Mechanical Conditioning (MeCo) Score Progressively Increases Through the Metastatic Cascade in Breast Cancer via Circulating Tumor Cells
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Patient Cohorts and Sample Collection
2.2. CTC Isolation Techniques
2.3. RNA Sequencing and Gene Expression Analysis
2.4. PAM50 Subtyping and MeCo Score Calculation
3. Results
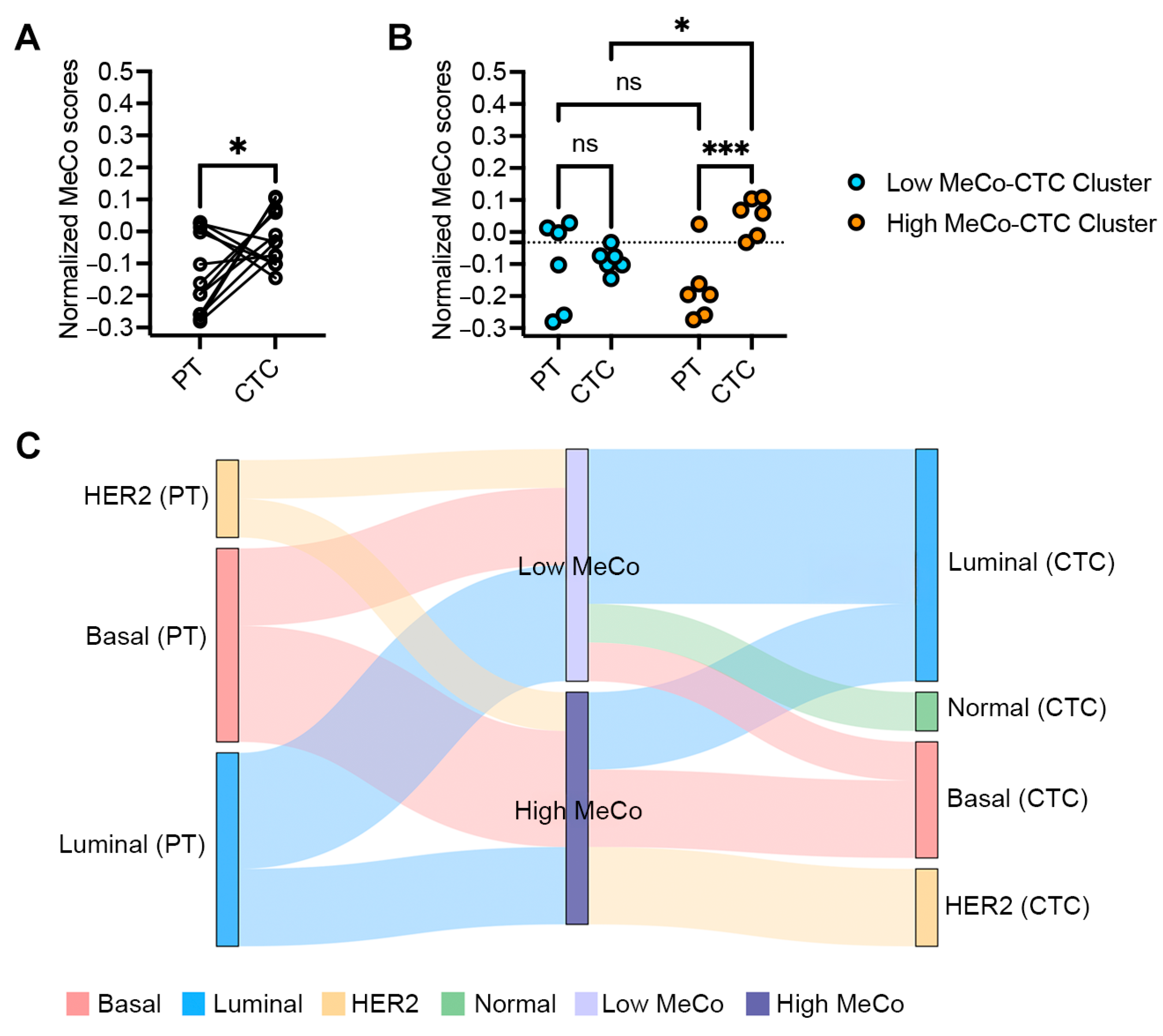
3.1. MeCo Score Comparison Between Primary Tumors and CTCs in Early-Stage Breast Cancer
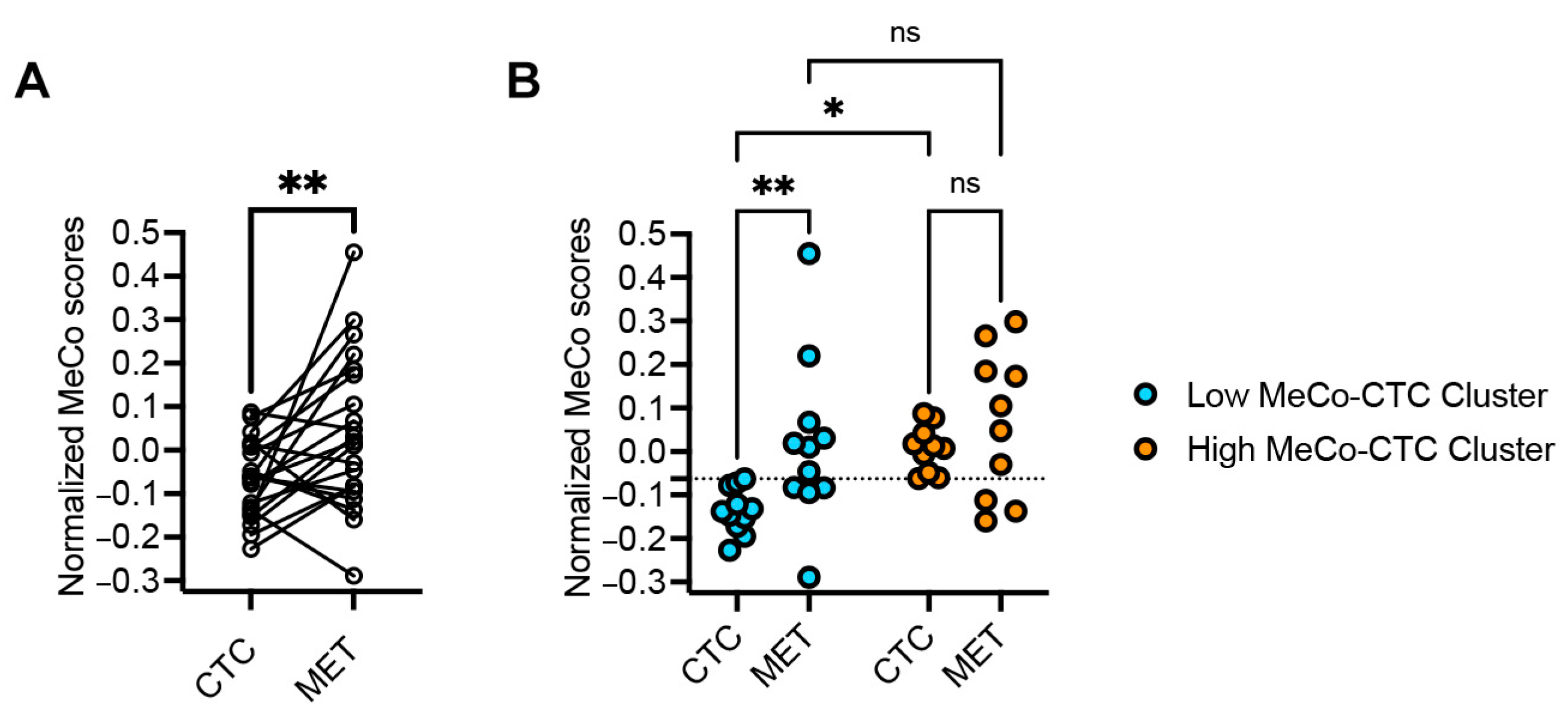
3.2. MeCo Score Comparison Between CTCs and Metastatic Sites in Stage IV Breast Cancer
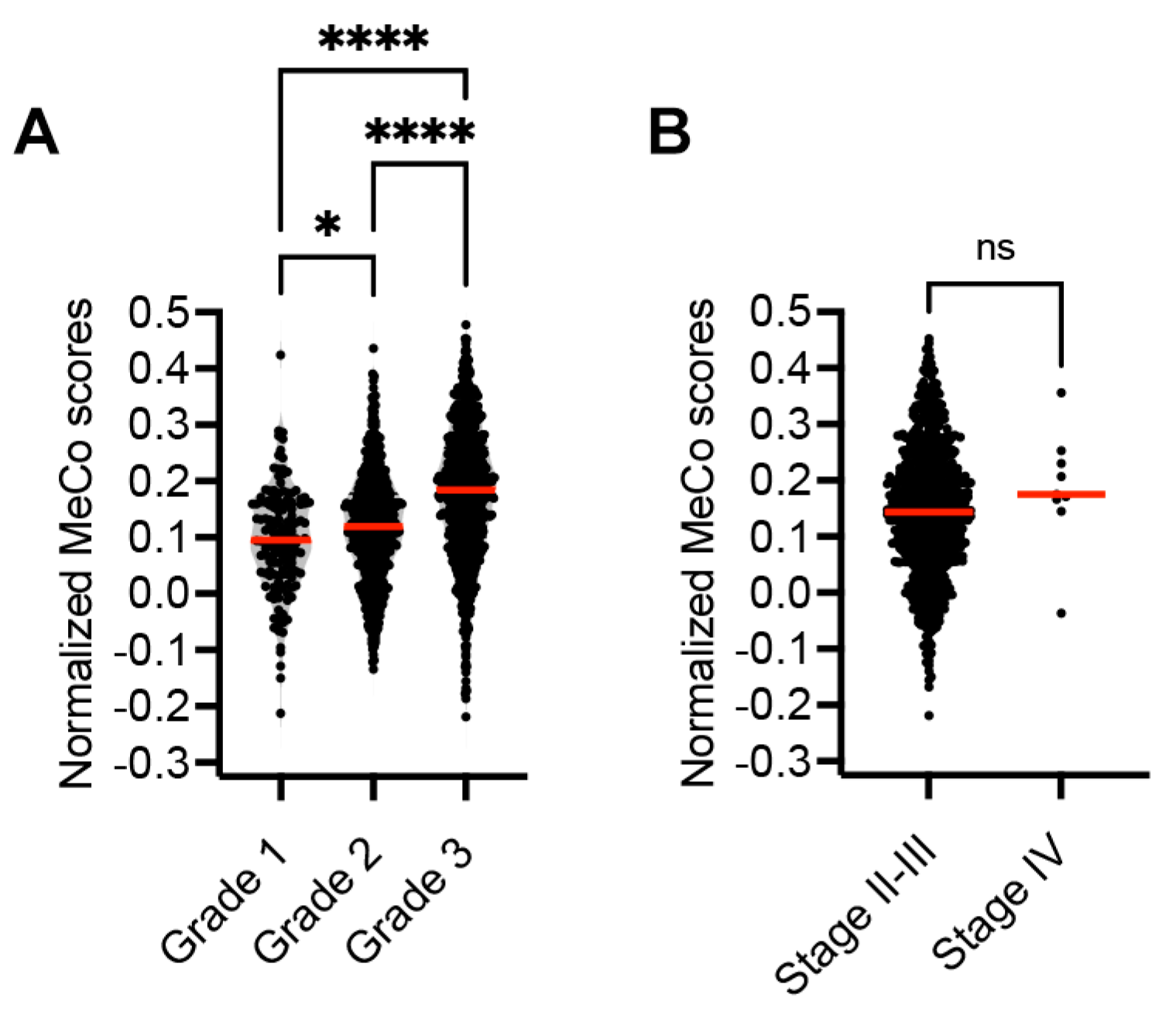
3.3. Correlation Between MeCo Scores and Established Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cambria, E.; Coughlin, M.F.; Floryan, M.A.; Offeddu, G.S.; Shelton, S.E.; Kamm, R.D. Linking cell mechanical memory and cancer metastasis. Nat. Rev. Cancer 2024, 24, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Borriello, L.; Karagiannis, G.S.; Duran, C.L.; Coste, A.; Oktay, M.H.; Entenberg, D.; Condeelis, J.S. The role of the tumor microenvironment in tumor cell intravasation and dissemination. Eur. J. Cell Biol. 2020, 99, 151098. [Google Scholar] [CrossRef]
- Huang, C.; Rajfur, Z.; Borchers, C.; Schaller, M.D.; Jacobson, K. JNK phosphorylates paxillin and regulates cell migration. Nature 2003, 424, 219–223. [Google Scholar] [CrossRef]
- Linke, J.A.; Munn, L.L.; Jain, R.K. Compressive stresses in cancer: Characterization and implications for tumour progression and treatment. Nat. Rev. Cancer 2024, 24, 768–791. [Google Scholar] [CrossRef]
