Brain Metastases from Primary Cardiac Tumors: A Systematic Review of Diagnosis, Treatment, and Prognosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Literature Search Strategy
2.3. Eligibility Criteria
2.4. Data Extraction and Synthesis
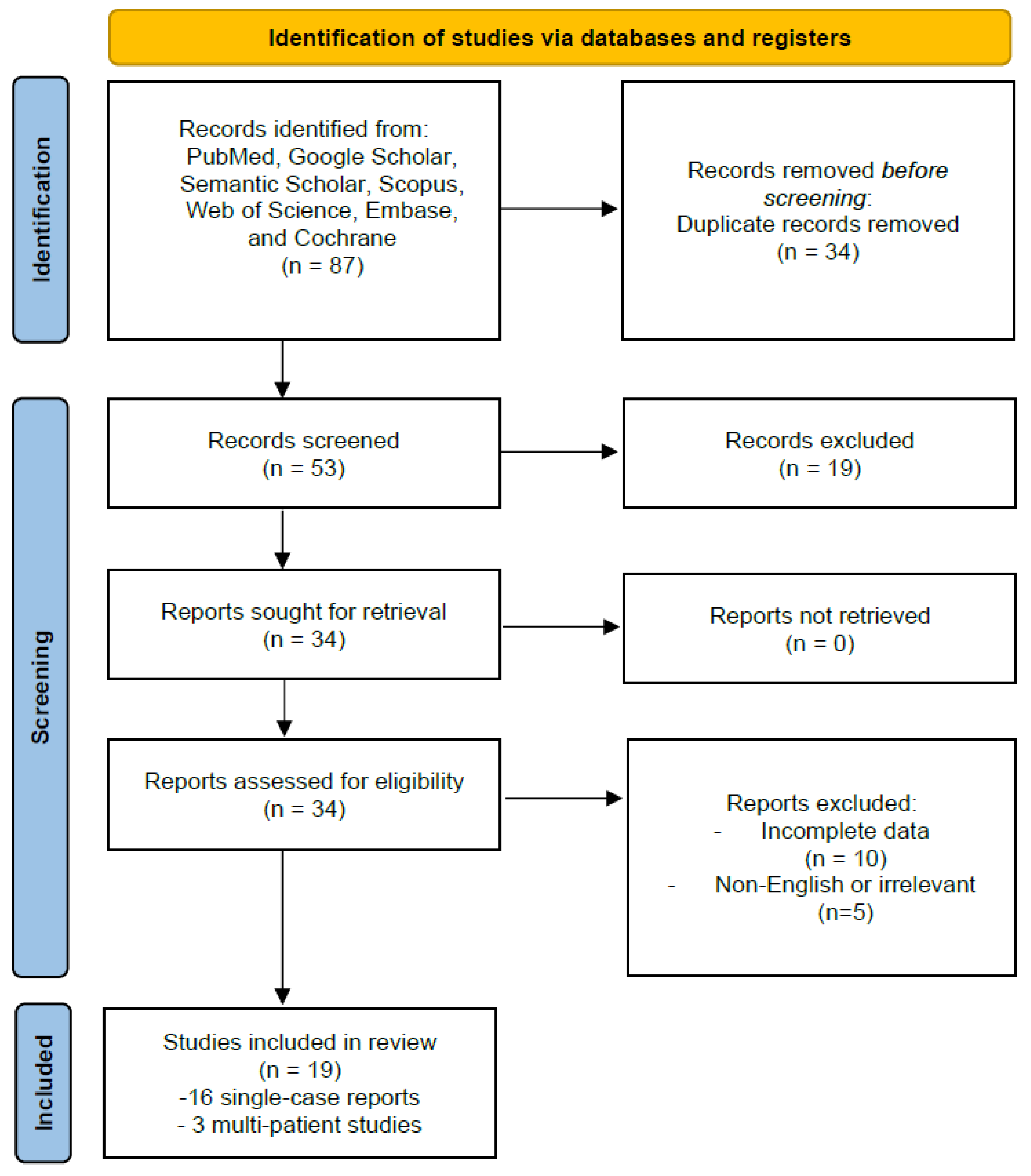
2.5. PRISMA Framework
3. Results
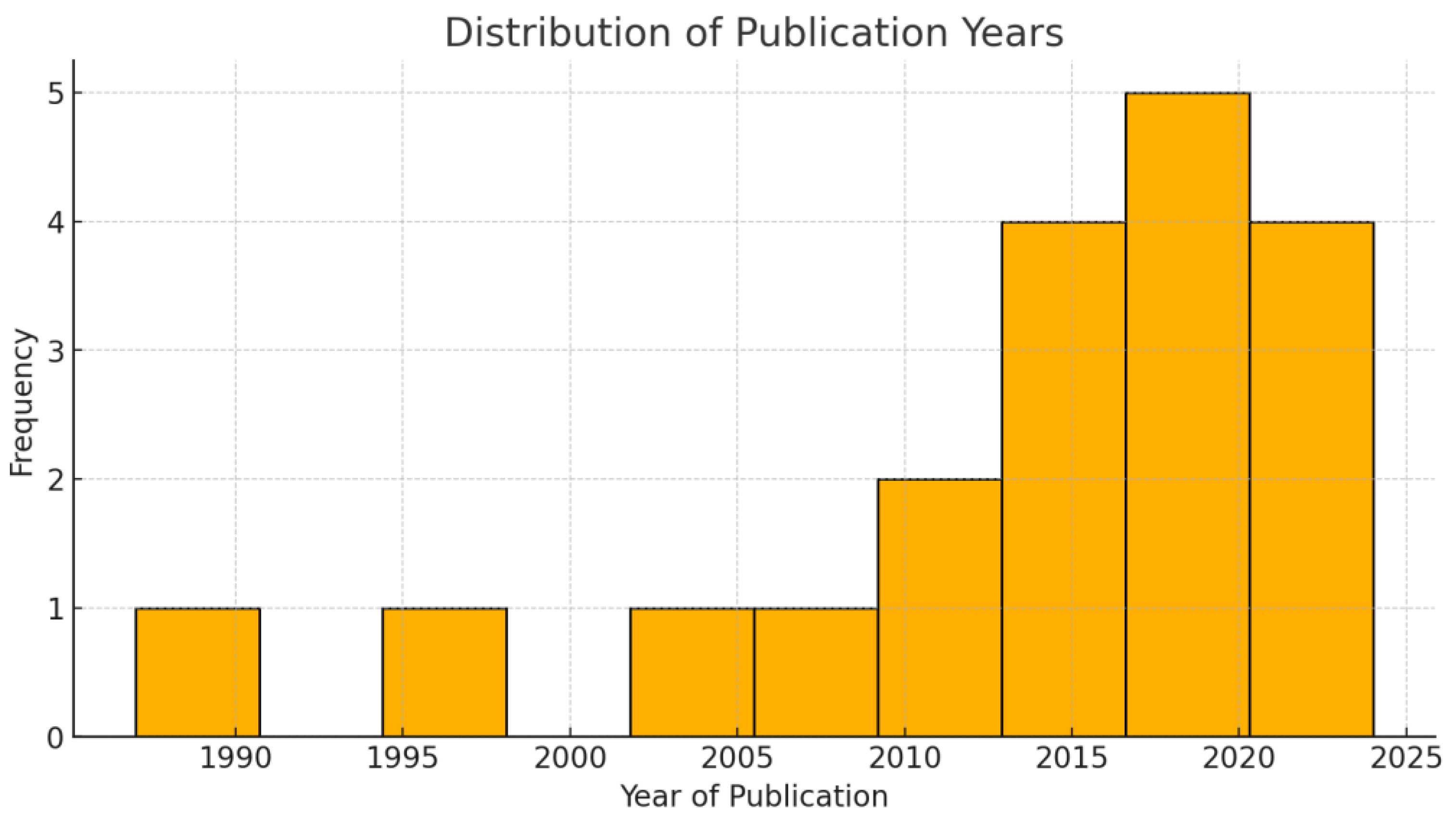
