Beyond Exosomes: An Ultrapurified Phospholipoproteic Complex (PLPC) as a Scalable Immunomodulatory Platform for Reprogramming Immune Suppression in Metastatic Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
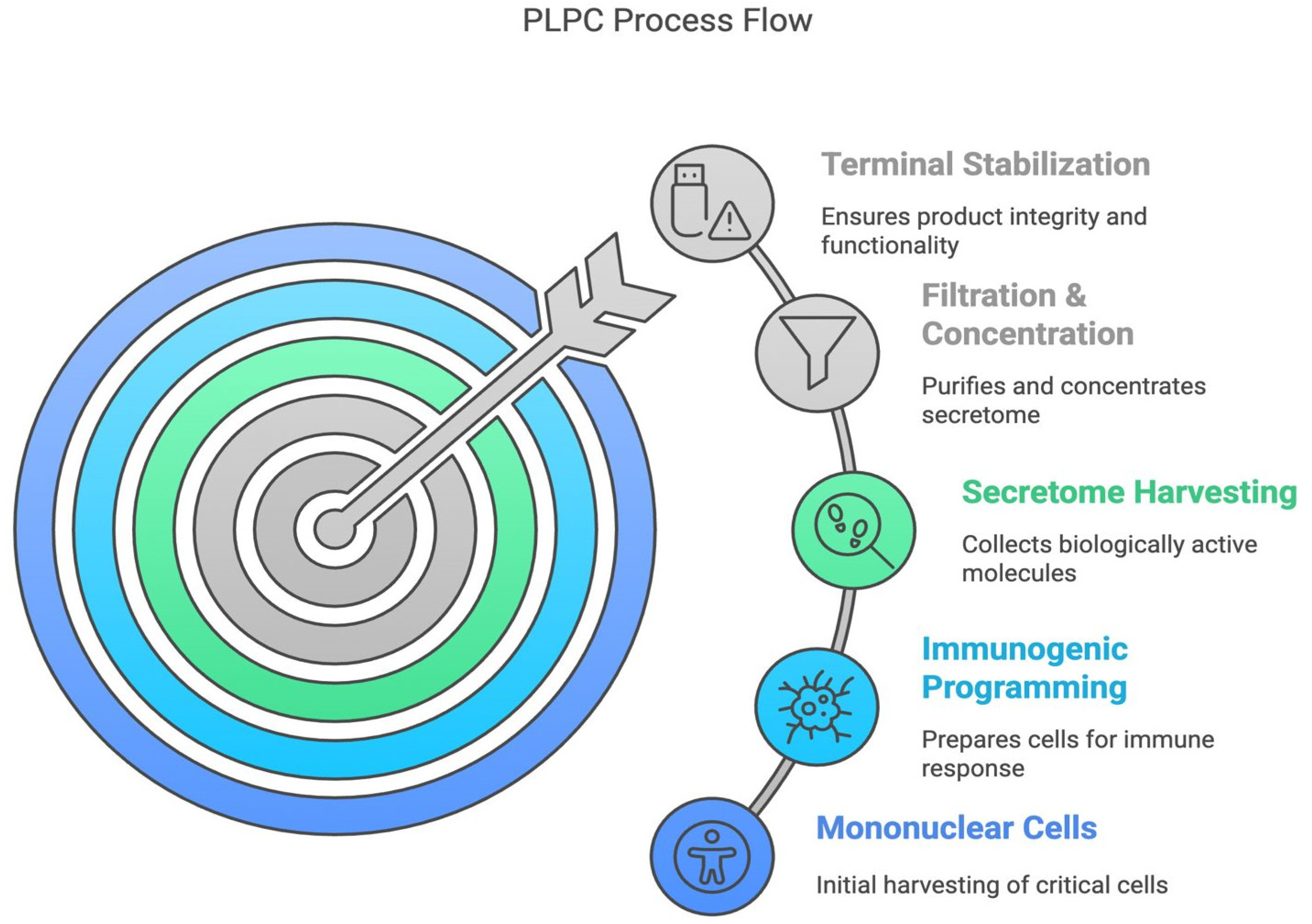
2.1. Cell Source and Dendritic Differentiation
2.2. Secretome Collection and Initial Processing
2.3. PLPC Production and Final Stabilization
2.4. Proteomic Characterization and Comparative Structural Analysis
2.5. Functional Assays in Tumor and Non-Tumor Cell Lines
2.6. Ex Vivo Immunological Analysis and Cytokine Profiling
