Effects of the COVID-19 Pandemic and Post-Pandemic Changes on the Diagnosis, Treatment, and Mortality of Hepatocellular Carcinoma in a Tertiary Center in Western Romania
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Variables
2.2. Diagnosis of HCC and Staging
2.3. Diagnosis of Cirrhosis and Comorbidities
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Coronavirus Disease 2019 Situation Report 51. Available online: https://www.who.int/publications/m/item/situation-report---51 (accessed on 15 September 2024).
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Wagatsuma, K.; Nojima, M.; Matsumoto, T.; Matsuura, M.; Iijima, H.; Matsuoka, K.; Ohmiya, N.; Ishihara, S.; Hirai, F.; et al. Anxiety and Behavioral Changes in Japanese Patients with Inflammatory Bowel Disease Due to COVID-19 Pandemic: A National Survey. J. Gastroenterol. 2023, 58, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Makuza, J.D.; Wong, S.; Morrow, R.L.; Binka, M.; Darvishian, M.; Jeong, D.; Adu, P.A.; Cua, G.; Yu, A.; Velásquez García, H.A.; et al. Impact of COVID-19 Pandemic on Hepatocellular Carcinoma Surveillance in British Columbia, Canada: An Interrupted Time Series Study. J. Viral Hepat. 2024, 31, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Parra, N.S.; Ross, H.M.; Khan, A.; Wu, M.; Goldberg, R.; Shah, L.; Mukhtar, S.; Beiriger, J.; Gerber, A.; Halegoua-DeMarzio, D. Advancements in the diagnosis of hepatocellular carcinoma. Int. J. Transl. Med. 2023, 3, 51–65. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236, Correction in: J. Hepatol. 2019, 70, 817. [Google Scholar] [CrossRef]
- Singal, A.G.; Zhang, E.; Narasimman, M.; Rich, N.E.; Waljee, A.K.; Hoshida, Y.; Yang, J.D.; Reig, M.; Cabibbo, G.; Nahon, P.; et al. HCC Surveillance Improves Early Detection, Curative Treatment Receipt, and Survival in Patients with Cirrhosis: A Meta-Analysis. J. Hepatol. 2022, 77, 128–139. [Google Scholar] [CrossRef]
- Wolf, E.; Rich, N.E.; Marrero, J.A.; Parikh, N.D.; Singal, A.G. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: A systematic review and meta-analysis. Hepatology 2021, 73, 713–725. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer. Available online: https://gco.iarc.fr/today/en/dataviz/maps-heatmap?mode=population&zoom=5&cancers=11&types=0 (accessed on 15 September 2024).
- Akbulut, S.; Garzali, I.U.; Hargura, A.S.; Aloun, A.; Yilmaz, S. Screening, Surveillance, and Management of Hepatocellular Carcinoma During the COVID-19 Pandemic: A Narrative Review. J. Gastrointest. Cancer 2023, 54, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.J.; Rozenberg-Ben-Dror, K.; Jacob, D.A.; Berry, K.; Ioannou, G.N. The COVID-19 pandemic highlights opportunities to improve hepatocellular carcinoma screening and diagnosis in a national health system. Am. J. Gastroenterol. 2022, 117, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Geh, D.; Watson, R.; Sen, G.; French, J.J.; Hammond, J.; Turner, P.; Hoare, T.; Anderson, K.; McNeil, M.; McPherson, S.; et al. COVID-19 and Liver Cancer: Lost Patients and Larger Tumours. BMJ Open Gastroenterol. 2022, 9, e000794. [Google Scholar] [CrossRef]