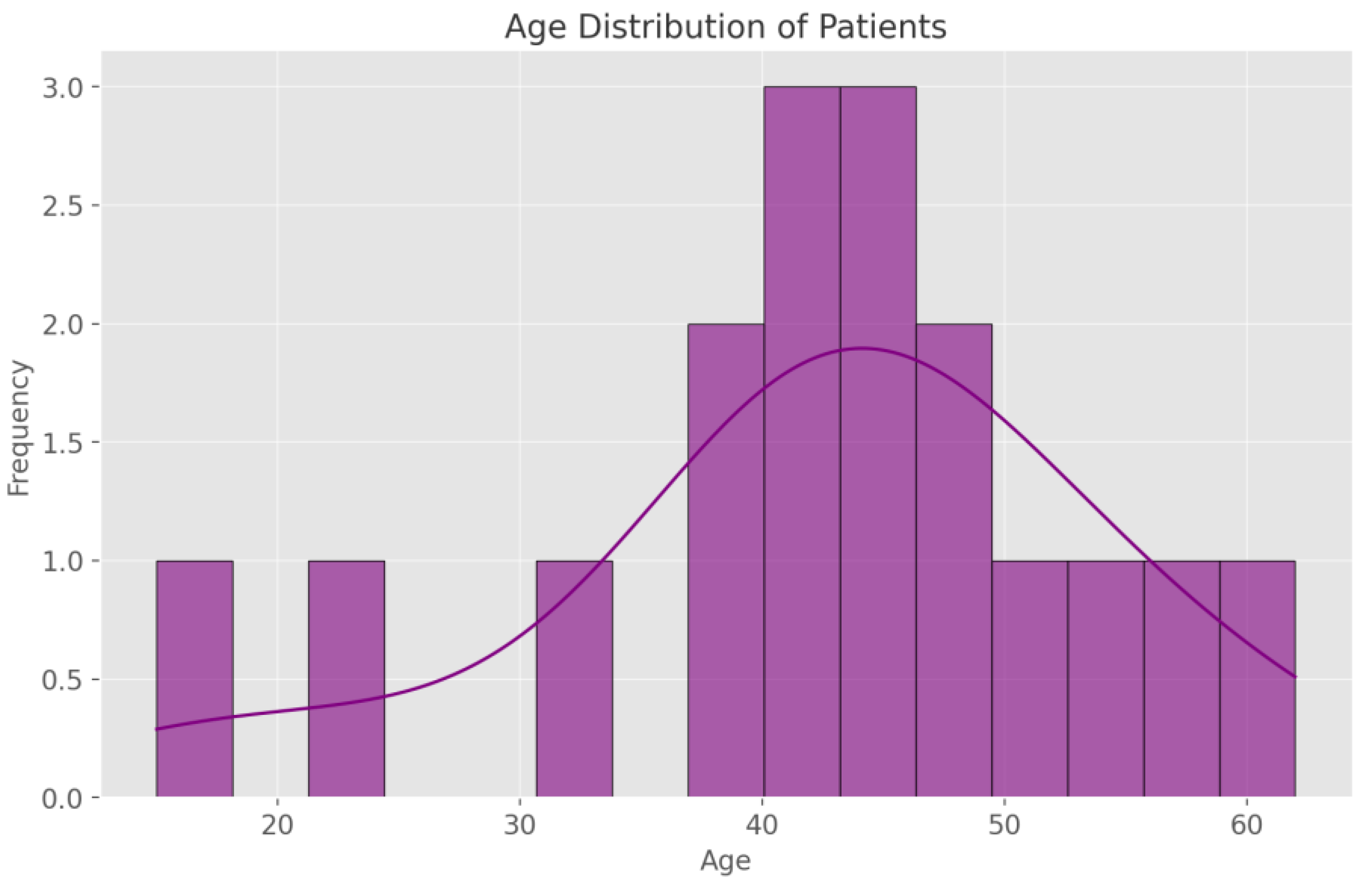
3.1. Demographic Data
3.2. Clinical Presentation and Symptomatology
