Simple Summary
We know little about how common bone metastases (cancer spreading to bones from its original site) are in biliary tract cancers (BTC), or if they are more common in some BTC types compared to others. To learn more, we reviewed the medical notes of 197 patients with BTC treated in a large UK cancer centre. We studied details of patients’ BTCs, if they had bone metastases, and how they responded to treatment. In patients with incurable BTC, we found no difference in survival for patients with bone metastases compared to those without. Patients with special cancer-related genetic alterations (called ‘actionable alterations’) were just as likely to have bone metastases than those without, and with similar survival rates. When targeting these alterations with matched anti-cancer treatments, over 50% of patients had a survival of over 29.9 months since their cancer diagnosis, which is longer than that reported in many other studies.
Abstract
Background: Bone metastasis (BM) prevalence is underreported in biliary tract cancers (BTC). This study aimed to assess BM prevalence in a real-world BTC population, alongside examining its relationship to prognosis and genomic alterations. Methods: Patients with histology-proven BTC as reviewed at a university cancer centre between January 2019 and August 2022 were assessed. Data extracted from records included BTC subtype, molecular profiling and systemic anti-cancer therapy (SACT) use. Stratification by BTC subtype and metastasis sites occurred. Median overall survival (mOS) was defined as time from relapse or metastases to death. Survival analysis was conducted using the Cox Proportional Hazard model. Results: Of 197 patients, 74 (37.6%) had intrahepatic and 67 (34%) had extrahepatic cholangiocarcinoma. Thirty-four patients had BM (17.3%), with 14 identified at initial diagnosis. OS was not influenced by bone (HR 1.15; p = 0.48) or liver metastases (HR 1.09; p = 0.6). Stratifying for age and gender, no significant difference in OS was observed. Actionable alterations were equally likely in patients with (52.4%) and without BM (58.5%). Age of BTC onset (<65 or ≥65) did not significantly influence prevalence of actionable alterations. Patients receiving matched, targeted SACT had a mOS of 29.9 months, compared to 13.3 months in those with actionable alterations but no SACT matching (HR 0.35; p < 0.005). Conclusions: In advanced BTC, BM do not affect OS. Across all cohorts, actionable alterations improved OS when treated with matched SACT.
1. Introduction
Biliary tract cancers (BTC) represent just 1% of all cancers [1,2,3]. Although the term is often used interchangeably with cholangiocarcinoma, BTC also include gallbladder cancers (GBC) and ampullary cancers with pancreatobiliary differentiation [3,4]. Cholangiocarcinoma is subcategorised into intrahepatic (iCCA) and extrahepatic cholangiocarcinoma (eCCA), with eCCA further divided into peri-hilar and distal [2,3,4].
Frequently diagnosed at advanced or inoperable stages, BTC patients have poor prognoses and limited responses to systemic anti-cancer therapies (SACT) [2,3]. Even with curative treatments, over 50% of patients relapse [4]. Across all stages, the five-year overall survival (OS) remains below 20% [4]. Considering the limited efficacy observed with licensed SACT, studies assessing the efficacy of alternative, herbal and plant-based agents are also increasing in number, including randomised trials with large sample sizes [5,6].
While metastases to lymph nodes, lungs and liver are well-documented, particularly in cholangiocarcinoma, the prevalence of bone metastases (BM) remains poorly understood [7,8]. The available literature is often limited to studies on iCCA or data from the Surveillance, Epidemiology, and End Results (SEER) programme, which may lack granularity [7,8,9,10,11,12,13]. Additionally, landmark BTC clinical trials did not report metastatic disease sites in participants with advanced disease [14,15,16]. Little is known about the impact BM have on survival outcomes, or their relationship with actionable molecular aberrations [7,9,10,12,13].
Using a real-world BTC cohort, this study aimed to estimate the true prevalence of BM, evaluate survival outcomes with standard-of-care treatments, and explore the relationship between BM, prognosis and genomic alterations.
2. Materials and Methods
This study aimed to characterise the prevalence of BM in a real-world BTC population and compare survival outcomes with those reported in both randomised controlled trials (RCT) and observational cohorts. Additionally, we examined whether BM presence influenced OS and the frequency of actionable genomic alterations.
All patients with histologically confirmed BTC assessed or treated at University College London Hospital NHS Foundation Trust between January 2019 and August 2022 were included. BTC diagnoses were categorised as follows: iCCA, eCCA, cholangiocarcinoma not otherwise specified (NOS), ampullary cancer, and GBC. Data were collected from electronic health records and chemotherapy databases.
Data extracted included: demographics, BTC subtype, TNM staging, sites of metastases (at diagnosis and during disease course), operability status, molecular profiling, SACT use, clinical trial participation, presence of BM-related and non-BM-related hypercalcaemia, presence of skeletal-related events in those with BM, diagnosis date, relapse and survival timepoints. Metastasis sites were confirmed using cross-sectional imaging, including CT, MRI and/or FDG-PET modalities. Molecular profiling was defined as next-generation sequencing on serum or tissue samples, conducted locally or accessed via commercial or trial-based services.
