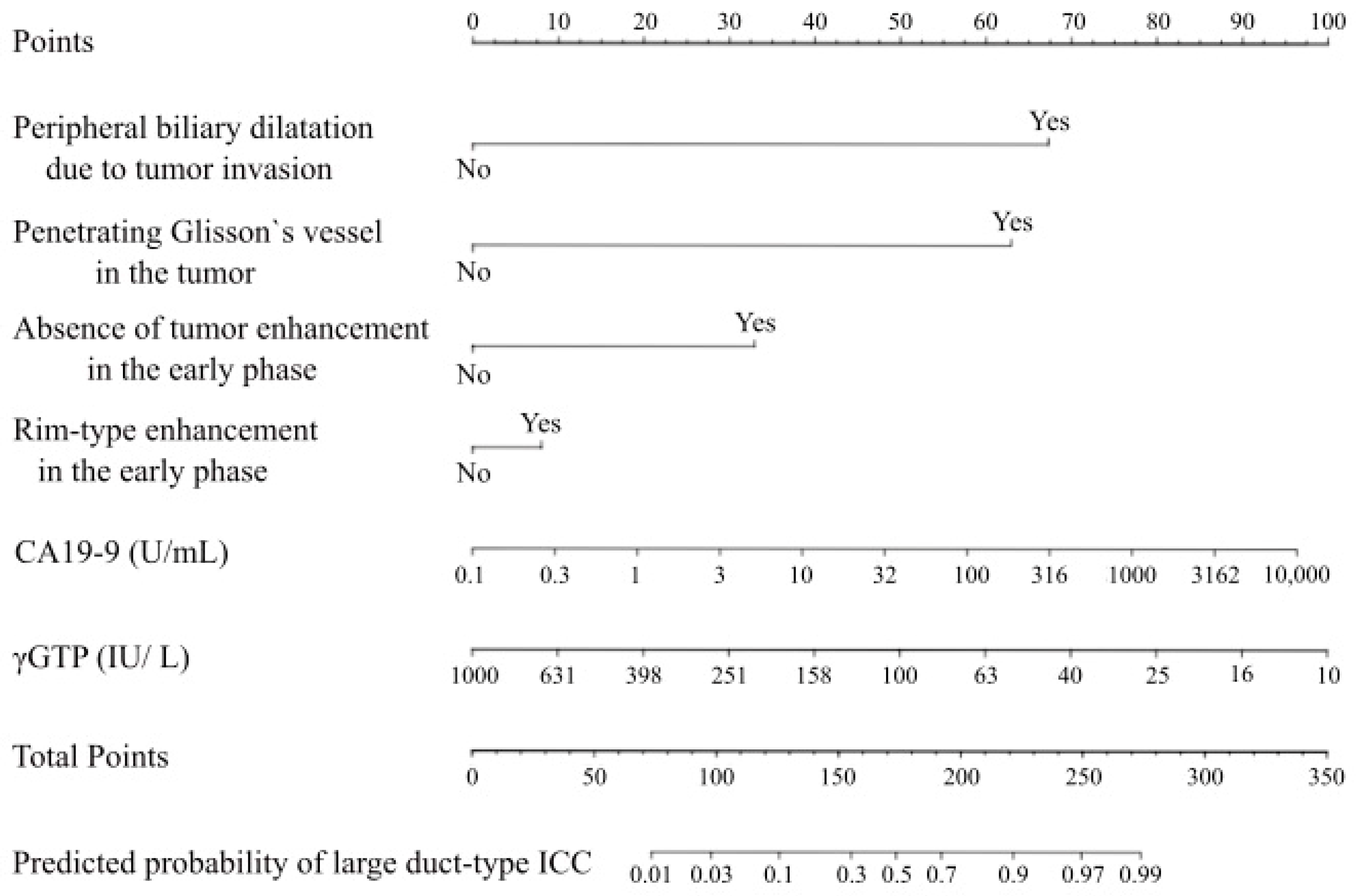
3.2. Preoperative Diagnostic Nomogram to Predict the Subclassifications of ICCs
3.3. Sensitivity and Specificity Using the Developed Nomogram
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| γGTP | Gamma-glutamyl transpeptidase |
| AUC | Area under the ROC curve |
| CA19-9 | Carbohydrate antigen 19-9 |
| CI | Confidence interval |
| CT | Computed tomography |
| H&E | Hematoxylin and eosin |
| ICC | Intrahepatic cholangiocarcinoma |
| LND | Lymph node dissection |
| LNM | Lymph node metastasis |
| MF | Mass forming |
| PI | Periductal infiltration |
| ROC | Receiver operating characteristic |
| WHO | World Health Organization |
References
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Liau, J.Y.; Tsai, J.H.; Yuan, R.H.; Chang, C.N.; Lee, H.J.; Jeng, Y.M. Morphological subclassification of intrahepatic cholangiocarcinoma: Etiological, clinicopathological, and molecular features. Mod. Pathol. 2014, 27, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Misumi, K.; Shibahara, J.; Arita, J.; Sakamoto, Y.; Hasegawa, K.; Kokudo, N.; Fukayama, M. Distinct clinicopathologic and genetic features of 2 histologic subtypes of intrahepatic cholangiocarcinoma. Am. J. Surg. Pathol. 2016, 40, 1021–1030. [Google Scholar] [CrossRef]
- Kubo, S.; Nakanuma, Y.; Takemura, S.; Sakata, C.; Urata, Y.; Nozawa, A.; Nishioka, T.; Kinoshita, M.; Hamano, G.; Terajima, H.; et al. Case series of 17 patients with cholangiocarcinoma among young adult workers of a printing company in Japan. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 479–488. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kubo, S.; Nakanuma, Y.; Sato, Y.; Takemura, S.; Tanaka, S.; Hamano, G.; Ito, T.; Terajima, H.; Yamada, T.; et al. Pathological spectrum of bile duct lesions from chronic bile duct injury to invasive cholangiocarcinoma corresponding to bile duct imaging findings of occupational cholangiocarcinoma. J. Hepato-Biliary-Pancreat. Sci. 2016, 23, 92–101. [Google Scholar] [CrossRef]