- Ribaldone, D.G.; Caviglia, G.P.; Gaia, S.; Rolle, E.; Risso, A.; Campion, D.; Brunocilla, P.R.; Saracco, G.M.; Carucci, P. Effect of COVID-19 Pandemic on Hepatocellular Carcinoma Diagnosis: Results from a Tertiary Care Center in North-West Italy. Curr. Oncol. 2022, 29, 1422–1429. [Google Scholar] [CrossRef]
- Okushin, K.; Tateishi, R.; Hirakawa, S.; Tachimori, H.; Uchino, K.; Nakagomi, R.; Yamada, T.; Nakatsuka, T.; Minami, T.; Sato, M.; et al. The Impact of COVID-19 on the Diagnosis and Treatment of HCC: Analysis of a Nationwide Registry for Advanced Liver Diseases (REAL). Sci. Rep. 2024, 14, 2826. [Google Scholar] [CrossRef]
- Liang, J.; Lee, Y.T.; Yeo, Y.H.; Luu, M.; Ayoub, W.; Kuo, A.; Trivedi, H.; Vipani, A.; Gaddam, S.; Kim, H.; et al. Impact of the Early COVID-19 Pandemic on Incidence and Outcomes of Hepatocellular Carcinoma in the United States. Clin. Transl. Gastroenterol. 2024, 15, e00723. [Google Scholar] [CrossRef]
- Bilican, G.; Özgül, S.; Ekmen, N.; Moral, K.; Küçük, H.; Dumanlı, S.; Abiyev, A.; Karakan, T.; Kekilli, M. Effect of COVID-19 Pandemic on Hepatocellular Carcinoma Diagnosis: Results from a Single Turkey Center Study. J. Gastrointest. Liver Dis. 2023, 32, 367–370. [Google Scholar] [CrossRef]
- Murai, K.; Hikita, H.; Kodama, T.; Kaibori, M.; Nishimura, Y.; Tatsumi, T.; Yamada, T.; Kanto, T.; Mochida, S.; Takehara, T. The Impact of the COVID-19 Pandemic on Hepatocellular Carcinoma Diagnosis and Treatment in Japan: A Multicenter Collaborative Observational Study. Hepatol. Res. 2024, 54, 439–450. [Google Scholar] [CrossRef]
- Kaur, B.; Yeo, Y.H.; Liang, J.; Luu, M.; Ayoub, W.; Kuo, A.; Trivedi, H.; Sankar, K.; Gong, J.; Hendifar, A.; et al. COVID-19 Pandemic Impact on Diagnosis, Stage, and Treatment of Hepatocellular Carcinoma in the United States. Gastro Hep Adv. 2023, 3, 230–237. [Google Scholar] [CrossRef]
- Pomej, K.; Scheiner, B.; Hartl, L.; Balcar, L.; Meischl, T.; Mandorfer, M.; Reiberger, T.; Müller, C.; Trauner, M.; Pinter, M. COVID-19 Pandemic: Impact on the Management of Patients with Hepatocellular Carcinoma at a Tertiary Care Hospital. PLoS ONE 2021, 16, e0256544. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver—Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef]