‘Actionable’ molecular alterations were defined as those with matched targeted treatments with evidence of efficacy in peer-reviewed literature, accessible through the English National Health Service (NHS), RCT or pharmaceutical compassionate access programs. This study considered the following alterations as actionable: BRCA alterations, FGFR fusions or mutations, KRAS G12C mutation, HER2 overexpression or mutation, IDH1 mutation and BRAF V600E mutations. Clinical trial involvement data were extracted from trial records. Data was stored following University College London Hospital NHS Foundation Trust and NHS England data protection rules.
Following data anonymisation, analyses were conducted using Microsoft Excel (version 16.96.1), R (version 4.2.3), and RStudio (version 2022.12.0.353). Continuous variables were summarised using means and standard deviations. Categorical variables were summarised using medians and interquartile ranges. Outcomes were stratified by multiple variables, including age, gender, BTC subtype, metastasis sites, presence of actionable alterations, SACT received and clinical trial involvement. Curative treatment was defined as successful surgical resection, with or without adjuvant SACT.
Survival analyses were conducted for the incurable population. For inoperable and de novo metastatic patients, OS was defined as time from primary diagnosis to death of any cause. For the relapsed population, OS was defined as time from relapse to death of any cause. Survival analysis was performed using the Cox Proportional Hazard model, taking censorship into account. Survival data were displayed using Kaplan–Meier curves. Categorical variables were compared using the Chi-squared test. Statistical significance was set at p < 0.05.
This service evaluation was performed as an audit within University College London Hospitals NHS Foundation Trust. Only retrospective data collected as part of routine standard care was included. As per local guidelines, research ethics council approval or informed patient consent was not required.
3. Results
3.1. Patient Characteristics
Between January 2019 and August 2022, 197 BTC patients were identified—71 patients were local to UCLH, while 126 were referrals for clinical trials or second opinions. The most common histological subtype was cholangiocarcinoma (n = 142; 72.1%). Table 1 lists population demographics and characteristics. At diagnosis, only 74 patients (37.6%) presented with resectable disease (Figure 1; Table 1), with 61 (82.4%) proceeding to surgery and 32 (52.5%) receiving adjuvant SACT (26 received capecitabine; 42.6%). Reasons for not receiving adjuvant therapy included patient choice, poor post-operative performance status, early post-operative disease progression or inoperable disease discovered intraoperatively. Overall, 168 patients were defined as incurable BTC for subsequent survival analyses, based on inoperable, metastatic or relapsed disease.

Table 1.
Real-World Study Biliary Tract Cancer Population Demographics.
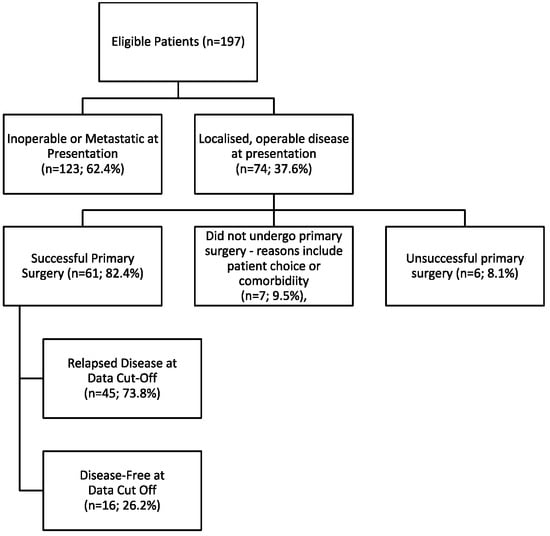
Figure 1.
Consort Diagram Outlining Disease Status of Included Patients.
3.2. Cohort Survival Analysis
Of the 123 patients with inoperable or metastatic disease at primary diagnosis, 122 had adequate survival data. Median OS was 13.7 months (95% CI 11.2–19.2 months; Figure 2A). Forty-three patients with relapsed disease had sufficient outcome data and a median OS of 18.3 months (95% CI 13.8–37.2 months). There was no statistical difference in OS between these groups (HR 0.68; 95% CI: 0.46–1.0; p = 0.05). For the overall incurable cohort (inoperable, de novo metastatic and relapsed patients), 165 patients had sufficient outcome data; median OS was 15.1 months (95% CI: 12.5–19.5 months; Figure 2A). No survival differences were observed when patients in the incurable cohort were stratified by age (<65 versus ≥65) and gender (Figure S1 and Figure S2, respectively).
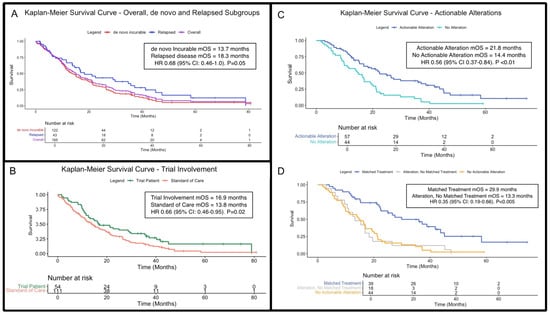
Figure 2.