- Nam, J.G.; Lee, J.M.; Joo, I.; Ahn, S.J.; Park, J.Y.; Lee, K.B.; Han, J.K. Intrahepatic mass-forming cholangiocarcinoma: Relationship between computed tomography characteristics and histological subtypes. J. Comput. Assist. Tomogr. 2018, 42, 340–349. [Google Scholar] [CrossRef]
- Kinoshita, M.; Sato, Y.; Shinkawa, H.; Kimura, K.; Ohira, G.; Nishio, K.; Tanaka, R.; Kurihara, S.; Kushiyama, S.; Tani, N.; et al. Impact of tumor subclassifications for identifying an appropriate surgical strategy in patients with intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2024, 31, 2579–2590. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, C.; Ni, X.; Huang, P.; Wu, F.; Yang, C.; Zeng, M. Preoperative subcategorization based on magnetic resonance imaging in intrahepatic cholangiocarcinoma. Cancer Imaging 2023, 23, 15. [Google Scholar] [CrossRef]
- Aishima, S.; Fujita, N.; Mano, Y.; Kubo, Y.; Tanaka, Y.; Taketomi, A.; Shirabe, K.; Maehara, Y.; Oda, Y. Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: Carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am. J. Surg. Pathol. 2011, 35, 590–598. [Google Scholar] [CrossRef]
- Song, G.; Shi, Y.; Meng, L.; Ma, J.; Huang, S.; Zhang, J.; Wu, Y.; Li, J.; Lin, Y.; Yang, S.; et al. Single-cell transcriptomic analysis suggests two molecularly distinct subtypes of intrahepatic cholangiocarcinoma. Nat. Commun. 2022, 13, 1642. [Google Scholar] [CrossRef]
- Seo, N.; Kim, D.Y.; Choi, J.Y. Cross-sectional imaging of intrahepatic cholangiocarcinoma: Development, growth, spread, and prognosis. AJR Am. J. Roentgenol. 2017, 209, W64–W75. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.P.; Sheng, R.F.; Yang, C.; Zeng, M.S. Combined arterial and delayed enhancement patterns of MRI assist in prognostic prediction for intrahepatic mass-forming cholangiocarcinoma (IMCC). Abdom. Radiol. 2022, 47, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Aishima, S.; Oda, Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: Different characters of perihilar large duct type versus peripheral small duct type. J. Hepato-Biliary-Pancreat. Sci. 2015, 22, 94–100. [Google Scholar] [CrossRef]