- Toyoda, H.; Johnson, P.J. The ALBI score: From liver function in patients with HCC to a general measure of liver function. JHEP Rep. 2022, 4, 100557. [Google Scholar] [CrossRef]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, M.Y.; Kang, S.H.; Baik, S.K. Application of ultrasound for the diagnosis of cirrhosis/portal hypertension. J. Med. Ultrason. 2022, 49, 321–331. [Google Scholar] [CrossRef]
- Ferraioli, G.; Barr, R.G.; Berzigotti, A.; Sporea, I.; Wong, V.W.; Reiberger, T.; Karlas, T.; Thiele, M.; Cardoso, A.C.; Ayonrinde, O.T.; et al. WFUMB Guideline/Guidance on Liver Multiparametric Ultrasound: Part 1. Update to 2018 Guidelines on Liver Ultrasound Elastography. Ultrasound Med. Biol. 2024, 50, 1071–1087. [Google Scholar] [CrossRef]
- Morra, R.; Munteanu, M.; Imbert-Bismut, F.; Messous, D.; Ratziu, V.; Poynard, T. FibroMAX: Towards a new universal biomarker of liver disease? Expert Rev. Mol. Diagn. 2007, 7, 481–490. [Google Scholar] [CrossRef]
- Harreiter, J.; Roden, M. Diabetes mellitus—Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2023) [Diabetes mellitus: Definition, classification, diagnosis, screening and prevention (Update 2023)]. Wien Klin. Wochenschr. 2023, 135, 7–17. [Google Scholar] [CrossRef]
- Brouwers, S.; Sudano, I.; Kokubo, Y.; Sulaica, E.M. Arterial hypertension. Lancet 2021, 398, 249–261. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Vardas, E.P.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Tousoulis, D. Atrial Fibrillation: Pathogenesis, Predisposing Factors, and Genetics. Int. J. Mol. Sci. 2021, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Pastena, P.; Frye, J.T.; Ho, C.; Goldschmidt, M.E.; Kalogeropoulos, A.P. Ischemic cardiomyopathy: Epidemiology, pathophysiology, outcomes, and therapeutic options. Heart Fail. Rev. 2024, 29, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef]
- Peng, Y.; Qi, X.; Guo, X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine 2016, 95, e2877. [Google Scholar] [CrossRef]
- Furumaya, A.; van Delden, O.M.; de Wilde, R.F.; de Vos-Geelen, J.; van der Geest, L.G.; Dutch Hepatocellular & Cholangiocarcinoma Group (DHCG) and the COVID & Cancer-NL consortium. Impact of COVID-19 on incidence, treatment, and survival of patients with hepatocellular carcinoma in the Netherlands. Disaster Med. Public Health Prep. 2024, 18, e243. [Google Scholar] [CrossRef]
- Inchingolo, R.; Acquafredda, F.; Tedeschi, M.; Laera, L.; Surico, G.; Surgo, A.; Fiorentino, A.; Spiliopoulos, S.; de’Angelis, N.; Memeo, R. Worldwide Management of Hepatocellular Carcinoma During the COVID-19 Pandemic. World J. Gastroenterol. 2021, 27, 3780–3789. [Google Scholar] [CrossRef]