Kaplan–Meier overall survival (OS) curves for patients with incurable disease. (A) OS curves for the incurable cohort (purple), alongside de novo incurable (red) and relapsed disease (blue) subgroups (p = 0.054). (B) OS for clinical trial enrolment (green) vs. standard-of-care treatments (orange; p = 0.02). (C) OS stratified by presence (blue) and absence (turquoise) of actionable genetic alterations (regardless of whether patients received matched, targeted treatments; p < 0.01). (D) OS further stratified by receipt of treatment matched to actionable alterations, if applicable (p < 0.005). Abbreviations: BTC = biliary tract cancers; HR = hazard ratio; OS = overall survival; mOS = median overall survival.
The clinical trial participation rate was 30.5% overall. Patients with incurable BTC enrolled in clinical trials (n = 54 with sufficient outcome data) had a significantly longer median OS of 16.9 months (95% CI: 12.5–29.8), compared to 13.8 months (95%CI: 11.2–19.2) for those receiving standard treatment (n = 111; HR 0.66, 95%CI: 0.46–0.95, p = 0.02; Figure 2B). Table S1 lists included trials, including enrolment numbers.
3.3. Actionable Genetic Alterations
Of the 108 patients who underwent genetic profiling, all had incurable BTC and 59 (54.6%) had actionable alterations (Table 2). Stratified by age at diagnosis, a higher proportion of patients in the <65 years group had identified actionable genetic alterations, compared with those in the older group (65 or older; 61.0% vs. 52.3%), though this did not reach statistical significance (p = 0.49; Table 2).

Table 2.
Real-World Biliary Tract Cancer Population Genetic Profiling Assessment and Results.
Overall, 57 patients had actionable alterations and survival outcomes with a median OS of 21.8 months (95% CI: 15.3–36.0), compared to 14.4 months (95% CI: 9.83–20.1) in the 44 patients without actionable alterations and available survival data (HR 0.56; 95% CI: 0.37–0.84; p <0.01; Figure 2C).
Among patients with actionable alterations, 69.5% subsequently received matched targeted treatment, with a median OS of 29.9 months (95% CI: 22.5–45.1; Figure 2D) compared to 13.3 months (95% CI: 10.5–19.5) for patients with actionable alterations who did not receive targeted treatments (HR 0.35 [95% CI 0.19–0.66]; p < 0.005).
3.4. Receipt of Systemic Anti-Cancer Therapies and Associated Outcomes
A total of 139 patients (82.7%) with incurable disease received SACT in metastatic and relapsed disease settings, and 137 had available outcome data with a median OS of 17.0 months (95% CI: 15.1–21.4). In comparison, those with sufficient survival data who did not receive SACT (n = 27) had a median OS of 3.2 months (95% CI: 2.8–9.2), a statistically significant difference (HR 0.29 [95% CI: 0.19–0.44]; p < 0.001; Figure 3A), and 83.7% (n = 103) and 82.2% (n = 37) received SACT in the metastatic/inoperable and relapsed disease settings, respectively. The reasons for not receiving palliative SACT were primarily poor performance status or patient choice. Focusing on those receiving platinum/gemcitabine combinations with sufficient outcome data, the median OS was 17.0 months (n = 126; 95% CI: 15.1–21.8) compared to 5.9 months (95% CI: 3.8–11.2) in patients not receiving platinum/gemcitabine (n = 39; HR 0.38 [95% CI 0.26–0.57]; p < 0.001).
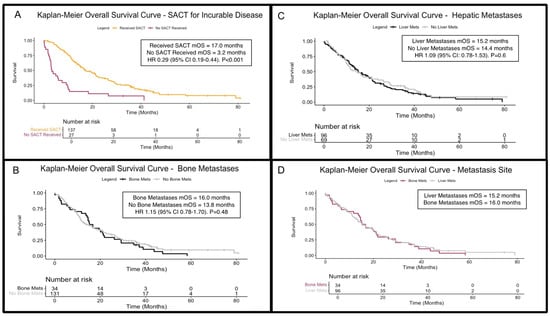
Figure 3.
Kaplan–Meier overall survival (OS) curves for patients with incurable disease. (A) OS curves for patients receiving (amber) or not receiving (maroon) systemic anti-cancer therapies in the incurable (metastatic/inoperable/relapsed) setting (p < 0.001). (B) OS stratified by the presence (black) and absence (grey) of bone metastases (p = 0.48). (C) OS stratified by the presence (black) and absence (grey) of liver metastases (p = 0.6). (D) Comparison of OS in patients with liver metastases (grey) to those with bony metastases (purple), showing comparable outcomes. Abbreviations: HR = hazard ratio; mets = metastases; SACT = systemic anti-cancer therapy/therapies; mOS = median overall survival.