- Harris, M.A.; Savas, P.; Virassamy, B.; O’malley, M.M.R.; Kay, J.; Mueller, S.N.; Mackay, L.K.; Salgado, R.; Loi, S. Towards targeting the breast cancer immune microenvironment. Nat. Rev. Cancer 2024, 24, 554–577. [Google Scholar] [CrossRef]
- Watson, A.W.; Grant, A.D.; Parker, S.S.; Hill, S.; Whalen, M.B.; Chakrabarti, J.; Harman, M.W.; Roman, M.R.; Forte, B.L.; Gowan, C.C.; et al. Breast tumor stiffness instructs bone metastasis via maintenance of mechanical conditioning. Cell Rep. 2021, 35, 109293. [Google Scholar] [CrossRef] [PubMed]
- Vivo, M.; Rosti, V.; Cervone, S.; Lanzuolo, C. Chromatin plasticity in mechanotransduction. Curr. Opin. Cell Biol. 2024, 88, 102376. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zheng, Y.; Hu, G.; Xin, Y.; Li, K.; Zhang, C.; Chen, X.; Zhang, B.; Li, X.; Hu, B.; et al. Local soft niches in mechanically heterogeneous primary tumors promote brain metastasis via mechanotransduction-mediated HDAC3 activity. Sci. Adv. 2025, 11, eadq2881. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Cohen, E.N.; Jayachandran, G.; Moore, R.G.; Cristofanilli, M.; Lang, J.E.; Khoury, J.D.; Press, M.F.; Kim, K.K.; Khazan, N.; Zhang, Q.; et al. A Multi-Center Clinical Study to Harvest and Characterize Circulating Tumor Cells from Patients with Metastatic Breast Cancer Using the Parsortix((R)) PC1 System. Cancers 2022, 14, 5238. [Google Scholar] [CrossRef]