- Crăciun, R.; Ștefănescu, H.; Sporea, I.; Săndulescu, L.D.; Gheorghe, L.; Trifan, A.; Spârchez, Z.; Dănilă, M.; Rogoveanu, I.; Cerban, R.; et al. The Staging of Newly Diagnosed Hepatocellular Carcinoma in Romania. A National Multicentric Study. J. Gastrointest. Liver Dis. 2024, 33, 212–217. [Google Scholar] [CrossRef]
- Paik, J.M.; Shah, D.; Eberly, K.; Golabi, P.; Henry, L.; Younossi, Z.M. Changes in mortality due to chronic liver diseases (CLD) during the COVID-19 pandemic: Data from the United States’ National Vital Statistics System. PLoS ONE 2024, 19, e0289202. [Google Scholar] [CrossRef]
- Moga, T.V.; Foncea, C.; Bende, R.; Popescu, A.; Burdan, A.; Heredea, D.; Danilă, M.; Miutescu, B.; Ratiu, I.; Bizerea-Moga, T.O.; et al. Impact of COVID-19 on Patients with Decompensated Liver Cirrhosis. Diagnostics 2023, 13, 600. [Google Scholar] [CrossRef]
- Singal, A.G.; Kilgore, K.M.; Shvets, E.; Parikh, N.D.; Mehta, N.; Ozbay, A.B.; Teigland, C.; Hafez, O.; Schroeder, A.; Yang, A.; et al. Impact of Social Determinants of Health on Hepatocellular Carcinoma Surveillance, Treatment, and Health Care Costs. Hepatol. Commun. 2024, 8, e0517. [Google Scholar] [CrossRef] [PubMed]
- APASL COVID-19 Task Force; Lau, G.; Sharma, M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol. Int. 2020, 14, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Amaddeo, G.; Brustia, R.; Allaire, M.; Lequoy, M.; Hollande, C.; Regnault, H.; Blaise, L.; Ganne-Carrié, N.; Séror, O.; Larrey, E.; et al. Impact of COVID-19 on the Management of Hepatocellular Carcinoma in a High-Prevalence Area. JHEP Rep. 2021, 3, 100199. [Google Scholar] [CrossRef] [PubMed]
- De Souza, S.; Kahol de Jong, J.; Perone, Y.; Shetty, S.; Qurashi, M.; Vithayathil, M.; Shah, T.; Ross, P.; Temperley, L.; Yip, V.S.; et al. Impact of COVID-19 on 1-Year Survival Outcomes in Hepatocellular Carcinoma: A Multicenter Cohort Study. Cancers 2023, 15, 3378. [Google Scholar] [CrossRef]
- Li, D.; Jia, A.Y.; Zorzi, J.; Griffith, P.; Kim, A.K.; Dao, D.; Anders, R.A.; Georgiades, C.; Liddell, R.P.; Hong, K.; et al. Impact of the COVID-19 Pandemic on Liver Cancer Staging at a Multidisciplinary Liver Cancer Clinic. Ann. Surg. Open 2022, 3, e207. [Google Scholar] [CrossRef]
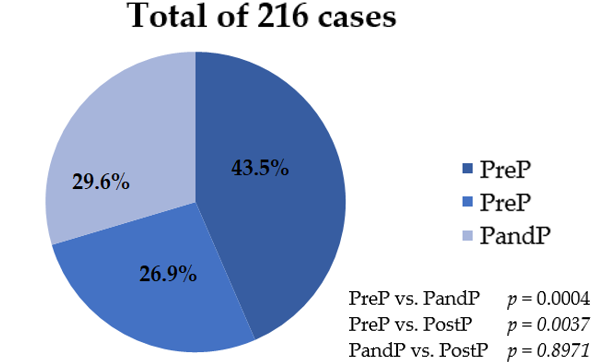

| Parameter | Overall (n = 216) | I. PreP Group (n = 94) | II. PandP Group (n = 58) | III. PostP Group (n = 64) | p Values | ||
|---|---|---|---|---|---|---|---|
| I/II | I/III | II/III | |||||
| Mean age (years) | 67.3 ± 8.7 | 67 ± 8.8 | 66 ± 9.6 | 68.9 ± 7.7 | 0.5120 | 0.1634 | 0.2283 |
| Gender (%) | |||||||
| Females | 60 (27.8%) | (26) 27.7% | 14 (24.1%) | 20 (31.3%) | 0.7642 | 0.7562 | 0.4943 |
| Males | 156 (72.2%) | (68) 72.3% | 44 (75.9%) | 44 (68.7%) | 0.7642 | 0.7562 | 0.4943 |
| Etiology (%) | |||||||
| ALD | 45 (20.8%) | 18 (19.1%) | 13 (22.4%) | 14 (21.9%) | 0.7768 | 0.8194 | 0.8793 |