2.7. Exploratory Functional Assessment in a Non-Clinical Biological Environment
2.8. Statistical and Bioinformatic Analyses
3. Results
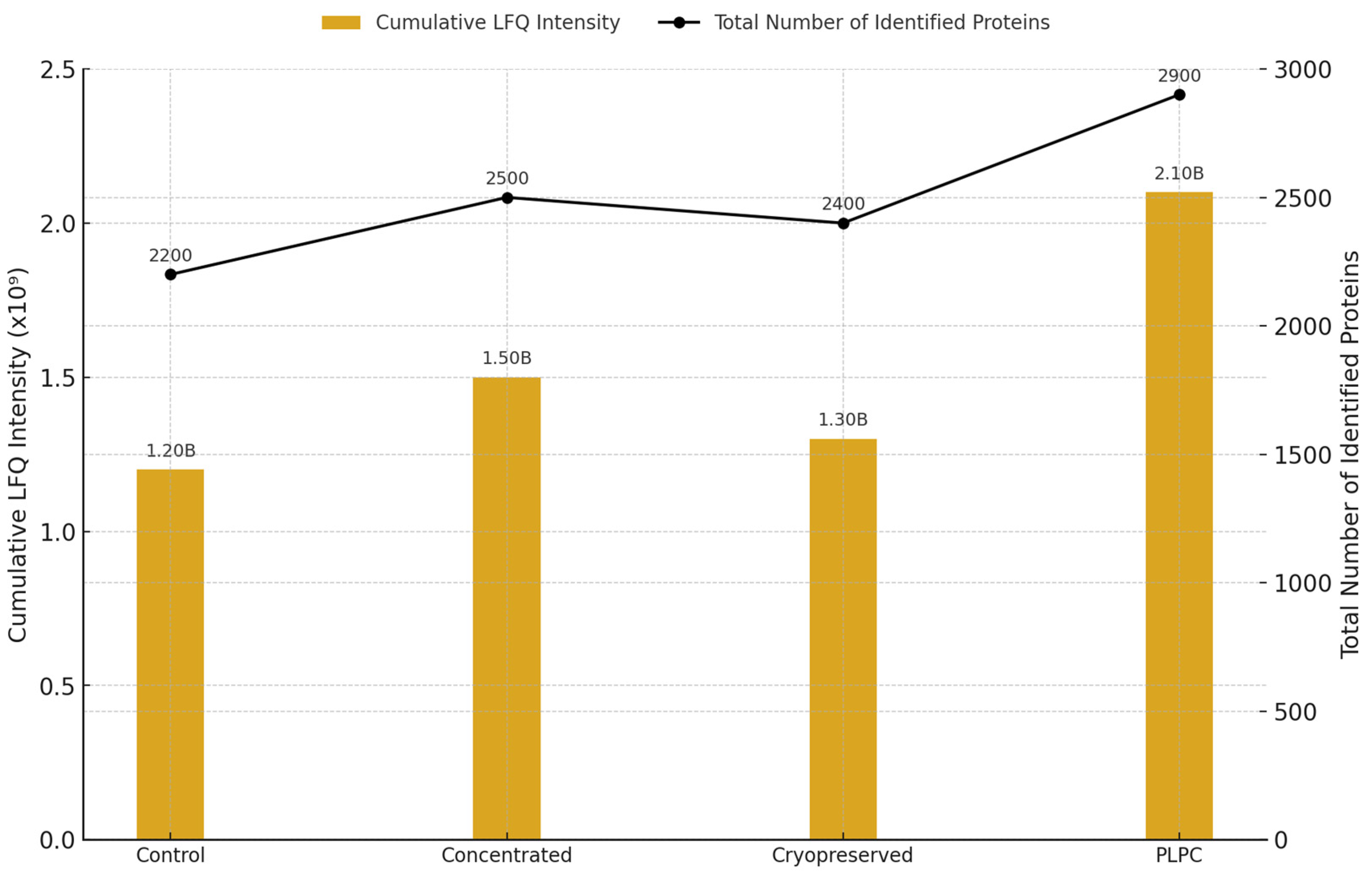
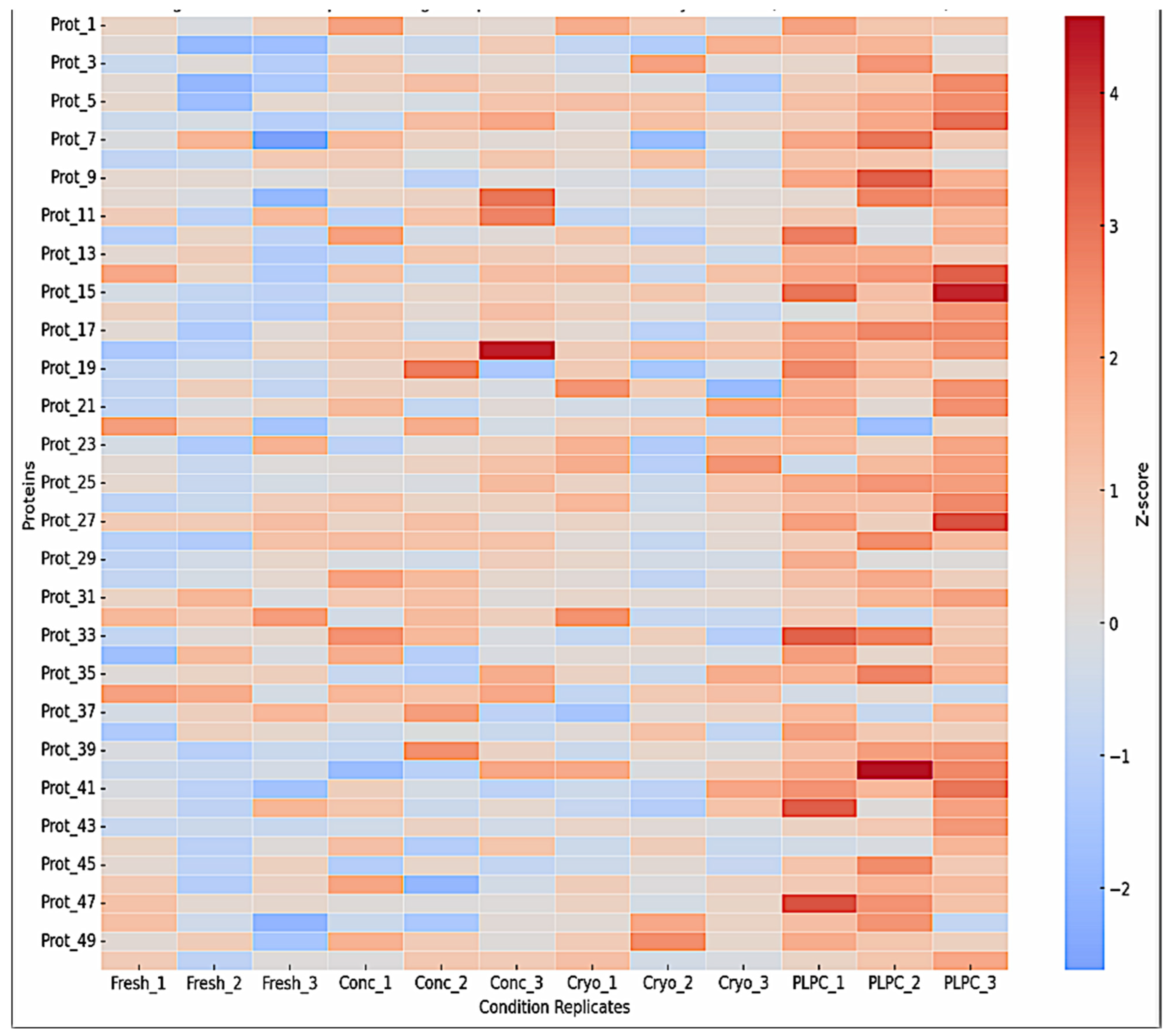
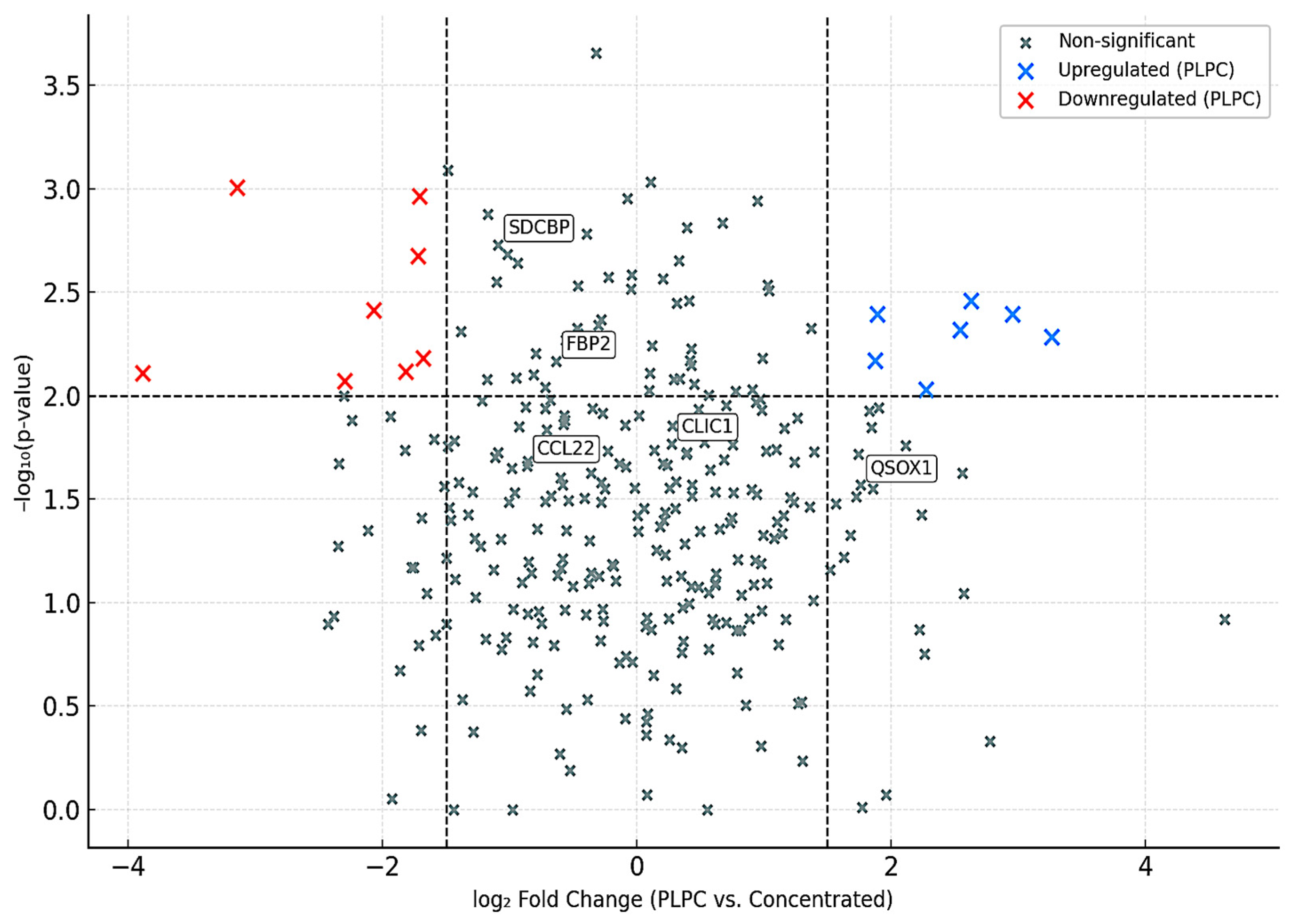
3.1. Proteomic Composition of the PLPC Compared with Other Secretome-Derived Fractions
- •
- QSOX1 (4.1× increase): an enzyme involved in oxidative folding and apoptosis via redox stress;
- •
- CCL22 (2.9× increase): a chemokine involved in dendritic–T cell crosstalk;
- •