3.5. Bone Metastases, Liver Metastases and Relation to Overall Survival
Bone metastases were identified in 34 patients (17.3%) during their disease course, with 14 cases detected at first presentation (Table 3). Patients with BM had a median OS of 16.0 months (95% CI: 14.2–21.8), while 131 patients without BM had sufficient survival outcome data and a median OS of 13.8 months (95% CI: 11.5–21.1). There was no significant survival difference between the two groups (HR 1.15; 95% CI: 0.78–1.70; p = 0.48; Figure 3B). When stratifying by time of BM onset, those with BM at initial presentation had a median OS of 14.8 months (95% CI 2.8–29.2); those developing BM later in their disease course had a median OS of 19 months (95% CI 14.2–37.2). The survival difference did not reach statistical significance (Figure S3; HR 2.0; 95% CI 0.97–4.15; p = 0.06).

Table 3.
Bone and Liver Metastases in the Biliary Tract Cancer Real-World Cohort. Breakdown by Malignant Subtype and Actionable Alteration Status.
To better understand the morbidity associated with BTC-induced BM, we assessed the prevalence of skeletal-related events and symptoms. Eighteen (52.9%) of the 34 patients reported bone pain and 15 (44.1%) had evidence of pathological fractures. Sixteen patients (47.1%) with BM received radiotherapy, with the intent of symptom control. Of the four patients who experienced metastatic spinal cord compression, all received radiotherapy to treat this, with one patient also undergoing neurosurgical intervention. Two other patients underwent surgery for femoral metastatic deposits. BM-associated hypercalcaemia occurred in three patients (8.8%). Alongside those receiving systematic therapies (bisphosphonates or denosumab) for hypercalcaemia, eight patients also received these for the presence BM-related symptoms or fracture prevention.
In those who underwent genetic profiling, actionable alterations were equally likely in the presence and absence of BM (52.4% vs. 58.5%; p = 0.95). For patients with both BM and actionable alterations (n = 11), the median OS was 19.5 months (95% CI: 15.2–not assessable). For patients with BM and no alterations (n = 10), the median OS was 14.4 months (95% CI: 6.7–not assessable). There was no significant survival difference between these groups (HR 0.95; 95% CI: 0.69–1.32; p = 0.8).
Of the 102 (51.8%) patients with liver metastases (LM), 61 cases (31.0% of total cohort, 59.8% of those with LM overall) were detected at diagnosis (Table 3). Of those, 96 patients had LM and sufficient outcome data, with a median OS of 15.2 months (95% CI: 13.2–21.1), compared to 14.4 months (95% CI: 10.5–23.9) without LM (n = 69). Comparing these cohorts, the difference was not significant (HR 1.09 [95% CI: 0.78–1.53]; p = 0.6; Figure 3C). Survival for patients with BM and LM was comparable (Figure 3D).
4. Discussion
4.1. Cohort Generalisability
In this real-world BTC cohort, subtype distribution was comparable to those in the ABC-02 (NCT00262769), ABC-06 (NCT01926236), and TOPAZ-1 trials (NCT03875235; [14,15,16]). In ABC-06 and TOPAZ-1, approximately 50% of patients had iCCA, a greater proportion than in our study. While TOPAZ-1 equally recruited from Asia, Europe, and the USA, both ABC-02 and ABC-06 were UK-based trials. The BTC subtype proportions in our cohort showed similarities to an English descriptive study of over 50,000 BTC patients identified through the National Cancer Registration Dataset, encompassing patients from diverse socioeconomic and ethnic backgrounds across all NHS England hospitals [1]. Although OS was assessed, the lack of stratification by treatment intent and metastases presence generally limits direct comparisons with this study.
Our cohort included more females than males, both in the overall and incurable populations. Although overall there was an almost equal split of patients aged <65 versus ≥65, in the incurable population more patients were aged >65. These observations are similar to those reported in other European large observational studies; survival outcomes in these subgroups are discussed further below [1,17].
Similar to another UK-based BTC study showing that higher emergency presentation rates at diagnosis correlated with a lower likelihood of curative surgery [18], most patients in this English cohort presented via emergency care pathways, which are generally associated with advanced disease stages and poorer outcomes. Although in our study, route of presentation and referral data was not collected, 62.4% of our patients had inoperable or metastatic disease at diagnosis, suggesting late presentation, likely via emergency pathways.
4.2. Survival in Incurable Cases—Systemic Anti-Cancer Therapy Use
In our study, only 37.6% of BTC patients presented with operable disease, consistent with previous reported estimates of 35% or lower [19,20]. Additionally, 73.8% of patients in our study relapsed after surgery, with or without adjuvant treatments [4,20,21,22]. This is higher than in the BILCAP trial, where five-year relapse rates were 66% with adjuvant capecitabine and 69% with observation. Only 52.5% of our cohort received any adjuvant SACT post-resection, with 42.6% receiving adjuvant capecitabine. The updated BILCAP survival results were not published until 2022, which may explain the less frequent use of adjuvant capecitabine in our study, potentially contributing to our observed higher relapse rates [22].