- Alam, R.; Reva, A.; Edwards, D.G.; Lege, B.M.; Munoz-Arcos, L.S.; Reduzzi, C.; Singh, S.; Hao, X.; Wu, Y.-H.; Tian, Z.; et al. Bone-Induced Her2 Promotes Secondary Metastasis in HR+/Her2- Breast Cancer. Cancer Discov. 2025, 15, 818–837. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.E.; Scott, J.H.; Wolf, D.M.; Novak, P.; Punj, V.; Magbanua, M.J.M.; Zhu, W.; Mineyev, N.; Haqq, C.M.; Crothers, J.R.; et al. Expression profiling of circulating tumor cells in metastatic breast cancer. Breast Cancer Res. Treat. 2015, 149, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ring, A.; Campo, D.; Porras, T.B.; Kaur, P.; Forte, V.A.; Tripathy, D.; Lu, J.; Kang, I.; Press, M.F.; Jeong, Y.J.; et al. Circulating Tumor Cell Transcriptomics as Biopsy Surrogates in Metastatic Breast Cancer. Ann. Surg. Oncol. 2022, 29, 2882–2894. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.E.; Ring, A.; Porras, T.; Kaur, P.; Forte, V.A.; Mineyev, N.; Tripathy, D.; Press, M.F.; Campo, D. RNA-Seq of Circulating Tumor Cells in Stage II-III Breast Cancer. Ann. Surg. Oncol. 2018, 25, 2261–2270. [Google Scholar] [CrossRef]
- Fina, E.; Cleris, L.; Dugo, M.; Lecchi, M.; Ciniselli, C.M.; Lecis, D.; Bianchi, G.V.; Verderio, P.; Daidone, M.G.; Cappelletti, V. Gene signatures of circulating breast cancer cell models are a source of novel molecular determinants of metastasis and improve circulating tumor cell detection in patients. J. Exp. Clin. Cancer Res. 2022, 41, 78. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Melisko, M.; Roy, R.; Sosa, E.V.; Hauranieh, L.; Kablanian, A.; Eisenbud, L.E.; Ryazantsev, A.; Au, A.; Scott, J.H.; et al. Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res. 2013, 73, 7134–7143. [Google Scholar] [CrossRef]