- FBP2 (3.8× increase): a regulator of immunometabolic activity;
- •
- CLIC1 (2.4× increase): an apoptosis-associated ion channel;
- •
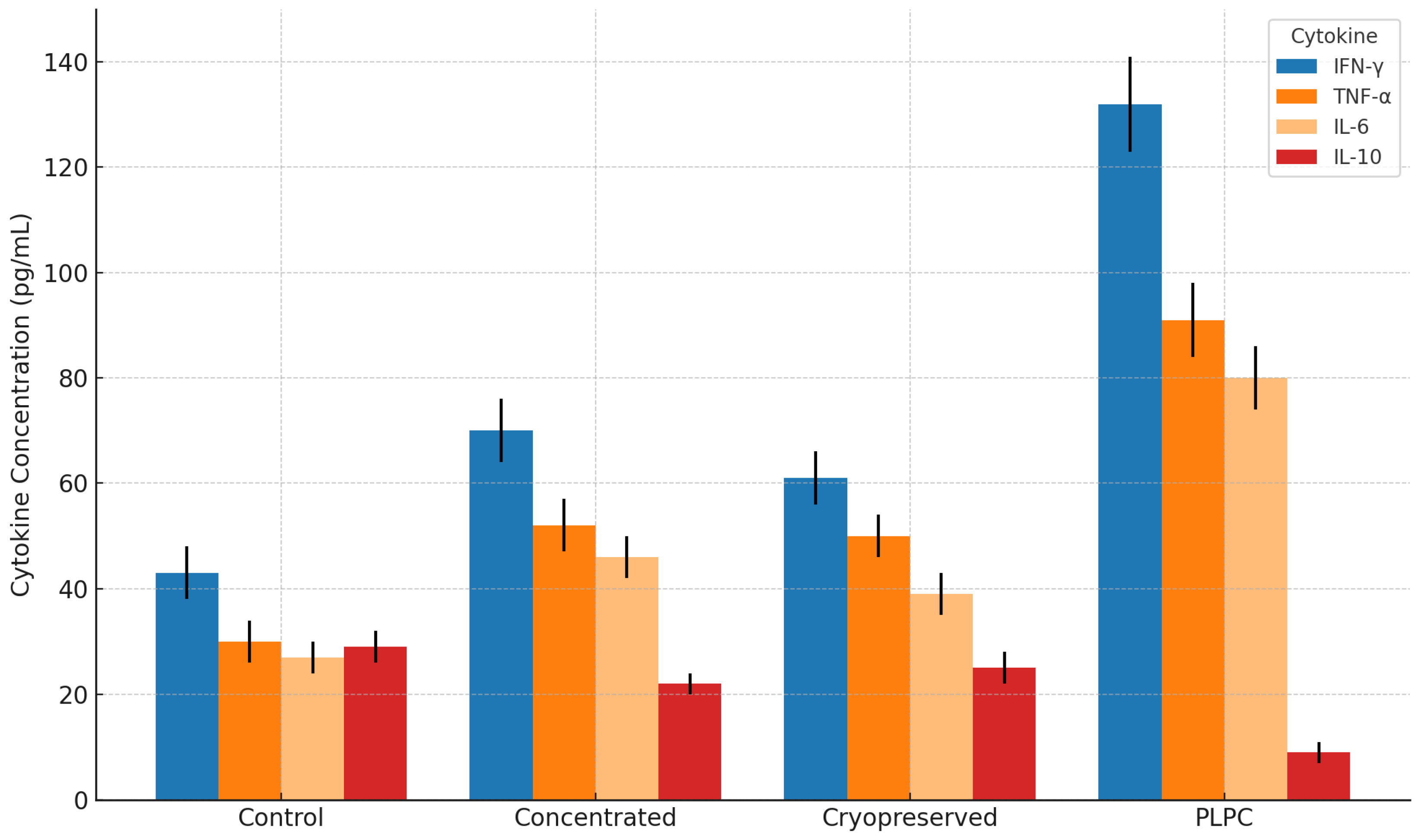
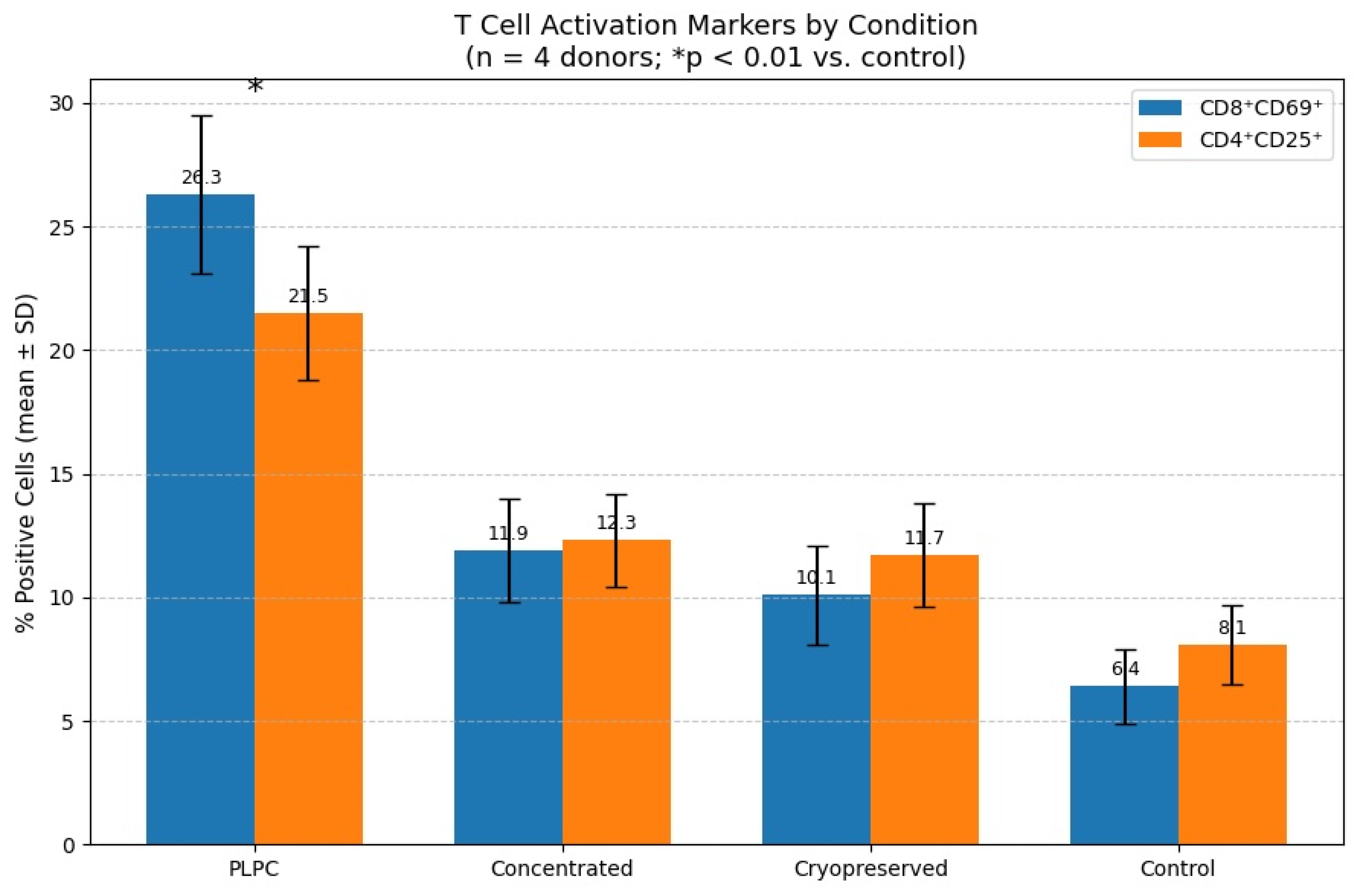
3.2. Functional Immune Profile: Cytokines and T Cell Activation
3.3. Tumor Cell Apoptosis and Non-Tumor Safety
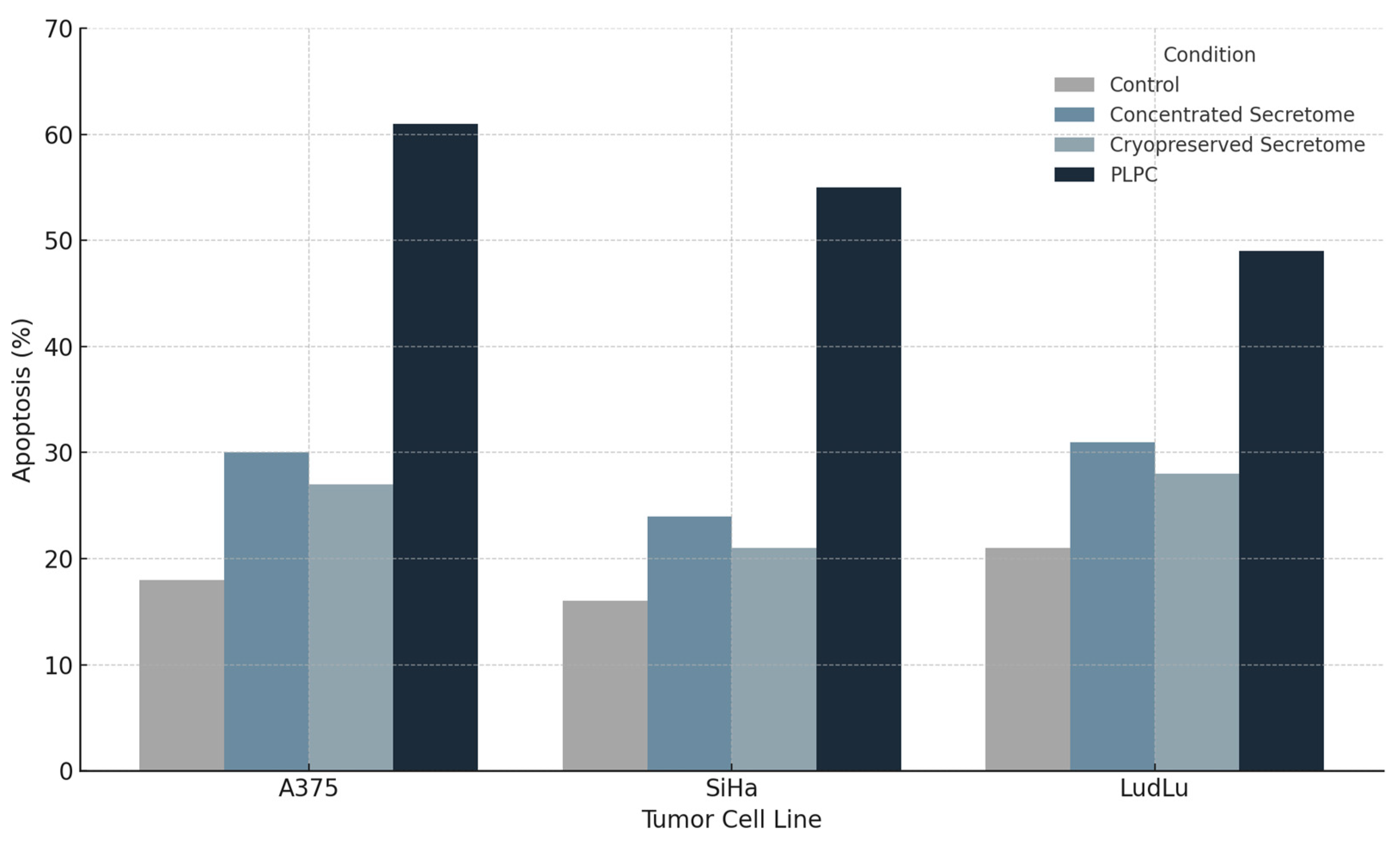
3.3.1. Apoptosis Induction in Tumor Cell Lines
- •
- A375: from 18.2% ± 2.4 (control) to 61.3% ± 3.2 (p < 0.001);
- •
- SiHa: from 15.6% ± 1.9 to 55.4% ± 2.8;
- •
- LudLu: from 21.1% ± 2.7 to 49.1% ± 3.6.