Patients receiving platinum/gemcitabine in the incurable setting in our study had a median OS of 17.5 months, exceeding survival estimates from early trials including ABC-02 [15]. Though this could be considered to reflect the increasing availability of subsequent treatment lines, it is not fully explained, as the recently published TOPAZ-1 trial also reported a lower median OS of 11.3 months in their platinum/gemcitabine control arm [14,23]. Our survival estimates also surpass reports in other observational studies, though direct comparisons are challenging, as studies include variable BTC subtypes, and some predate the use of cisplatin and gemcitabine, a standard first-line therapy [10,12,24].
Compared to large cohort studies and real-world datasets, treatment rates were higher in our tertiary centre, with 82.7% of patients with incurable disease receiving SACT (excluding adjuvant chemotherapy). In contrast, a European Network for the Study of Cholangiocarcinoma Registry study of 2334 patients found that only 26.2% of those with unresectable disease received chemotherapy, with a median OS of 10.6 months. However, this network study focused on selected centres and excluded gallbladder and ampullary cancers, so may not be representative of wider European populations [8]. SEER data from the USA estimated that 55.7% of patients with metastatic GBC received SACT [10].
An English Cancer Registry study of almost 9000 cholangiocarcinoma patients found that only 19.9% of non-operative patients received SACT [18]. Among those with initially resectable disease, the study did not differentiate between adjuvant and palliative SACT, limiting direct comparison with our study. We acknowledge that as our centre is a leading research institution, this may have influenced treatment access and outcomes.
4.3. Survival in Incurable Cases—Age and Gender Stratification
When stratifying for age and gender, no difference in OS was noted in our study (Figure S1 and Figure S2). Interestingly, Tataru et al.’s English, registry-based study (mentioned above) reported superior OS in both male and younger patients [1]. Two different, US-based, large studies of cholangiocarcinoma patients have also demonstrated superior OS in patients aged <50, compared to those over 50. One of these studies assessed survival in those with incurable disease, noting age to be an independent variable influencing survival [25], whereas the other study showed age to independently predict OS across all disease stages [26]. Indeed, the lack of OS difference regarding both gender and age in our study may be explained by a lower sample size, inclusion of patients with gallbladder and ampullary cancers and/or the highly selected patient cohort in this tertiary centre compared to the aforementioned studies, which are either large multi-centre studies or based on national registry data.
4.4. Molecular Profiling
In our study, over 50% of patients underwent genetic profiling, with 54.6% identified as having an actionable, targetable alteration. Even among patients without actionable alterations, the median OS exceeded 14 months, likely reflecting this group’s overall fitness, particularly as access to profiling is often through eligible enrolment into clinical trials. Consistent with previous findings, our data suggest that the presence of an actionable alteration alone did not confer a prognostic advantage without a matched targeted treatment [27]. However, when matched to targeted treatment, the median OS was 29.9 months (95% CI 22.5–45.1)—one of the highest reported to date [27,28].
Studies assessing differences in outcomes between younger and older patients with BTCs are increasing in number, proposing a possible relationship between age of cancer onset and underlying molecular driver mechanisms [25,26]. In keeping with results from large, US-based studies of cholangiocarcinoma patients [25,29], we show that the proportion of actionable mutations is influenced by age of diagnosis, though this did not reach significance in our cohort; small sample sizes may have influenced this. Though Pappas et al. [25] did not report the proportion of patients with actionable alterations by age group, they did show that younger patients had a higher frequency of targeted therapy use, alongside a higher frequency of FGFR2, BRAF and ATM alterations. When considered alongside their finding that OS was greater in patients <50 years old, this further highlights a possible association between age, actionable genetic alterations and OS. These findings are supported by a study of over 9000 iCCA patients with available tissue genomic profiling data, reporting that FGFR2 rearrangements increase in frequency in those <45, particularly in females [29]. As just 19 patients were aged <45 in our cohort, and only 12 of these had molecular profiling, we did not explore this age cut-off further. Our findings, showing that OS was not influenced by age alone, may be explained by the lack of a significant difference between the proportion of actionable alterations between younger and older age groups. Indeed, this relationship should be assessed further in future studies.
Even in our tertiary centre with access to a broad clinical trial portfolio, 45% of patients did not undergo molecular profiling. Following UK health technology assessment approval of pemigatinib and ivosidenib in late 2021 and 2024, respectively, standard-of-care genetic testing for FGFR2 and IDH-1 is now provided on the NHS [30,31,32,33]. Our study largely predated these events. Until recently, whole genome sequencing was only available through clinical trials or private funding. Since 2023, the NHS England National Genomic Testing Directory permits molecular profiling on NHS BTC patients. However, to qualify for testing, patients must have exhausted all standard-of-care treatments, therefore assuming them to be metastatic [34]. No equivalent testing is currently available for NHS Scotland patients, and so any testing outside of trial profiling must be personally and privately funded [35]. Furthermore, access to matched targeted agents in patients with atypical identifiable alterations remains a challenge UK-wide and is generally not funded by the NHS. No agreements for molecular profiling are in place for patients with resectable or curable disease, mainly due to the limited availability of licensed targeted treatments in this setting. These issues are also compounded by known limitations relating to the availability of sufficient tissue for testing in eligible populations. Indeed, when these factors are considered in the context of significantly prolonged survival in patients receiving matched targeted treatments, as shown in this study, limited access to molecular profiling within public healthcare systems results in implications for patients, primarily relating to equity of access to both molecular testing and subsequent targeted treatments in BTC cohorts.