- Yamada, M.; Yamamoto, Y.; Sugiura, T.; Kakuda, Y.; Ashida, R.; Tamura, S.; Okamura, Y.; Ito, T.; Ohgi, K.; Nakanuma, Y.; et al. Comparison of the clinicopathological features in small bile duct and bile ductular type intrahepatic cholangiocarcinoma. Anticancer Res. 2019, 39, 2121–2127. [Google Scholar] [CrossRef]
- Choi, S.B.; Kim, K.S.; Choi, J.Y.; Park, S.W.; Choi, J.S.; Lee, W.J.; Chung, J.B. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: Association of lymph node metastasis and lymph node dissection with survival. Ann. Surg. Oncol. 2009, 16, 3048–3056. [Google Scholar] [CrossRef]
- Lee, A.J.; Chun, Y.S. Intrahepatic cholangiocarcinoma: The AJCC/UICC 8th edition updates. Chin. Clin. Oncol. 2018, 7, 52. [Google Scholar] [CrossRef]
- Li, D.Y.; Zhang, H.B.; Yang, N.; Quan, Y.; Yang, G.S. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: Results of a monocentric series. World J. Gastroenterol. 2013, 19, 9084–9091. [Google Scholar] [CrossRef]
- Sahara, K.; Tsilimigras, D.I.; Merath, K.; Bagante, F.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; Alexandrescu, S.; Poultsides, G.A.; et al. Therapeutic index associated with lymphadenectomy among patients with intrahepatic cholangiocarcinoma: Which patients benefit the most from nodal evaluation? Ann. Surg. Oncol. 2019, 26, 2959–2968. [Google Scholar] [CrossRef]
- Lavanchy, D. Evolving epidemiology of hepatitis C virus. Clin. Microbiol. Infect. 2011, 17, 107–115. [Google Scholar] [CrossRef]