| HBV | 37 (17.1%) | 17 (18.1%) | 7 (12.1%) | 13 (20.3%) | 0.4496 | 0.8890 | 0.3287 |
| HBV + HCV | 5 (2.3%) | 2 (2.1%) | 2 (3.5%) | 1 (1.6%) | 0.9982 | 0.7130 | 0.9278 |
| HBV + HDV | 12 (5.6%) | 6 (6.4%) | 3 (5.2%) | 3 (4.7%) | 0.9608 | 0.9181 | 0.7710 |
| HCV | 88 (40.8%) | 39 (41.5%) | 23 (39.7%) | 26 (40.6%) | 0.9605 | 0.9587 | 0.9333 |
| MASLD | 29 (13.4%) | 12 (12.8%) | 10 (17.2%) | 7 (10.9%) | 0.6089 | 0.9114 | 0.4577 |
| Performance index | 1 [0–3] | 1 [0–3] | 1 [0–3] | 1 [0–3] | 0.7474 | 0.8544 | 0.5517 |
| Platelet count (×103/µL) | 175 [33–715] | 142.5 [33–388] | 142.5 [42–438] | 186 [60–715] | 0.6904 | 0.0280 | 0.0061 |
| ALT(UI/L) | 45 [8–816] | 49.5 [10–566] | 44.5 [10–685] | 38 [8–816] | 0.1367 | 0.0249 | 0.3848 |
| ALB (mg/dL) | 2.9 [1.2–5.2] | 2.75 [1.2–4.7] | 2.75 [1.6–4.5] | 3.15 [1.2–5.2] | 0.3229 | 0.0020 | 0.0507 |
| AFP (ng/mL) | 73 [0.1–191,000] | 63.1 [1.5–191,000] | 215.9 [0.1–99,500] | 63.5 [0.1–29,800] | 0.2822 | 0.5649 | 0.1632 |
| Serum sodium (mg/dL) | 138 [114–147] | 137 [124–144] | 138 [126–146] | 138 [114–147] | 0.0612 | 0.0144 | 0.4956 |
| Environment: | |||||||
| Rural | 79 (36.6%) | 38 (40.4%) | 20 (34.5%) | 21 (32.8%) | 0.5785 | 0.4225 | 0.9947 |
| Urban | 137 (63.4%) | 56 (59.6%) | 38 (65.5%) | 43 (67.2%) | 0.5785 | 0.4225 | 0.9947 |
| Cirrhosis (%) | |||||||
| Yes | 200 (92.6%) | 91 (96.8%) | 51 (87.9%) | 58 (90.6%) | 0.0701 | 0.1938 | 0.8503 |
| No | 16 (7.4%) | 3 (3.2%) | 7 (12.1%) | 6 (9.4%) | 0.0701 | 0.1938 | 0.8503 |
| Newly diagnosed cirrhosis | |||||||
| Yes | 90 (45%) | 40 (44%) | 24 (47.1%) | 26 (44.8%) | 0.8568 | 0.9416 | 0. 9618 |
| No | 110 (55%) | 51 (56%) | 27 (52.9%) | 32 (55.2%) | 0.8568 | 0.9416 | 0.9618 |
| Child-Pugh grade (%) | |||||||
| A [5–6] | 66 (30.6%) | 28 (29.8%) | 17 (29.3%) | 21 (32.8%) | 0.9066 | 0.8220 | 0.8250 |
| B [7–9] | 81 (37.5%) | 37 (39.4%) | 19 (32.8%) | 25 (39.1%) | 0.5181 | 0.8981 | 0.5928 |
| C [10–15] | 69 (31.9%) | 29 (30.8%) | 22 (37.9%) | 18 (28.2%) | 0.4691 | 0.8621 | 0.3439 |
| MELD score | 18 [11–33] | 19 [15–33] | 17 [13–26] | 18 [11–30] | 0.2235 | 0.4587 | 0.8480 |
| Comorbidities | |||||||
| CC | 52 (24.1%) | 21 (22.3%) | 12 (20.7%) | 19 (29.7%) | 0.9761 | 0.3876 | 0.3514 |
| HTN | 93 (43.1%) | 39 (41.5%) | 20 (34.5%) | 34 (53.1%) | 0.4909 | 0.2029 | 0.0597 |
| CKD | 10 (4.6%) | 7 (7.4%) | 1 (1.7%) | 2 (3.1%) | 0.2465 | 0.4250 | 0.9306 |
| DM | 72 (33.4%) | 38 (40.4%) | 15 (25.9%) | 19 (29.7%) | 0.995 | 0.2277 | 0.7908 |
| ALBI score | −1.81 ± 0.47 | −1.83 ± 0.38 | −1.81 ± 0.55 | −1.76 ± 0.57 | 0.7915 | 0.3556 | 0.6236 |
| Number of HCC lesions: | |||||||
| 1 lesion | 100 (46.3%) | 47 (50%) | 22 (37.9%) | 31 (48.5%) | 0.1978 | 0.9816 | 0.3189 |