- •
- QSOX1, a redox-active oxidoreductase that promotes disulfide bond formation and ROS-mediated apoptosis;
- •
- CLIC1, an intracellular chloride channel implicated in mitochondrial destabilization;
- •
- Annexin A1, a phospholipid-binding protein involved in apoptotic clearance and immunomodulation [47].
3.3.2. Safety Evaluation in Non-Tumor Human Cells
- •
- HEK293: human embryonic kidney epithelium;
- •
- BEWO: placental trophoblast (syncytiotrophoblast lineage);
- •
- HMC3: microglia-derived human macrophages.
- •
- HEK293: 94.1% ± 1.8;
- •
- BEWO: 92.4% ± 1.9;
- •
- HMC3: 93.5% ± 1.7.
3.3.3. Implications for Clinical Translation
- •
- Pre-conditioning regimens for checkpoint inhibitors;
- •
- Adjuvant platforms for dendritic vaccines;
- •
- Maintenance of immunomodulation post-remission.
3.4. Comparative Functional Performance of the PLPC
3.4.1. Selective Proteomic Enrichment in the PLPC vs. the Concentrated Secretome
- •
- QSOX1 (4.1× increase): a redox enzyme linked to disulfide bond formation and apoptotic priming;
- •
- CCL22 (2.9× increase): a chemokine involved in dendritic–T cell communication;
- •
- FBP2 (3.8× increase): a metabolic enzyme implicated in immune polarization;
- •
- SDCBP (2.1× increase): a scaffold protein associated with vesicle formation and ICAM signaling.
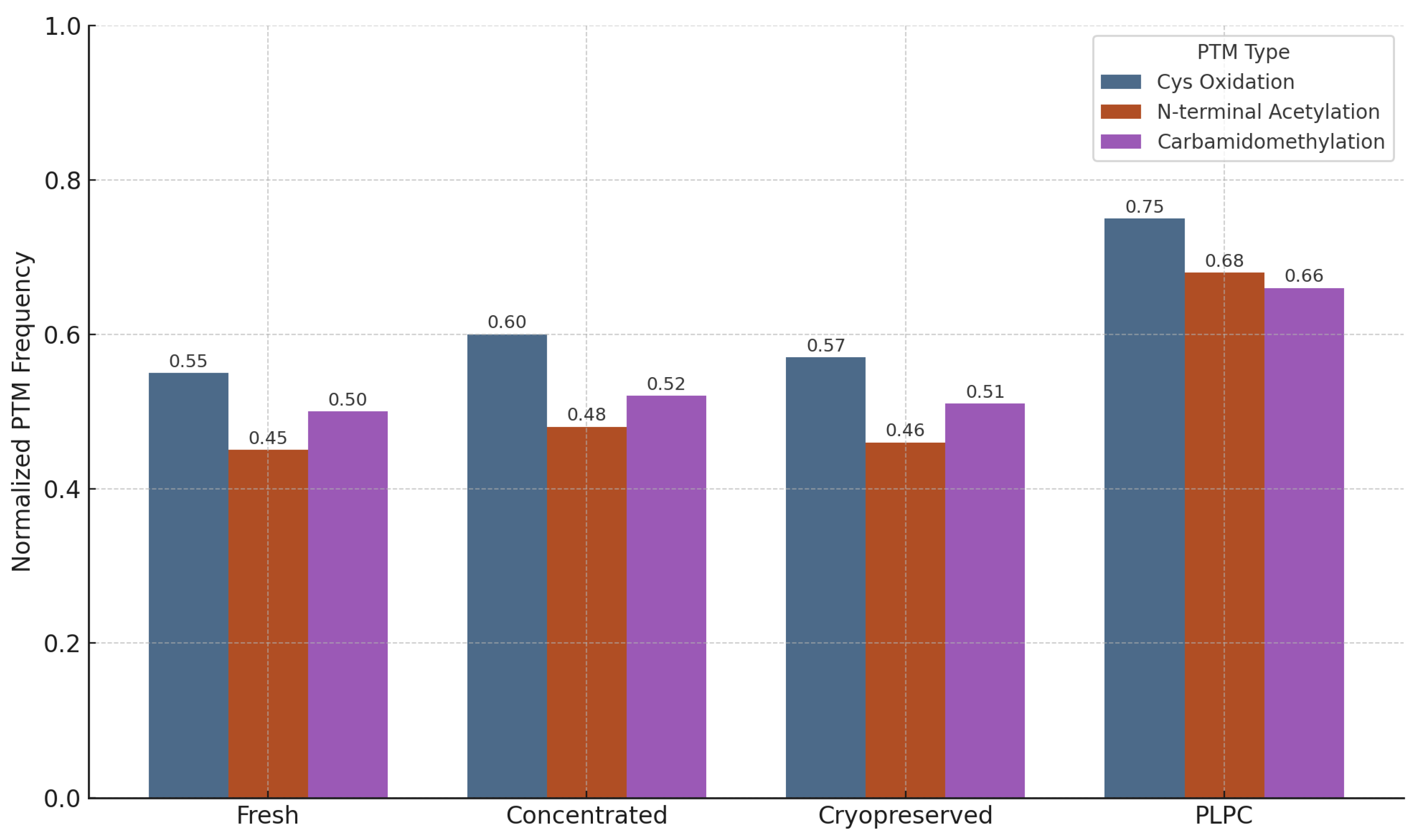
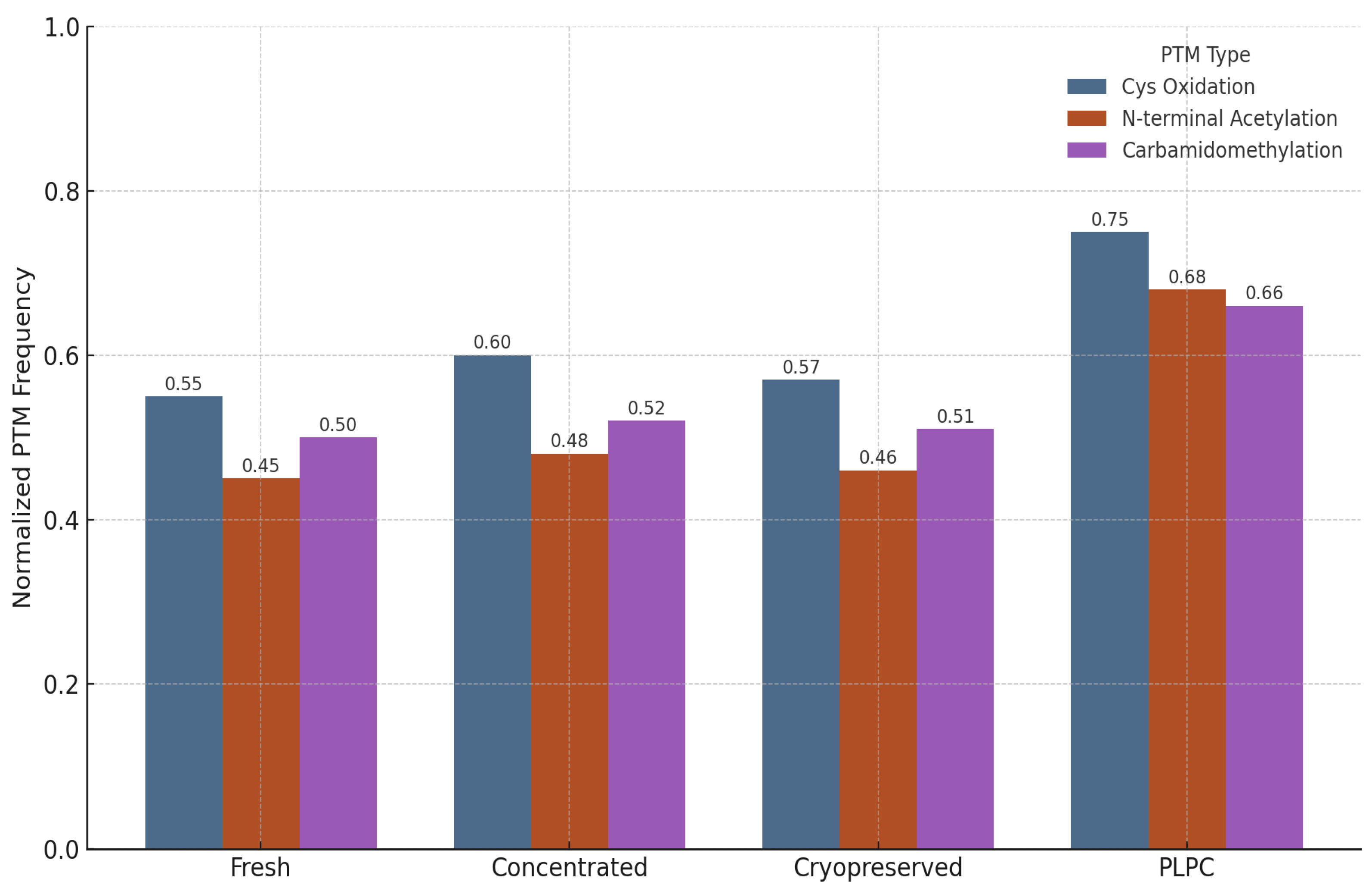
3.4.2. Preservation of Post-Translational Modifications (PTMs)
- Cysteine oxidation and disulfide bond formation—markers of oxidative folding and redox signaling integrity.