- Parker, J.S.; Mullins, M.; Cheang, M.C.; Leung, S.; Voduc, D.; Vickery, T.; Davies, S.; Fauron, C.; He, X.; Hu, Z.; et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol. 2009, 27, 1160–1167. [Google Scholar] [CrossRef]
- Gendoo, D.M.A.; Ratanasirigulchai, N.; Schröder, M.S.; Paré, L.; Parker, J.S.; Prat, A.; Haibe-Kains, B. Genefu: An R/Bioconductor package for computation of gene expression-based signatures in breast cancer. Bioinformatics 2016, 32, 1097–1099. [Google Scholar] [CrossRef]
- Rueda, O.M.; Sammut, S.-J.; Seoane, J.A.; Chin, S.-F.; Caswell-Jin, J.L.; Callari, M.; Batra, R.; Pereira, B.; Bruna, A.; Ali, H.R.; et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature 2019, 567, 399–404. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Jørgensen, C.L.T.; Larsson, A.-M.; Forsare, C.; Aaltonen, K.; Jansson, S.; Bradshaw, R.; Bendahl, P.-O.; Rydén, L. PAM50 Intrinsic Subtype Profiles in Primary and Metastatic Breast Cancer Show a Significant Shift toward More Aggressive Subtypes with Prognostic Implications. Cancers 2021, 13, 1592. [Google Scholar] [CrossRef] [PubMed]
- Quintela-Fandino, M.; Bermejo, B.; Zamora, E.; Moreno, F.; García-Saenz, J.Á.; Pernas, S.; Martínez-Jañez, N.; Jiménez, D.; Adrover, E.; de Andrés, R.; et al. High Mechanical Conditioning by Tumor Extracellular Matrix Stiffness Is a Predictive Biomarker for Antifibrotic Therapy in HER2-Negative Breast Cancer. Clin. Cancer Res. 2024, 30, 5094–5104. [Google Scholar] [CrossRef] [PubMed]
- Castro-Giner, F.; Aceto, N. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med. 2020, 12, 31. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef]