4.5. Bone Metastases
Our study estimated that 17.3% of patients develop BM during their disease course, with 7.1% presenting with BM at primary diagnosis. BM incidence in BTC is sparsely reported in the existing literature, as key RCTs (such as ABC-02, ABC-06 and TOPAZ-1) did not specify sites of metastatic burden [14,15,16]. However, several observational studies have reported BM prevalence in specific BTC subgroups (Table 4; [7,8,9,10,11,12,13]). A Brazilian study including all BTC reported a similar BM prevalence of 19.2%, though it only included patients receiving first-line chemotherapy, thereby excluding supportively managed patients [12]. Across other studies, estimations of BM prevalence range from 6.2% to 29.7% depending on BTC subtype, disease stage at diagnosis and sample size. In our dataset, cholangiocarcinoma (especially iCCA) had a higher BM prevalence than GBC (Table 3), though small subgroup sizes limit definitive conclusions. Direct comparisons with other studies are also challenging due to study heterogeneity (Table 4).

Table 4.
Existing Published Studies Assessing the Role of Bone Metastases in Biliary Tract Cancers.
Large population studies and meta-analyses have evaluated the impact of metastasis site on cancer outcomes. In breast, renal and prostate cancers, BM are associated with a worse survival than lymph node metastases, but a better survival than visceral (including liver) and central nervous system metastases [36,37,38]. In BTC, most observational studies (summarised in Table 4) suggest BM correlate with poorer prognoses across all subtypes, though methodologies vary, particularly in distinguishing isolated versus non-isolated BM [7,10,11,12,13]. In contrast, our study found BM did not significantly impact survival compared to other metastatic sites, including LM. However, even though BM presence did not influence survival in our study, we show evidence of significant morbidity and symptom burden associated with their presence, with over 50% reporting pain associated with BM and almost 50% receiving radiotherapy to bone for symptoms.
To our knowledge, this study is the first to report that patients with BM were equally likely to have actionable alterations than those without. Patients with concurrent BM and actionable genomic alterations had comparable survival to those with BM alone. However, small sample sizes in all subgroups may have affected statistical significance and survival estimates.
4.6. Study Strengths and Limitations
Our study is the first to examine the relationship between BM and actionable alterations, offering novel insights for future research. Despite its contributions, our study has limitations. Reliance on electronic health records restricted data availability, including no assessable time points from BM confirmation to death for survival analysis. Potential clinical coding errors may have affected patient identification, particularly in cases where patients died soon after diagnosis, leading to missing or inaccurate coding. Subgroup sample sizes were small in certain cases, influencing statistical significance and result interpretation. Finally, as this study was conducted in a tertiary cancer centre with an active clinical trial portfolio, our cohort may not be fully representative of real-world practice.
5. Conclusions
In patients with BTC, actionable alterations were equally likely with or without BM, and the presence of BM did not affect OS in our cohort. While actionable alterations facilitated access to targeted treatment and better survival, this benefit was not unique to patients with BM. Larger collaborative studies with standardised methodologies are needed to determine BM prevalence rates more accurately.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17101639/s1, Figure S1: Kaplan-Meier Overall Survival Curves for Patients with Incurable Disease, Stratified by Age; Figure S2: Kaplan-Meier Overall Survival Curves for Patients with Incurable Disease, Stratified by Gender; Figure S3: Kaplan-Meier Overall Survival Curves for Patients with Incurable Disease and Bone Metastases, Stratified by Onset of Bone Disease; Table S1: List of Enrolled Clinical Trials and Number of Enrolled Patients.