- N-terminal acetylation—associated with protein–membrane interactions and stabilization of immune-relevant conformations.
- Carbamidomethylation—proxy for the maintenance of protein backbone integrity during sample processing [57].
- •
- Cysteine oxidation motifs were preserved in 96% of the PLPC spectra versus 61% in the cryopreserved samples (p < 0.01).
- •
- N-terminal acetylation in vesicular membrane proteins was maintained in >85% of the PLPC replicates compared with ~60% in the concentrated secretome.
- •
- Carbamidomethylation stability was highest in the PLPC, indicating minimal degradation or preparation artifacts.
3.4.3. Functional and Regulatory Comparison with Other Platforms
- •
- Dendritic exosomes (DEXs): vesicles with immunogenic cargo but limited by cryodependence and low batch reproducibility;
- •
- Immunoliposomes: lipid vesicles with good physicochemical control but minimal direct immunopotency;
- •
- Chimeric antigen receptor T cells (CAR-T): potent genetically engineered cellular therapies with high manufacturing complexity and individualized protocols [60].
4. Discussion
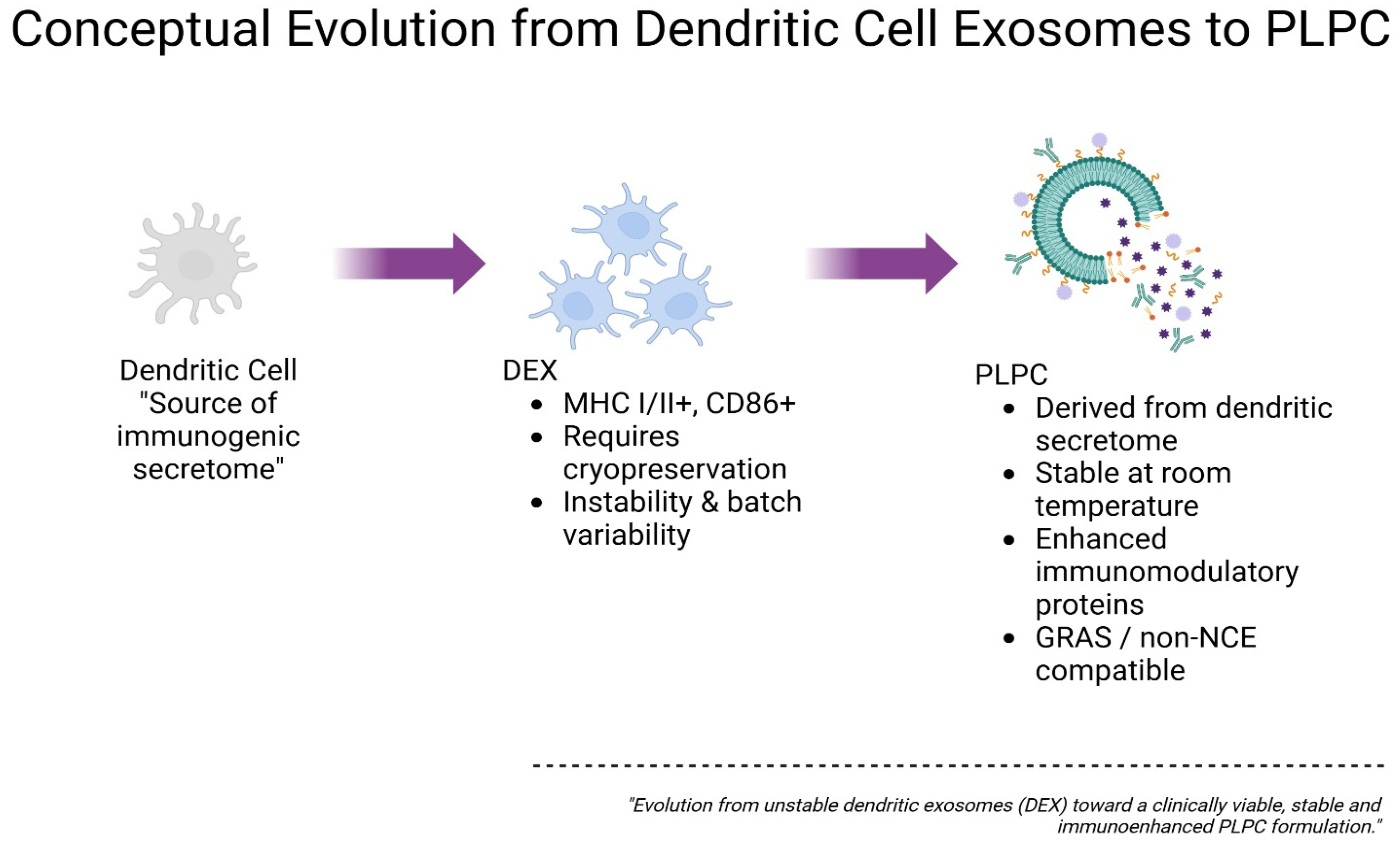
4.1. Biostructural Rationale and Conceptual Evolution of the PLPC Versus Conventional Exosomal Platforms
- •
- Compositional heterogeneity due to variability in dendritic cell maturation states and culture conditions;
- •
- Loss of bioactivity following repeated freeze–thaw cycles and long-term cryostorage;
- •
- Scalability limitations related to autologous processing, donor-to-donor variability, and lack of standardized protocols;
- •
- Low cargo-loading efficiency and vesicle fragmentation during large-scale production attempts;
- •
- Absence of validated GMP-compatible workflows for reproducible DEX batch manufacturing [64].
- •
- Remove vesicle-ambiguous or immunologically inert components;
- •
- Retain and concentrate vesicle-bound proteins with validated immune-activating potential;
- •
- Preserve vesicle structural fidelity without reliance on cryopreservation or exposure to denaturing conditions.
- •
- Superior structural preservation in the PLPC compared with fresh, concentrated, and cryopreserved secretomes;
- •
- Significantly reduced inter-batch variability (CV < 12%), enhancing reproducibility;
- •
- Higher retention rates of oxidized cysteine motifs and N-terminal acetylation sites critical for vesicle-mediated immune interactions.
4.2. Functional Immune Reprogramming and Cytokine Plasticity in Suppressive Environments
4.3. Differential Cytotoxicity and Trans-Tissue Safety Profile in Human Models
4.4. Regulatory Considerations, Routes of Administration, and Clinical Integration Prospects
- •
- Donor dependency and associated biological variability;
- •
- Batch inconsistency with high inter-lot heterogeneity;
- •
- Cryopreservation logistics that limit shelf life and distribution;
- •
- Regulatory ambiguity across EMA, FDA, and hybrid jurisdictional frameworks [71].
- •
- Sublingual films (targeting direct mucosal immune interfaces);
- •
- Intradermal applications (microneedle arrays or fractional injections);
- •
- Endonasal delivery (targeting the respiratory mucosa and neuroimmune axis);
- •
- Topical formulations (for local immunotherapy applications);
- •
- Low-volume injectable formats (for targeted systemic deployment) [73].
- •
- Outpatient care settings;
- •
- Decentralized or resource-limited environments;
- •
- Maintenance-phase immunotherapies;
- •
- Neoadjuvant protocols and combined immunotherapeutic regimens [74].