- Wheler, J.J.; Janku, F.; Naing, A.; Li, Y.; Stephen, B.; Zinner, R.; Subbiah, V.; Fu, S.; Karp, D.; Falchook, G.S.; et al. Cancer Therapy Directed by Comprehensive Genomic Profiling: A Single Center Study. Cancer Res. 2016, 76, 3690–3701. [Google Scholar] [CrossRef]
- Shan, N.L.; Gould, B.; Wang, X.; Bonora, G.; Blenman, K.; Foldi, J.; Campos, G.E.; Walsh, M.; Du, P.; Pusztai, L. Circulating tumor DNA fraction predicts residual cancer burden post-neoadjuvant chemotherapy in triple negative breast cancer. J. Liq. Biopsy 2024, 6, 100168. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Swigart, L.B.; Ahmed, Z.; Sayaman, R.W.; Renner, D.; Kalashnikova, E.; Hirst, G.L.; Yau, C.; Wolf, D.M.; Li, W.; et al. Clinical significance and biology of circulating tumor DNA in high-risk early-stage HER2-negative breast cancer receiving neoadjuvant chemotherapy. Cancer Cell 2023, 41, 1091–1102 e4. [Google Scholar] [CrossRef]
- Davidson, B.A.; Croessmann, S.; Park, B.H. The breast is yet to come: Current and future utility of circulating tumour DNA in breast cancer. Br. J. Cancer 2021, 125, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.C.; Brisson, B.K.; Dekky, B.; Berger, A.C.; Yen, W.; Mauldin, E.A.; Loebel, C.; Gillette, D.; Assenmacher, C.-A.; Quincey, C.; et al. Prognostic and therapeutic implications of tumor-restrictive type III collagen in the breast cancer microenvironment. NPJ Breast Cancer 2024, 10, 86. [Google Scholar] [CrossRef]
- Wullkopf, L.; West, A.-K.V.; Leijnse, N.; Cox, T.R.; Madsen, C.D.; Oddershede, L.B.; Erler, J.T. Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol. Biol. Cell 2018, 29, 2378–2385. [Google Scholar] [CrossRef]