Author Contributions
Conceptualization, K.H.E.-S. and J.B.; Data curation, J.K., R.A. and P.M.; Formal analysis, K.H.E.-S.; Methodology, K.H.E.-S. and J.B.; Writing—original draft, K.H.E.-S., J.K., R.A. and P.M.; Writing—review & editing, K.H.E.-S., J.K., R.A., P.M. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data supporting reported results can be accessed, in accordance with NHS England and University College London Hospital NHS Foundation Trust data protection rules, through direct application to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tataru, D.; Khan, S.A.; Hill, R.; Morement, H.; Wong, K.; Paley, L.; Toledano, M.B. Cholangiocarcinoma across England: Temporal changes in incidence, survival and routes to diagnosis by region and level of socioeconomic deprivation. JHEP Rep. 2024, 6, 100983. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Bridgewater, J.; Edeline, J.; Kelley, R.K.; Klümpen, H.J.; Malka, D.; Primrose, J.N.; Rimassa, L.; Stenzinger, A.; Valle, J.W.; et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Bridgewater, J.A.; Goodman, K.A.; Kalyan, A.; Mulcahy, M.F. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology Publications: Alexandria, VA, USA, 2016; pp. e194–e203. [Google Scholar] [CrossRef]
- Lamarca, A.; Edeline, J.; Goyal, L. How I treat biliary tract cancer. ESMO Open 2022, 7, 100378. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, G.; Cheng, C.S.; Yeerken, R.; Chan, Y.T.; Fu, Z.; Zheng, Y.C.; Feng, Y.; Wang, N. Traditional Chinese medicine for the treatment of cancers of hepatobiliary system: From clinical evidence to drug discovery. Mol. Cancer 2024, 23, 218. [Google Scholar] [CrossRef]
- Tritripmongkol, P.; Plengsuriyakarn, T.; Tarasuk, M.; Na-Bangchang, K. In vitro cytotoxic and toxicological activities of ethanolic extract of Kaempferia galanga Linn. and its active component, ethyl-p-methoxycinnamate, against cholangiocarcinoma. J. Integr. Med. 2020, 18, 326–333. [Google Scholar] [CrossRef]
- Garajová, I.; Gelsomino, F.; Salati, M.; Leonardi, F.; De Lorenzo, S.; Granito, A.; Tovoli, F. Bone Metastases from Intrahepatic Cholangiocarcinoma Confer Worse Prognosis. Curr. Oncol. 2023, 30, 2613. [Google Scholar] [CrossRef]
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 2022, 76, 1109–1121. [Google Scholar] [CrossRef]
- Santini, D.; Brandi, G.; Aprile, G.; Russano, M.; Cereda, S.; Leone, F.; Lonardi, S.; Fornaro, L.; Scartozzi, M.; Silvestris, N.; et al. Bone metastases in biliary cancers: A multicenter retrospective survey. J. Bone Oncol. 2018, 12, 33. [Google Scholar] [CrossRef]
- Gera, K.; Kahramangil, D.; Fenton, G.A.; Martir, D.; Rodriguez, D.N.; Ijaz, Z.; Lin, R.Y.; Rogers, S.C.; Ramnaraign, B.H.; George, T.J.; et al. Prognosis and Treatment Outcomes of Bone Metastasis in Gallbladder Adenocarcinoma: A SEER-Based Study. Cancers 2023, 15, 5055. [Google Scholar] [CrossRef]
- Cheng, R.; Du, Q.; Ye, J.; Wang, B.; Chen, Y. Prognostic value of site-specific metastases for patients with advanced intrahepatic cholangiocarcinoma. Medicine 2019, 98, e18191. [Google Scholar] [CrossRef]
- Felismino, T.C.; Araujo Oliveira, F.M.; Zanco Fogassa, C.A.; Santerini, S.N.; Fonseca de Jesus, V.H.; Riechelmann, R.S.P.; Fernandez Coimbra, F.J.; Lopes Mello, C.A. Evaluation of prognostic factors in patients undergoing first-line chemotherapy for advanced biliary tract cancer: A retrospective analysis from a South American cancer centre. ecancermedicalscience 2022, 16, 1345. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, P.; Zhu, Z.; Ning, Z.; Xu, L.; Zhuang, L.; Sheng, J.; Meng, Z. Site-Specific Metastases of Intrahepatic Cholangiocarcinoma and its Impact on Survival: A Population-Based Study. Future Oncol. 2019, 15, 2125–2137. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Ruth, A.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evidence 2022, 1, EVIDoa2200015. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Rahman, R.; Ludvigsson, J.F.; von Seth, E.; Lagergren, J.; Bergquist, A.; Radkiewicz, C. Age trends in biliary tract cancer incidence by anatomical subtype: A Swedish cohort study. Eur. J. Cancer 2022, 175, 291–298. [Google Scholar] [CrossRef]
- Jose, S.; Zalin-Miller, A.; Knott, C.; Paley, L.; Tataru, D.; Morement, H.; Toledano, M.B.; Khan, S.A. Cohort study to assess geographical variation in cholangiocarcinoma treatment in England. World J. Gastrointest. Oncol. 2023, 15, 2077–2092. [Google Scholar] [CrossRef]
- Kefas, J.; Bridgewater, J.; Vogel, A.; Stein, A.; Primrose, J. Adjuvant therapy of biliary tract cancers. Ther. Adv. Med. Oncol. 2023, 15, 17588359231163785. [Google Scholar] [CrossRef]