- •
- Metastatic, checkpoint-resistant tumors;
- •
- Minimal residual disease;
- •
- Maintenance of immunomodulation post-therapy;
- •
- Combined regimens with checkpoint inhibitors, dendritic vaccines, or adoptive cell transfers.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| Abbreviation | Definition |
| DC | Dendritic cell |
| DEX | Dendritic-cell-derived exosome |
| PLPC | Phospholipoproteic complex |
| PBMC | Peripheral blood mononuclear cell |
| FEC-GM | Granulocyte–macrophage colony-stimulating factor |
| TNF-α | Tumor necrosis factor alpha |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IL-1β | Interleukin 1 beta |
| IFN-γ | Interferon gamma |
| CD | Cluster of differentiation |
| CD4+, CD8+ | T lymphocyte subtypes (Helper and Cytotoxic T cells) |
| CD25, CD69 | T cell activation markers |
| HLA-DR | Human leukocyte antigen isotype DR |
| ELISA | Enzyme-linked immunosorbent assay |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay |
| Annexin V/PI | Annexin V and Propidium Iodide (apoptosis assay markers) |
| ROS | Reactive oxygen species |
| PCA | Principal component analysis |
| LFQ | Label-free quantification |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| FDR | False discovery rate |
| PTM | Post-translational modification |
| NTA | Nanoparticle tracking analysis |
| SDCBP | Syndecan-binding protein (syntenin-1) |
| QSOX1 | Quiescin sulfhydryl oxidase 1 |
| CCL22 | C-C motif chemokine ligand 22 |
| FBP2 | Fructose-bisphosphatase 2 |
| CLIC1 | Chloride intracellular channel protein 1 |
| ANXA1 | Annexin A1 |
| HSP70 | Heat-shock protein 70 |
| BEWO | Human placental trophoblast cell line |
| HEK293 | Human embryonic kidney 293 cells |
| HMC3 | Human microglial cell line |
| A375 | Human melanoma cell line |
| SiHa | Human cervical cancer cell line |
| LudLu | Human lung adenocarcinoma cell line |
| CAR-T | Chimeric antigen receptor T cells |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| iRECIST | Immune Response Evaluation Criteria in Solid Tumors |
| GRAS | Generally Recognized As Safe |
| NCE | New Chemical Entity |
References
- Bai, R.; Cui, J. Development of immunotherapy strategies targeting tumor microenvironment is fiercely ongoing. Front. Immunol. 2022, 13, 890166. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, T.; Hou, B.; Huang, X. An iPSC-derived exosome-pulsed dendritic cell vaccine boosts antitumor immunity in melanoma. Mol. Ther. 2023, 31, 2376–2390. [Google Scholar] [CrossRef]
- Tuluwengjiang, G.; Rasulova, I.; Ahmed, S.; Kiasari, B.A.; Sârbu, I.; Ciongradi, C.I.; Omar, T.M.; Hussain, F.; Jawad, M.J.; Castillo-Acobo, R.Y.; et al. Dendritic cell-derived exosomes (Dex): Underlying the role of exosomes derived from diverse DC subtypes in cancer pathogenesis. Pathol. Res. Pract. 2024, 254, 155097. [Google Scholar] [CrossRef]
- Molatefi, R.; Talebi, S.; Samei, A.; Roshanravan, N.; Manshouri, S.; Hashemi, B.; Ghobadi Dana, V.; Mosharkesh, E.; Bahar, M.A.; Khajoei, S.; et al. Clues of HLAs, metabolic SNPs, and epigenetic factors in T cell-mediated drug hypersensitivity reactions. Heliyon 2024, 10, 1–12. [Google Scholar] [PubMed] [PubMed Central]
- Romagnoli, G.G.; Zelante, B.B.; Toniolo, P.A.; Migliori, I.K.; Barbuto, J.A.M. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front. Immunol. 2015, 5, 692. [Google Scholar] [CrossRef] [PubMed]
- Achouri, I.E.; Rhoden, A.; Hudon, S.; Gosselin, R.; Simard, J.-S.; Abatzoglou, N. Non-invasive detection technologies of solid foreign matter and their applications to lyophilized pharmaceutical products: A review. Talanta 2021, 224, 121885. [Google Scholar] [CrossRef] [PubMed]
- Risha, Y.; Minic, Z.; Ghobadloo, S.M.; Berezovski, M.V. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci. Rep. 2020, 10, 13572. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Morisaki, T.; Kubo, M.; Onishi, H.; Hirano, T.; Morisaki, S.; Eto, M.; Monji, K.; Takeuchi, A.; Nakagawa, S.; Tanaka, H.; et al. Efficacy of intranodal neoantigen peptide-pulsed dendritic cell vaccine monotherapy in patients with advanced solid tumors: A retrospective analysis. Anticancer Res. 2021, 41, 4101–4115. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Peng, Y.; Du, Y.; Yang, Z.; Qi, X. Dendritic cell-derived exosomes loaded neoantigens for personalized cancer immunotherapies. J Control. Release 2023, 353, 423–433. [Google Scholar] [CrossRef]
- Kanlikilicer, P. Exosome-related methods and potential use as vaccines. Methods Mol. Biol. 2022, 2435, 35–41. [Google Scholar]
- Zhang, Y.; Zuo, B.; Yu, Z.; Zhao, K.; Zhang, Y.; He, K.; Seow, Y.; Yin, H. Complete remission of tumors in mice with neoantigen-painted exosomes and anti-PD-1 therapy. Mol. Ther. 2023, 31, 3579–3593. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Betzer, O.; Elmaliach-Pnini, N.; Motiei, M.; Sadan, T.; Cohen-Berkman, M.; Dagan, O.; Popovtzer, A.; Yosepovich, A.; Barhom, H.; et al. ‘Golden’ exosomes as delivery vehicles to target tumors and overcome intratumoral barriers: In vivo tracking in a model for head and neck cancer. Biomater. Sci. 2021, 9, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Liu, H.; Yan, H.; Che, R.; Jin, Y.; Yang, X.; Zhou, X.; Yang, H.; Ge, K.; Liang, X.-J.; et al. A CAR T-inspiring platform based on antibody-engineered exosomes from antigen-feeding dendritic cells for precise solid tumor therapy. Biomaterials 2022, 282, 121424. [Google Scholar] [CrossRef]
- Ai, K.; Liu, B.; Chen, X.; Huang, C.; Yang, L.; Zhang, W.; Weng, J.; Du, X.; Wu, K.; Lai, P. Optimizing CAR-T cell therapy for solid tumors: Current challenges and potential strategies. J. Hematol. Oncol. 2024, 17, 105. [Google Scholar] [CrossRef]
- Wang, J.S.; Schellenberg, S.J.; Demeros, A.; Lin, A.Y. Exosomes in review: A new frontier in CAR-T cell therapies. Neoplasia 2025, 62, 101147. [Google Scholar] [CrossRef]
- Chen, P.; Yang, W.; Nagaoka, K.; Huang, G.L.; Miyazaki, T.; Hong, T.; Li, S.; Igarashi, K.; Takeda, K.; Kakimi, K.; et al. An IL-12-based nanocytokine safely potentiates anticancer immunity through spatiotemporal control of inflammation to eradicate advanced cold tumors. Adv. Sci. 2023, 10, 2205139. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Ma, J.; Yu, Y.; Tao, Y.; Song, Z.; Li, J. Exosomes as carriers to stimulate an anti-cancer immune response in immunotherapy and as predictive markers. Biochem. Pharmacol. 2024, 232, 116699. [Google Scholar] [CrossRef]
- Jung, I.; Shin, S.; Baek, M.-C.; Yea, K. Modification of immune cell-derived exosomes for enhanced cancer immunotherapy: Current advances and therapeutic applications. Exp. Mol. Med. 2024, 56, 19–31. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Zitvogel, L.; Kroemer, G. The secret ally: Immunostimulation by anticancer drugs. Nat. Rev. Drug Discov. 2012, 11, 215–233. [Google Scholar] [CrossRef]
- Saw, P.E.; Liu, Q.; Wong, P.P.; Song, E. Cancer stem cell mimicry for immune evasion and therapeutic resistance. Cell Stem Cell 2024, 31, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Yu, S.; Yin, C.; Zhu, B.; Chen, Z.; Meng, Z.; Wang, P. Plasma IFN-γ-inducible chemokines CXCL9 and CXCL10 correlate with survival and chemotherapeutic efficacy in advanced pancreatic ductal adenocarcinoma. Pancreatology 2019, 19, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zheng, J.; Chen, Z.; Hu, D.; Li, S.; Zhuang, W.; He, Z.; Lin, G.; Wu, B.; Zhang, W.; et al. Clinical significance of tumor-infiltrating lymphocytes investigated using routine H&E slides in small cell lung cancer. Radiat. Oncol. 2022, 17, 127. [Google Scholar] [CrossRef]
- Lazzari, C.; Spagnolo, C.C.; Ciappina, G.; Di Pietro, M.; Squeri, A.; Passalacqua, M.I.; Marchesi, S.; Gregorc, V.; Santarpia, M. Immunotherapy in Early-Stage Non-Small Cell Lung Cancer (NSCLC): Current Evidence and Perspectives. Curr. Oncol. 2023, 30, 3684–3696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Han, W.; Dong, H.; Liu, X.; Su, X. The rising roles of exosomes in the tumor microenvironment reprogramming and cancer immunotherapy. MedComm 2024, 5, e541. [Google Scholar] [CrossRef]