- Schmit, N.; Nayagam, S.; Thursz, M.R.; Hallett, T.B. The global burden of chronic hepatitis B virus infection: Comparison of country-level prevalence estimates from four research groups. Int. J. Epidemiol. 2021, 50, 560–569. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, H.; Yu, M.H.; Lee, E.S.; Yoon, J.H.; Joo, I.; Lee, J.M. Subtype classification of intrahepatic cholangiocarcinoma using Liver MR imaging features and its prognostic value. Liver Cancer 2022, 11, 233–246. [Google Scholar] [CrossRef] [PubMed]


| Variables | Patients with Small Duct-Type ICC (n = 70) | Patients with Large Duct-Type ICC (n = 56) | p-Value |
|---|---|---|---|
| Age, years | 68 (32–89) | 70 (42–82) | 0.79 |
| Sex, male: female, n | 52:18 | 32:24 | 0.066 |
| Chronic liver disease, n | 39 | 25 | 0.29 |
| Viral hepatitis (HCV: HBV), n | 18 (14:4) | 13 (11:2) | 0.91 |
| Alcoholism, n | 11 | 3 | 0.088 |
| MASH, n | 11 | 11 | 0.73 |
| Hepatolithiasis, n | 0 | 3 | 0.085 |
| Laboratory test results, median (IQR) | |||
| Total bilirubin level, mg/dL | 0.6 (0.3–22.7) | 0.6 (0.2–1.6) | 0.72 |
| Albumin level, g/dL | 4.2 (3.2–4.8) | 4.1 (3.2–4.9) | 0.66 |
| AST level, U/L | 30 (11–164) | 26 (16–160) | 0.28 |
| ALT level, U/L | 26 (7–208) | 21 (11–148) | 0.64 |
| γGTP level, IU/L | 63 (13–951) | 104 (15–620) | 0.11 |
| Log(γGTP), log(IU/L) * | 4.1 (2.6–6.9) | 4.6 (2.7–6.4) | 0.11 |
| Prothrombin activity, % | 99 (59–150) | 102 (68–142) | 0.17 |
| Platelet count, ×104/μL | 18.7 (5.4–32.8) | 20.1 (5.9–51.1) | 0.57 |
| CRP level, mg/dL | 0.10 (0.01–7.12) | 0.22 (0.01– 8.5) | 0.088 |
| CEA level, ng/mL | 3.4 (0.7–56.9) | 4.1 (0.6–2108.5) | 0.070 |
| CA19-9 level, U/mL | 21 (2.0 –1554) | 146.5 (0.1–10,000) | <0.001 |
| Log(CA19-9), log(U/mL) * | 3.0 (0.7–7.4) | 5.0 (0.7–9.2) | <0.001 |
| Dynamic CT scan findings | |||
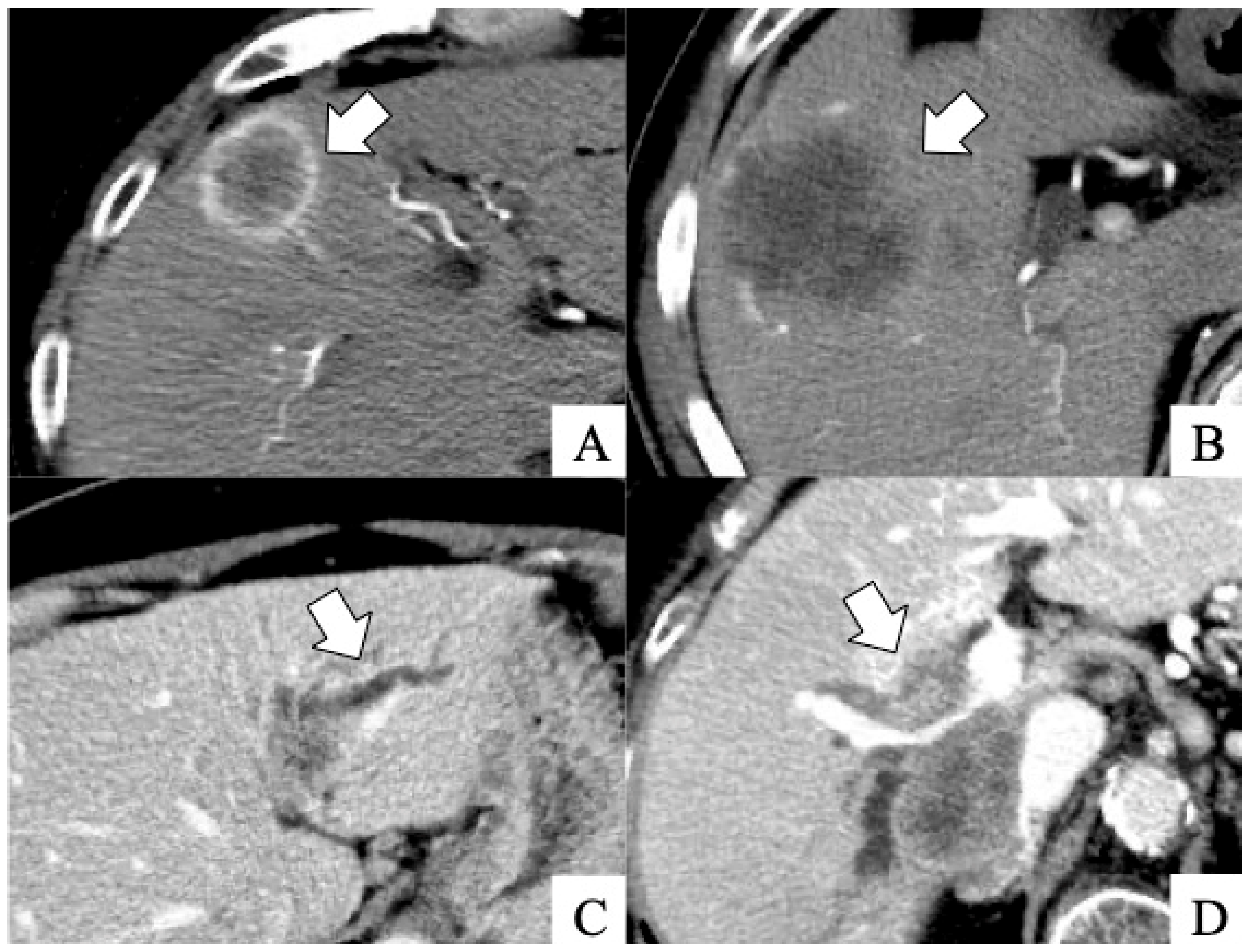
| Rim-type enhancement in the early phase, n | 51 | 23 | <0.001 |
| Absence of tumor enhancement in the early phase, n | 4 | 18 | <0.001 |
| Peripheral biliary dilatation due to tumor invasion, n | 8 | 39 | <0.001 |
| Penetrating Glisson’s vessel in the tumor, n | 8 | 39 | <0.001 |