- Hüsemann, Y.; Geigl, J.B.; Schubert, F.; Musiani, P.; Meyer, M.; Burghart, E.; Forni, G.; Eils, R.; Fehm, T.; Riethmüller, G.; et al. Systemic spread is an early step in breast cancer. Cancer Cell 2008, 13, 58–68. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouneimne, G.; Connors, C.; Watson, A.; Grant, A.; Campo, D.; Ring, A.; Kaur, P.; Lang, J.E. Mechanical Conditioning (MeCo) Score Progressively Increases Through the Metastatic Cascade in Breast Cancer via Circulating Tumor Cells. Cancers 2025, 17, 1632. https://doi.org/10.3390/cancers17101632
Mouneimne G, Connors C, Watson A, Grant A, Campo D, Ring A, Kaur P, Lang JE. Mechanical Conditioning (MeCo) Score Progressively Increases Through the Metastatic Cascade in Breast Cancer via Circulating Tumor Cells. Cancers. 2025; 17(10):1632. https://doi.org/10.3390/cancers17101632
Chicago/Turabian StyleMouneimne, Ghassan, Casey Connors, Adam Watson, Adam Grant, Daniel Campo, Alexander Ring, Pushpinder Kaur, and Julie E. Lang. 2025. "Mechanical Conditioning (MeCo) Score Progressively Increases Through the Metastatic Cascade in Breast Cancer via Circulating Tumor Cells" Cancers 17, no. 10: 1632. https://doi.org/10.3390/cancers17101632
APA StyleMouneimne, G., Connors, C., Watson, A., Grant, A., Campo, D., Ring, A., Kaur, P., & Lang, J. E. (2025). Mechanical Conditioning (MeCo) Score Progressively Increases Through the Metastatic Cascade in Breast Cancer via Circulating Tumor Cells. Cancers, 17(10), 1632. https://doi.org/10.3390/cancers17101632



_Mouneimne.png)