- Pi, Y.-N.; Xia, B.-R.; Jin, M.-Z.; Jin, W.-L.; Lou, G. Exosomes: Powerful weapon for cancer nano-immunoengineering. Biochem. Pharmacol. 2021, 186, 114487. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Msallam, R. Tissues and tumor microenvironment (TME) in 3D: Models to shed light on immunosuppression in cancer. Cells 2021, 10, 831. [Google Scholar] [CrossRef]
- Ros, J.; Balconi, F.; Baraibar, I.; Saoudi Gonzalez, N.; Salva, F.; Tabernero, J.; Elez, E. Advances in immune checkpoint inhibitor combination strategies for microsatellite stable colorectal cancer. Front. Oncol. 2023, 13, 1112276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sosnowska, A.; Chlebowska-Tuz, J.; Matryba, P.; Pilch, Z.; Greig, A.; Wolny, A.; Grzywa, T.M.; Rydzynska, Z.; Sokolowska, O.; Rygiel, T.P.; et al. Inhibition of arginase modulates T-cell response in the tumor microenvironment of lung carcinoma. OncoImmunology 2021, 10, 1956143. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xu, L.; Chen, Y.; Rong, Y.; Zou, Y.; Ge, S.; Wu, T.; Lai, Y.; Xu, Q.; Guo, W.; et al. IDO1 inhibition promotes activation of tumor-intrinsic STAT3 pathway and induces adverse tumor-protective effects. J. Immunol. 2024, 212, 1232–1243. [Google Scholar] [CrossRef]
- Zaiatz-Bittencourt, V.; Finlay, D.K.; Gardiner, C.M. Canonical TGF-β signaling pathway represses human NK cell metabolism. J. Immunol. 2019, 200, 3934–3941. [Google Scholar] [CrossRef]
- Delgoffe, G. Cancer Metabolism and the Immune System; American Association for Cancer Research: Philadelphia, PA, USA, 2021. [Google Scholar]
- Zemanek, T.; Danisovic, L.; Nicodemou, A. Exosomes, their sources, and possible uses in cancer therapy in the era of personalized medicine. J. Cancer Res. Clin. Oncol. 2024, 151, 16. [Google Scholar] [CrossRef] [PubMed]
- Dai, E.; Zhu, Z.; Wahed, S.; Qu, Z.; Storkus, W.J.; Guo, Z.S. Epigenetic modulation of antitumor immunity for improved cancer immunotherapy. Mol. Cancer 2021, 20, 171. [Google Scholar] [CrossRef]
- Nakamura, K.; Yagyu, S.; Hirota, S.; Tomida, A.; Kondo, M.; Shigeura, T.; Hasegawa, A.; Tanaka, M.; Nakazawa, Y. Autologous antigen-presenting cells efficiently expand piggyBac transposon CAR-T cells with predominant memory phenotype. Mol. Ther. Methods Clin. Dev. 2021, 21, 315–324. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Yu, Q.; Chen, Y.; Sun, Y.; Zhu, Q.; Yang, J.; Jiang, R. PLAC8 is an innovative biomarker for immunotherapy participating in remodeling the immune microenvironment of renal clear cell carcinoma. Front. Oncol. 2023, 13, 1207551. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W.; et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015, 527, 249–253. [Google Scholar] [CrossRef]
- Beumer-Chuwonpad, A.; Taggenbrock, R.L.R.E.; Ngo, T.A.; van Gisbergen, K.P.J.M. The potential of tissue-resident memory T cells for adoptive immunotherapy against cancer. Cells 2021, 10, 2234. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.; Pathak, S.; Palani, V.; Acar, A.; Banerjee, A.; Al-Dewik, N.I.; Essa, M.M.; Mohammed, S.G.A.A.; Qoronfleh, M.W. Current Technologies and Future Perspectives in Immunotherapy towards a Clinical Oncology Approach. Biomedicines 2024, 12, 217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khojandi, N.; Connelly, L.; Piening, A.; Hoft, S.G.; Pherson, M.; Donlin, M.J.; DiPaolo, R.J.; Teague, R.M. Single-cell analysis of peripheral CD8+ T cell responses in patients receiving checkpoint blockade immunotherapy for cancer. Cancer Immunol. Immunother. 2023, 72, 397–408. [Google Scholar] [CrossRef]
- Elwood, P.C.; Morgan, G.; Delon, C.; Protty, M.; Galante, J.; Pickering, J.; Watkins, J.; Weightman, A.; Morris, D. Aspirin and cancer survival: A systematic review and meta-analyses of 118 observational studies of aspirin and 18 cancers. Ecancermedicalscience 2021, 15, 1258. [Google Scholar] [CrossRef]
- Bouafia, A.; Lofek, S.; Bruneau, J.; Chentout, L.; Lamrini, H.; Trinquand, A.; Deau, M.-C.; Heurtier, L.; Meignin, V.; Picard, C.; et al. Loss of ARHGEF1 causes a human primary antibody deficiency. J. Clin. Investig. 2019, 129, 1047–1060. [Google Scholar] [CrossRef]
- Sacco, A.; Bruno, A.; Contursi, A.; Dovizio, M.; Tacconelli, S.; Ricciotti, E.; Guillem-Llobat, P.; Salvatore, T.; Di Francesco, L.; Fullone, R.; et al. Platelet-specific deletion of cyclooxygenase-1 ameliorates dextran sulfate sodium–induced colitis in mice. J. Pharmacol. Exp. Ther. 2019, 370, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Contursi, A.; Tacconelli, S.; Sacco, A.; Hofling, U.; Mucci, M.; Lamolinara, A.; Del Pizzo, F.; Ballerini, P.; Di Gregorio, P.; et al. The specific deletion of cyclooxygenase-1 in megakaryocytes/platelets reduces intestinal polyposis in ApcMin/+ mice. Pharmacol. Res. 2022, 185, 106506. [Google Scholar] [CrossRef]
- McNeil, J.J.; Gibbs, P.; Orchard, S.G.; Lockery, J.E.; Bernstein, W.B.; Cao, Y.; Ford, L.; Haydon, A.; Kirpach, B.; Macrae, F.; et al. Effect of aspirin on cancer incidence and mortality in older adults. J. Natl. Cancer Inst. 2021, 113, 258–265. [Google Scholar] [CrossRef]
- Gebhardt, T.; Park, S.L.; Parish, I.A. Stem-like exhausted and memory CD8+ T cells in cancer. Nat. Rev. Cancer 2023, 23, 780–798. [Google Scholar] [CrossRef]
- Brummelman, J.; Haftmann, C.; Núñez, N.G.; Alvisi, G.; Mazza, E.M.C.; Becher, B.; Lugli, E. Development, application and computational analysis of high-dimensional fluorescent antibody panels for single-cell flow cytometry. Nat. Protoc. 2019, 14, 1946–1969. [Google Scholar] [CrossRef]
- Alvisi, G.; Brummelman, J.; Puccio, S.; Mazza, E.M.C.; Tomada, E.P.; Losurdo, A.; Zanon, V.; Peano, C.; Colombo, F.S.; Scarpa, A.; et al. IRF4 instructs effector Treg differentiation and immune suppression in human cancer. J. Clin. Investig. 2020, 130, 3137–3150. [Google Scholar] [CrossRef] [PubMed]
- Demma, M.J.; Mapelli, C.; Sun, A.; Bodea, S.; Ruprecht, B.; Javaid, S.; Wiswell, D.; Muise, E.; Chen, S.; Zelina, J.; et al. Omomyc reveals new mechanisms to inhibit the myc oncogene. Mol. Cell Biol. 2019, 39, e00248-19. [Google Scholar] [CrossRef]
- Beaulieu, M.-E.; Jauset, T.; Massó-Vallés, D.; Martínez-Martín, S.; Rahl, P.; Maltais, L.; Zacarias-Fluck, M.F.; Casacuberta-Serra, S.; del Pozo, E.S.; Fiore, C.; et al. Intrinsic cell-penetrating activity propels Omomyc from proof of concept to viable anti-MYC therapy. Sci. Transl. Med. 2019, 11, eaar5012. [Google Scholar] [CrossRef] [PubMed]
- Struntz, N.B.; Chen, A.; Deutzmann, A.; Wilson, R.M.; Stefan, E.; Evans, H.L.; Ramirez, M.A.; Liang, T.; Caballero, F.; Wildschut, M.H.; et al. Stabilization of the Max homodimer with a small molecule attenuates Myc-driven transcription. Cell Chem. Biol. 2019, 26, 711.e14–723.e14. [Google Scholar] [CrossRef]
- Massó-Vallés, D.; Soucek, L. Blocking Myc to treat cancer: Reflecting on two decades of Omomyc. Cells 2020, 9, 883. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Lu, M.; Zhang, S.; Zheng, L. c-Myc maintains the self-renewal and chemoresistance properties of colon cancer stem cells. Oncol. Lett. 2019, 17, 4487–4493. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cai, B.; Ding, M.; Su, Z.; Liu, Y.; Shen, L. c-Myc overexpression promotes oral cancer cell proliferation and migration by enhancing glutaminase and glutamine synthetase activity. Am. J. Med. Sci. 2019, 358, 235–242. [Google Scholar] [CrossRef]
- Alimova, I.; Pierce, A.; Danis, E.; Donson, A.; Birks, D.K.; Griesinger, A.; Foreman, N.K.; Santi, M.; Soucek, L.; Venkataraman, S.; et al. Inhibition of MYC attenuates tumor cell self-renewal and promotes senescence in SMARCB1-deficient Group 2 atypical teratoid rhabdoid tumors to suppress tumor growth in vivo. Int. J. Cancer 2019, 144, 1983–1995. [Google Scholar] [CrossRef]