| Tumor size, cm | 3.9 (0.4–12.5) | 3.7 (0.8–13.0) | 0.81 |
| Median (interquartile range) | |||
| Imaging Finding | Sensitivity | p-Value a | Specificity | p-Value b |
|---|---|---|---|---|
| Developed nomogram, % | 77% | 94% | ||
| No rim-type enhancement in the early phase, % | 59% | 0.049 | 73% | 0.003 |
| Absence of tumor enhancement in the early phase, % | 32% | <0.001 | 94% | 1.00 |
| Peripheral biliary dilatation due to tumor invasion, % | 70% | 0.40 | 89% | 0.24 |
| penetrating Glisson’s vessel in the tumor, % | 70% | 0.40 | 89% | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, M.; Kinoshita, M.; Nonomiya, Y.; Kawai, R.; Shintani, A.; Sato, Y.; Kawaguchi, T.; Tanaka, R.; Kurihara, S.; Nishio, K.; et al. A Preoperative Diagnostic Nomogram to Predict Tumor Subclassifications of Intrahepatic Cholangiocarcinoma. Cancers 2025, 17, 1690. https://doi.org/10.3390/cancers17101690
Yoshida M, Kinoshita M, Nonomiya Y, Kawai R, Shintani A, Sato Y, Kawaguchi T, Tanaka R, Kurihara S, Nishio K, et al. A Preoperative Diagnostic Nomogram to Predict Tumor Subclassifications of Intrahepatic Cholangiocarcinoma. Cancers. 2025; 17(10):1690. https://doi.org/10.3390/cancers17101690
Chicago/Turabian StyleYoshida, Mizuki, Masahiko Kinoshita, Yuta Nonomiya, Ryota Kawai, Ayumi Shintani, Yasunori Sato, Takahito Kawaguchi, Ryota Tanaka, Shigeaki Kurihara, Kohei Nishio, and et al. 2025. "A Preoperative Diagnostic Nomogram to Predict Tumor Subclassifications of Intrahepatic Cholangiocarcinoma" Cancers 17, no. 10: 1690. https://doi.org/10.3390/cancers17101690
APA StyleYoshida, M., Kinoshita, M., Nonomiya, Y., Kawai, R., Shintani, A., Sato, Y., Kawaguchi, T., Tanaka, R., Kurihara, S., Nishio, K., Shinkawa, H., Kimura, K., Yamamoto, A., Kubo, S., & Ishizawa, T. (2025). A Preoperative Diagnostic Nomogram to Predict Tumor Subclassifications of Intrahepatic Cholangiocarcinoma. Cancers, 17(10), 1690. https://doi.org/10.3390/cancers17101690