- Lamarca, A.; Edeline, J.; McNamara, M.G.; Hubner, R.A.; Nagino, M.; Bridgewater, J.; Primrose, J.; Valle, J.W. Current standards and future perspectives in adjuvant treatment for biliary tract cancers. Cancer Treat. Rev. 2020, 84, 101936. [Google Scholar] [CrossRef]
- Primrose, J.N.; Neoptolemos, J.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef]
- Bridgewater, J.; Fletcher, P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Long-Term Outcomes and Exploratory Analyses of the Randomized Phase III BILCAP Study. J. Clin. Oncol. 2022, 40, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.-Y.; He, A.R.; Qin, S.; Chen, L.-T.; Okusaka, T.; Vogel, A.; Kim, J.W.; Suksombooncharoen, T.; Lee, M.A.; Kitano, M.; et al. 279MO Three-year survival, safety and extended long-term survivor (eLTS) analysis from the phase III TOPAZ-1 study of durvalumab (D) plus chemotherapy in biliary tract cancer (BTC). Ann. Oncol. 2024, 35, S117. [Google Scholar] [CrossRef]
- Nghiem, V.; Wood, S.; Ramachandran, R.; Williams, G.; Outlaw, D.; Paluri, R.; Kim YIl Gbolahan, O. Short- and Long-Term Survival of Metastatic Biliary Tract Cancer in the United States From 2000 to 2018. Cancer Control. 2023, 30, 10732748231211764. [Google Scholar] [CrossRef] [PubMed]
- Pappas, L.; Baiev, I.; Reyes, S.; Bocobo, A.G.; Jain, A.; Spencer, K.; Le, T.M.; Rahma, O.E.; Maurer, J.; Stanton, J.; et al. The Cholangiocarcinoma in the Young (CITY) Study: Tumor Biology, Treatment Patterns, and Survival Outcomes in Adolescent Young Adults with Cholangiocarcinoma. JCO Precis. Oncol. 2023, 7, e2200594. [Google Scholar] [CrossRef]
- Reddy, S.; Goksu, S.Y.; Sanford, N.N.; Kainthla, R.; Hsiehchen, D.; Sanjeevaiah, A.; Jones, A.L.; Karagkounis, G.; Al Mutar, S.; Ahn, C.; et al. Characteristics and clinical outcomes in young-onset cholangiocarcinoma. Cancer Med. 2023, 12, 14094–14103. [Google Scholar] [CrossRef]
- Kumar-Sinha, C.; Vats, P.; Tran, N.; Robinson, D.R.; Gunchick, V.; Wu, Y.M.; Cao, X.; Ning, Y.; Wang, R.; Rabban, E.; et al. Genomics driven precision oncology in advanced biliary tract cancer improves survival. Neoplasia 2023, 42, 100910. [Google Scholar] [CrossRef]
- Doleschal, B.; Taghizadeh, H.; Webersinke, G.; Piringer, G.; Schreil, G.; Decker, J.; Aichberger, K.J.; Kirchweger, P.; Thaler, J.; Petzer, A.; et al. Real world evidence reveals improved survival outcomes in biliary tract cancer through molecular matched targeted treatment. Sci. Rep. 2023, 13, 15421. [Google Scholar] [CrossRef]
- Hu, Z.I.; Ross, J.S.; Pavlick, D.; Hsiehchen, D. Age and Sex Affects the Frequency and Mutation Type of FGFR2 Alterations in Cholangiocarcinoma. JCO Oncol. Adv. 2024, 1, e2400027. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Pemigatinib for Treating Relapsed or Refractory Advanced Cholangiocarcinoma with FGFR2 Fusion or Rearrangement. Guidance; National Institute for Health and Care Excellence: London, UK, 2021. [Google Scholar]
- National Institute for Health and Care Excellence. Ivosidenib for Treating Advanced Cholangiocarcinoma with an IDH1 R132 Mutation after 1 or More Systemic Treatments. Guidance; National Institute for Health and Care Excellence: London, UK, 2024. [Google Scholar]
- Scottish Medicines Consortium. Pemigatinib (Pemazyre). 2022. Available online: https://scottishmedicines.org.uk/medicines-advice/pemigatinib-pemazyre-full-smc2399/ (accessed on 16 April 2025).
- Scottish Medicines Consortium. Ivosidenib (Tibsovo). 2024. Available online: https://scottishmedicines.org.uk/medicines-advice/ivosidenib-tibsovo-full-smc2615/ (accessed on 16 April 2025).
- NHS England. National Genomic Test Directory. 2025. Available online: https://www.england.nhs.uk/publication/national-genomic-test-directories/ (accessed on 16 April 2025).
- Scottish Strategic Network for Genomic Medicine. SSNGM Cancer Test Directory. National Services Scotland. 2023. Available online: https://www.nss.nhs.scot/publications/ssngm-cancer-test-directory/ (accessed on 16 April 2025).
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer 2019, 19, 1091. [Google Scholar] [CrossRef]
- Dudani, S.; de Velasco, G.; Wells, J.C.; Gan, C.L.; Donskov, F.; Porta, C.; Fraccon, A.; Pasini, F.; Lee, J.L.; Hansen, A.; et al. Evaluation of Clear Cell, Papillary, and Chromophobe Renal Cell Carcinoma Metastasis Sites and Association With Survival. JAMA Netw. Open 2021, 4, e2021869. [Google Scholar] [CrossRef]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men with Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).