- Gutierrez-Sandoval, R.; Rivadeneira, I.; Muñoz, P.I.; Iturra, F.J.; Krakowiak, F. DEX immunotherapy pharmaco-biotechnological advances in multimodal therapy for cancer. J. Pharm. Res. Rep. 2024, 5, 1–8. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- McAndrews, K.M.; Kalluri, R. Mechanisms associated with biogenesis of exosomes in cancer. Mol. Cancer 2019, 18, 52. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of exosome composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Pathan, M.; Fonseka, P.; Chitti, S.V.; Kang, T.; Sanwlani, R.; Van Deun, J.; Hendrix, A.; Mathivanan, S. Vesiclepedia 2019: A compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019, 47, D516–D519. [Google Scholar] [CrossRef]
- Wen, S.W.; Lima, L.G.; Lobb, R.J.; Norris, E.L.; Hastie, M.L.; Krumeich, S.; Möller, A. Breast cancer-derived exosomes reflect the cell-of-origin phenotype. Proteomics 2019, 19, e1800180. [Google Scholar] [CrossRef]
- Cheng, Y.; Schorey, J.S. Extracellular vesicles deliver Mycobacterium RNA to promote host immunity and bacterial killing. EMBO Rep. 2019, 20, e46613. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, R.G. Recent advances in the translational application of immunotherapy with pulsed dendritic cell-derived exosomes (DEX). J. Clin. Biomed. Res. 2024, 6, 1–8. [Google Scholar] [CrossRef]
- Wang, M.; Su, Z.; Barnie, P.A. Crosstalk among colon cancer-derived exosomes, fibroblast-derived exosomes, and macrophage phenotypes in colon cancer metastasis. Int. Immunopharmacol. 2020, 81, 106298. [Google Scholar] [CrossRef] [PubMed]
- Daßler-Plenker, J.; Kuttner, V.; Egeblad, M. Communication in tiny packages: Exosomes as means of tumor-stroma communication. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188340. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Yousefi, F.; Shabaninejad, Z.; Movahedpour, A.; Tehran, M.M.; Shafiee, A.; Moradizarmehri, S.; Hajighadimi, S.; Savardashtaki, A.; Mirzaei, H. Exosomes and cancer: From oncogenic roles to therapeutic applications. IUBMB Life 2020, 72, 724–748. [Google Scholar] [CrossRef]
- Pourhanifeh, M.H.; Mahjoubin-Tehran, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; Mirzaei, H.; Asemi, Z. MicroRNAs and exosomes: Small molecules with big actions in multiple myeloma pathogenesis. IUBMB Life 2020, 72, 314–333. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Naseri, Z.; Oskuee, R.K.; Forouzandeh-Moghadam, M.; Jaafari, M.R. Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem Cell Rev. Rep. 2020, 16, 541–556. [Google Scholar] [CrossRef]
- Huang, L.; Yang, L.; Ding, Y.; Jiang, X.; Xia, Z.; You, Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle 2020, 19, 339–353. [Google Scholar] [CrossRef]
- Che, J.; Wang, H.; Dong, J.; Wu, Y.; Zang, H.; Fu, L.; Zhang, J. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate neuroinflammation and oxidative stress through the NRF2/NF-κB/NLRP3 pathway. CNS Neurosci. Ther. 2024, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Q.; Liu, Y.; Ding, X.; Li, Q.; Qiu, F.; Wang, M.; Shen, Z.; Zheng, H.; Fu, G. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol. Cell Biochem. 2020, 465, 103–114. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jankovic, M.G.; Fellabaum, C.; Volarevic, A.; Djonov, V.; Arsenijevic, A.; Volarevic, V. Molecular mechanisms responsible for anti-inflammatory and immunosuppressive effects of mesenchymal stem cell-derived factors. Adv. Exp. Med. Biol. 2019, 1084, 187–206. [Google Scholar]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, H.; Hu, S.; Xu, F.; Liang, X. Exosomal miRNAs during chondrogenic differentiation. J. Cell Biochem. 2019, 120, 171–181. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates T cell responses in glioblastoma. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, X. Nanomaterials for cancer vaccine delivery. Cancer Biol. Med. 2021, 18, 352–371. [Google Scholar] [CrossRef]
- Titov, A.; Zmievskaya, E.; Ganeeva, I.; Valiullina, A.; Petukhov, A.; Rakhmatullina, A.; Miftakhova, R.; Fainshtein, M.; Rizvanov, A.; Bulatov, E. Adoptive immunotherapy beyond CAR T-cells. Cancers 2021, 13, 743. [Google Scholar] [CrossRef]
- Shemesh, C.S.; Hsu, J.C.; Hosseini, I.; Shen, B.Q.; Rotte, A.; Twomey, P.; Girish, S.; Wu, B. Personalized cancer vaccines: Clinical landscape and challenges. Mol. Ther. 2021, 29, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, M.; Chen, S.; Song, M.; Yue, Y. Biomimetic nanoparticles for tumor immunotherapy. Front. Bioeng. Biotechnol. 2022, 10, 989881. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.D.; Cabral, H.; Stylianopoulos, T.; Jain, R.K. Improving cancer immunotherapy with nanomedicines. Nat. Rev. Clin. Oncol. 2020, 17, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Aikins, M.E.; Xu, C.; Moon, J.J. Engineered nanoparticles for immunotherapy. Acc. Chem. Res. 2020, 53, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Li, Y.-M. Chemical strategies to boost cancer vaccines. Chem. Rev. 2020, 120, 11420–11478. [Google Scholar] [CrossRef]
- Roy, S.; Sethi, T.K.; Taylor, D.; Kim, Y.J.; Johnson, D.B. Breakthroughs in immune-oncology: Cancer vaccines. J. Leukoc. Biol. 2020, 108, 1455–1489. [Google Scholar] [CrossRef]


| Protein | Function | Condition Specificity | Fold Increase (vs. Cond. 2) | Immunological Relevance |
|---|---|---|---|---|
| QSOX1 | Redox regulation | PLPC only | 4.1× | Apoptosis, ROS-mediated stress |
| CCL22 | Chemokine | PLPC and Conc. | 2.9× | Immune attraction, Treg tuning |
| CLIC1 | Ion channel | Shared | 2.4× | Apoptosis, pH homeostasis |
| FBP2 | Glycolysis regulator | PLPC only | 3.8× | Metabolic–immune crosstalk |
| SDCBP | Vesicle scaffold | PLPC and Cryo | 2.1× | Vesicle formation, ICAM signaling |
| Cell Line | Untreated (%) | Concentrated Secretome (%) | Cryopreserved Secretome (%) | PLPC (%) |
|---|---|---|---|---|
| A375 | 18.2 | 29.8 | 25.7 | 61.3 |
| SiHa | 15.6 | 23.4 | 21.2 | 55.4 |
| LudLu | 21.1 | 30.6 | 26.9 | 49.1 |
| Cell Line | Viability (%) | Morphological Change | ROS Elevation | Annexin V⁺/PI⁺ (%) |
|---|---|---|---|---|
| HEK293 | 94.1 | None | No | <3% |
| BEWO | 92.4 | None | No | <2.5% |
| HMC3 | 93.5 | None | No | <2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Sandoval, R.; Gutiérrez-Castro, F.; Muñoz-Godoy, N.; Rivadeneira, I.; Sobarzo, A.; Iturra, J.; Krakowiak, F.; Alarcón, L.; Dorado, W.; Lagos, A.; et al. Beyond Exosomes: An Ultrapurified Phospholipoproteic Complex (PLPC) as a Scalable Immunomodulatory Platform for Reprogramming Immune Suppression in Metastatic Cancer. Cancers 2025, 17, 1658. https://doi.org/10.3390/cancers17101658
Gutierrez-Sandoval R, Gutiérrez-Castro F, Muñoz-Godoy N, Rivadeneira I, Sobarzo A, Iturra J, Krakowiak F, Alarcón L, Dorado W, Lagos A, et al. Beyond Exosomes: An Ultrapurified Phospholipoproteic Complex (PLPC) as a Scalable Immunomodulatory Platform for Reprogramming Immune Suppression in Metastatic Cancer. Cancers. 2025; 17(10):1658. https://doi.org/10.3390/cancers17101658
Chicago/Turabian StyleGutierrez-Sandoval, Ramon, Francisco Gutiérrez-Castro, Natalia Muñoz-Godoy, Ider Rivadeneira, Adolay Sobarzo, Jordan Iturra, Francisco Krakowiak, Luis Alarcón, Wilson Dorado, Andy Lagos, and et al. 2025. "Beyond Exosomes: An Ultrapurified Phospholipoproteic Complex (PLPC) as a Scalable Immunomodulatory Platform for Reprogramming Immune Suppression in Metastatic Cancer" Cancers 17, no. 10: 1658. https://doi.org/10.3390/cancers17101658
APA StyleGutierrez-Sandoval, R., Gutiérrez-Castro, F., Muñoz-Godoy, N., Rivadeneira, I., Sobarzo, A., Iturra, J., Krakowiak, F., Alarcón, L., Dorado, W., Lagos, A., Montenegro, D., Muñoz, I., Aguilera, R., & Toledo, A. (2025). Beyond Exosomes: An Ultrapurified Phospholipoproteic Complex (PLPC) as a Scalable Immunomodulatory Platform for Reprogramming Immune Suppression in Metastatic Cancer. Cancers, 17(10), 1658. https://doi.org/10.3390/cancers17101658