| 2 lesions | 20 (9.3%) | 8 (8.5%) | 5 (8.6%) | 7 (10.9%) | 0.7815 | 0.8189 | 0.9031 |
| 3 lesions | 10 (4.6%) | 2 (2.1%) | 6 (10.4%) | 2 (3.1%) | 0.0641 | 0.9016 | 0.2079 |
| Infiltrative (diffuse) | 16 (7.4%) | 8 (8.5%) | 3 (5.2%) | 5 (7.8%) | 0.6596 | 0.8904 | 0.8313 |
| Multiple lesions | 70 (32.4%) | 29 (30.9%) | 22 (37.9%) | 19(29.7%) | 0.4771 | 0.9879 | 0.449 |
| Tumor size (cm): | |||||||
| Single lesion | 5.5 ± 2.1 | 5.8 ± 3.1 | 4.8 ± 1.8 | 5.6 ± 1.9 | 0.8044 | 0.6280 | 0.7250 |
| Infiltrative/Multiple lesions | 6.1 ± 2.9 | 5.9 ± 1.8 | 6.1 ± 1.6 | 6.5 ± 2.8 | 0.6311 | 0.4562 | 0.5419 |
| TNM | |||||||
| IA | 12 (5.6%) | 9 (9.6%) | 1 (1.7%) | 2 (3.1%) | 0.1162 | 0.2087 | 0.9306 |
| IB | 58 (26.9%) | 21 (21.3%) | 17 (29.3%) | 20 31.3%) | 0.3566 | 0.2179 | 0.9159 |
| II | 37 (17.1%) | 20 (22.3%) | 9 (15.5%) | 8 (12.5%) | 0.4197 | 0.1759 | 0.8289 |
| IIIA | 38 (17.6%) | 17 (18.1%) | 11 (19%) | 10 (15.6%) | 0.9392 | 0.8457 | 0.7974 |
| IIIB | 45 (20.8%) | 16 (17%) | 13 (22.5%) | 16 (25%) | 0.5316 | 0.3044 | 0.9117 |
| IVA | 7 (3.2%) | 5 (5.3%) | 1 (1.7%) | 1 (1.6%) | 0.4963 | 0.4412 | 0.5036 |
| IVB | 19 (8.8%) | 6 (6.4%) | 6 (10.3%) | 7 (10.9%) | 0.5777 | 0.4741 | 0.8518 |
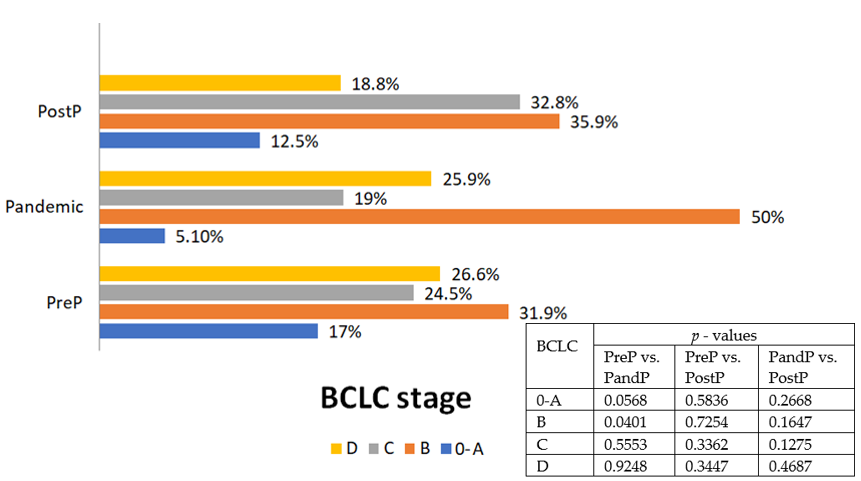
| HCC stage (BCLC) (%) | |||||||
| 0-A | 27 (12.5%) | 16 (17%) | 3 (5.2%) | 8 (12.5%) | 0.0595 | 0.5836 | 0.2763 |
| B | 82 (38%) | 30 (31.9%) | 29 (50%) | 23 (35.9%) | 0.0401 | 0.7254 | 0.1647 |
| C | 55 (25.5%) | 23 (24.5%) | 11 (19%) | 21 (32.8%) | 0.5553 | 0.3362 | 0.1275 |
| D | 52(24%) | 25 (26.6%) | 15 (25.8%) | 12 (18.8%) | 0.9356 | 0.3447 | 0.4766 |
| PVT (%) | 75 (34.7%) | 31 (33%) | 19 (32.8%) | 25 (39%) | 0.8791 | 0.5454 | 0.6006 |
| Benign | 7/75 (8%) | 3 (9.7%) | 2 (10.5%) | 1 (4%) | 0.6936 | 0.7632 | 0.6852 |
| Malignan | 69/75 (92%) | 90.3% (28) | 17 (89.5%) | 24 (96%) | 0.6936 | 0.7632 | 0.6852 |
| Treatment | Overall (n = 216) | PreP Group (n = 94) | Pandemic Group (n = 58) | PostP Group (n = 64) | p Values | ||
|---|---|---|---|---|---|---|---|
| I/II | I/III | II/III | |||||
| Systemic | 94 (43.5%) | 39 (41.5%) | 23 (39.7%) | 32 (50%) | 0.9605 | 0.3727 | 0.3372 |
| Surgery | 23 (10.7%) | 10 (10.6%) | 6 (10.3%) | 6 (9.4%) | 0.8307 | 0.9815 | 0.8905 |
| Percutaneous ablation | 20 (9.3%) | 11 (11.7%) | 5 (8.6%) | 5 (7.8%) | 0.7391 | 0.5966 | 0.8652 |
| TACE | 26 (12%) | 8 (8.5%) | 9 (15.5%) | 9 (14.1%) | 0.2865 | 0.3935 | 0.9698 |
| BSC | 53 (24.5%) | 26 (27.7%) | 15 (25.9%) | 12 (18.8%) | 0.9563 | 0.2737 | 0.4687 |
| BCLC classification according to treatment | |||||||
| BCLC stage | Systemic (n = 94) | Surgery (n = 23) | Percutaneous ablation (n = 20) | TACE (n = 26) | BSC (n = 53) | ||
| A | 2 (2.1%) | 7 (30.4%) | 14 (70%) | 3 (11.6%) | 1 (1.9%) | ||
| B | 41 (43.6%) | 16 (69.6%) | 2 (10%) | 23 (88.5%) | 0% | ||
| C | 51 (54.3%) | 0% | 4 (20%) | 0% | 0% | ||
| D | 0% | 0% | 0% | 0% | 52 (98.1%) | ||
| BCLC Stage | I.PreP Group (n = 94) | II.Pandemic Group (n = 58) | III.PostP Group (n = 64) | p Values | ||
|---|---|---|---|---|---|---|
| I/II | I/III | II/III | ||||
| 0-A | 8/16 (50%) | 2/3 (66.7%) | 2/8 (25%) | 0.9216 | 0.4642 | 0.5641 |
| B | 16/30 (53.4%) | 17/29 (58.6%) | 8/23 (34.8%) | 0.8887 | 0.2847 | 0.1537 |
| C | 14/23 (60.9%) | 6/11 (54.5%) | 8/21 (33.4%) | 0.9859 | 0.1280 | 0.4382 |
| D | 16/25 (64%) | 11/15 (73.4%) | 10/12 (84.4%) | 0.7903 | 0.3736 | 0.8265 |
| Baseline Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.303 (1.112–2.289) | 0.036 | ||
| AFP values | 1.601 (1.332–1.835) | 0.0034 | ||
| ALD etiology | 1.466 (1.679–2.815) | 0.04 | ||
| Child-Pugh stage | 1.704 (1.441–2.924) | 0.0036 | ||
| BCLC stage | 1.663 (1.032–2.406) | 0.0003 | 1.363 (1.032–2.406) | <0.0001 |
| TNM stage | 1.215 (1.002–2.342) | 0.0039 | ||
| Malignant PVT | 2.16 (1.902–3.974) | 0.0009 | 1.816 (1.330–2.724) | <0.0001 |
| Presence of comorbidities | 1.983 (1.421–3.268) | 0.001 | 1.623 (1.223–2.681) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burciu, C.; Miutescu, B.; Bende, R.; Burciu, D.; Moga, T.V.; Popescu, A.; Popa, A.; Bende, F.; Gadour, E.; Burdan, A.; et al. Effects of the COVID-19 Pandemic and Post-Pandemic Changes on the Diagnosis, Treatment, and Mortality of Hepatocellular Carcinoma in a Tertiary Center in Western Romania. Cancers 2025, 17, 1660. https://doi.org/10.3390/cancers17101660
Burciu C, Miutescu B, Bende R, Burciu D, Moga TV, Popescu A, Popa A, Bende F, Gadour E, Burdan A, et al. Effects of the COVID-19 Pandemic and Post-Pandemic Changes on the Diagnosis, Treatment, and Mortality of Hepatocellular Carcinoma in a Tertiary Center in Western Romania. Cancers. 2025; 17(10):1660. https://doi.org/10.3390/cancers17101660
Chicago/Turabian StyleBurciu, Calin, Bogdan Miutescu, Renata Bende, Deiana Burciu, Tudor Voicu Moga, Alina Popescu, Alexandru Popa, Felix Bende, Eyad Gadour, Adrian Burdan, and et al. 2025. "Effects of the COVID-19 Pandemic and Post-Pandemic Changes on the Diagnosis, Treatment, and Mortality of Hepatocellular Carcinoma in a Tertiary Center in Western Romania" Cancers 17, no. 10: 1660. https://doi.org/10.3390/cancers17101660
APA StyleBurciu, C., Miutescu, B., Bende, R., Burciu, D., Moga, T. V., Popescu, A., Popa, A., Bende, F., Gadour, E., Burdan, A., Iovanescu, D., Danila, M., & Sirli, R. (2025). Effects of the COVID-19 Pandemic and Post-Pandemic Changes on the Diagnosis, Treatment, and Mortality of Hepatocellular Carcinoma in a Tertiary Center in Western Romania. Cancers, 17(10), 1660. https://doi.org/10.3390/cancers17101660