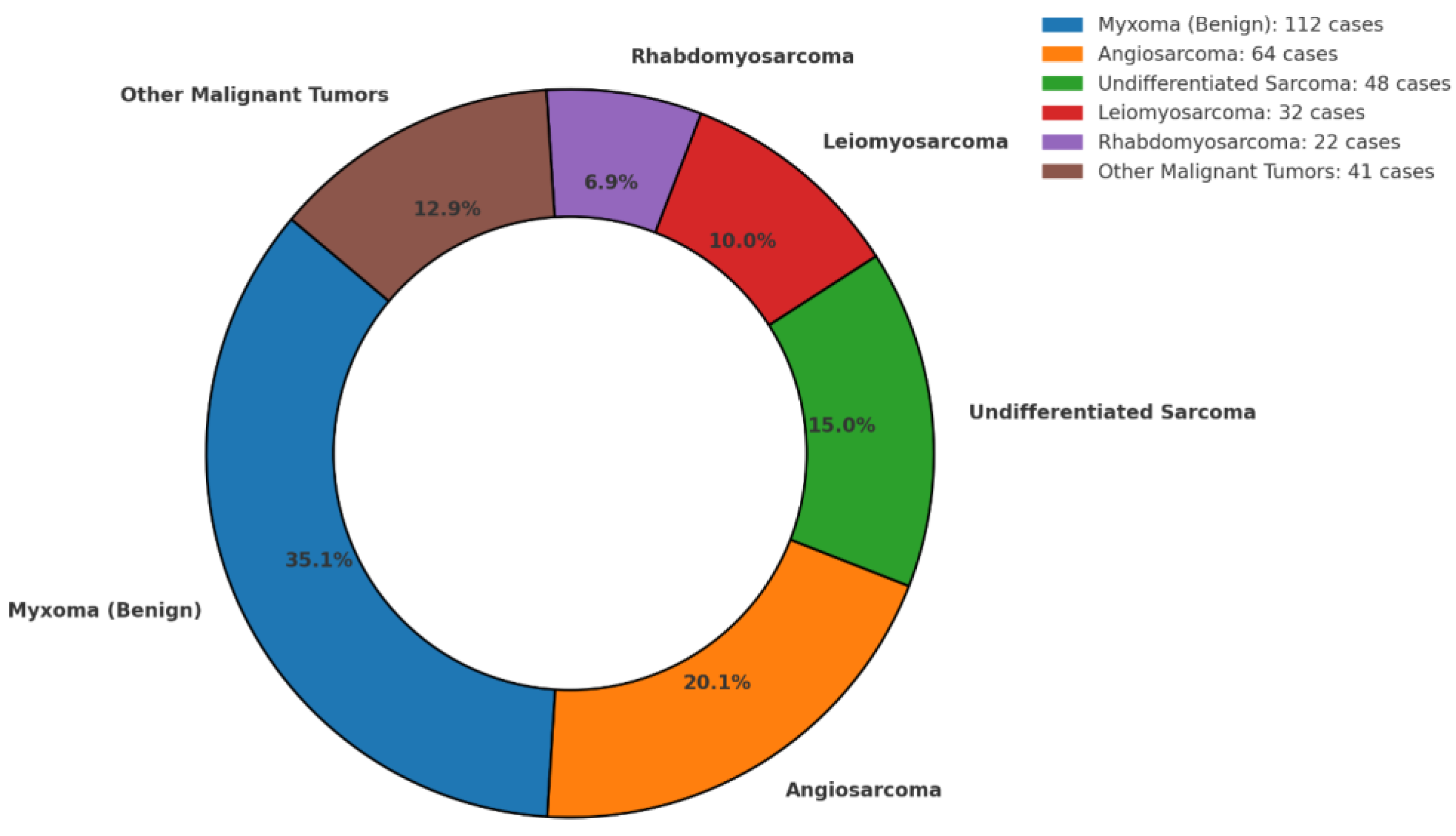
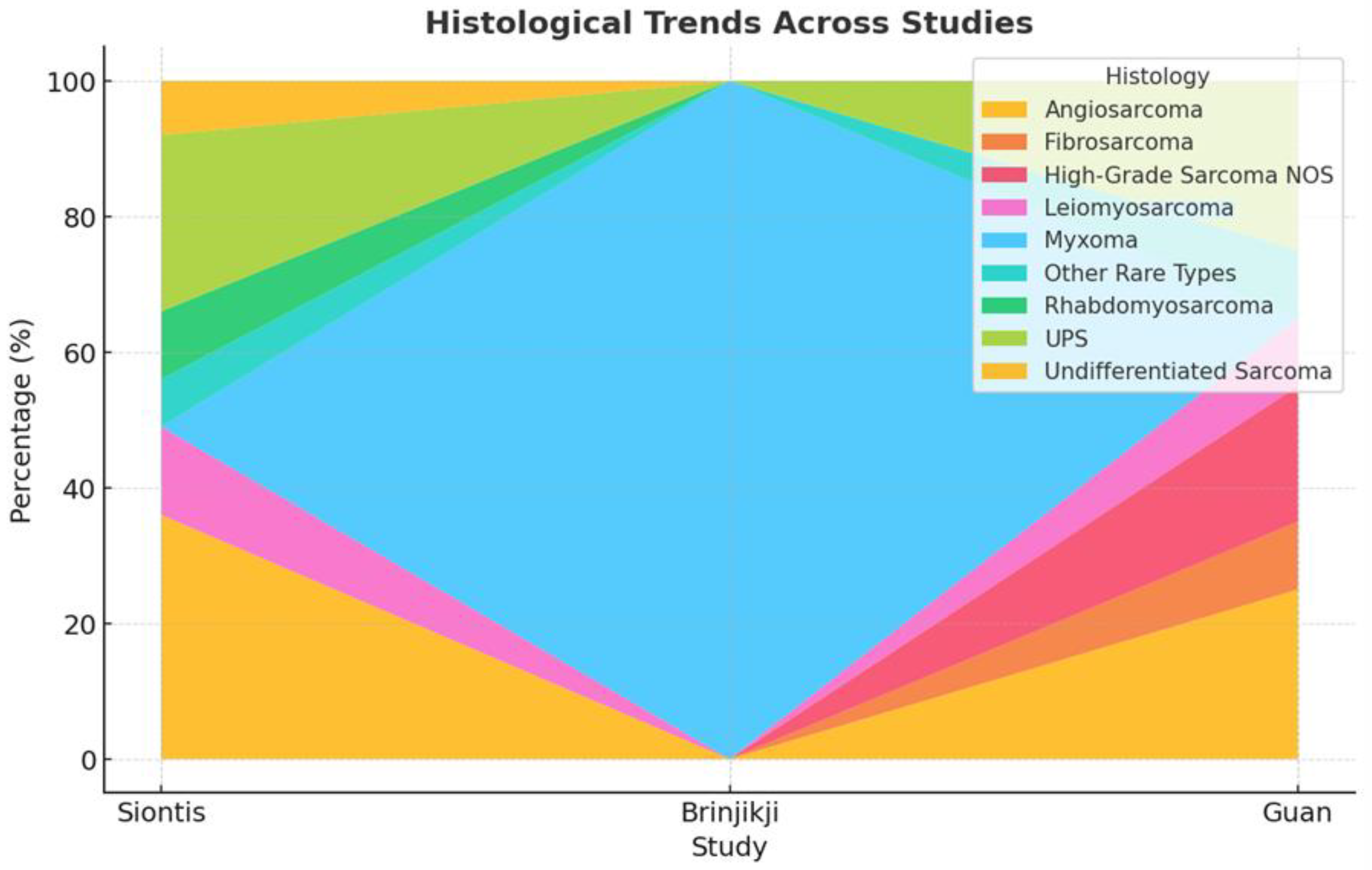
3.3. Histopathological Distribution
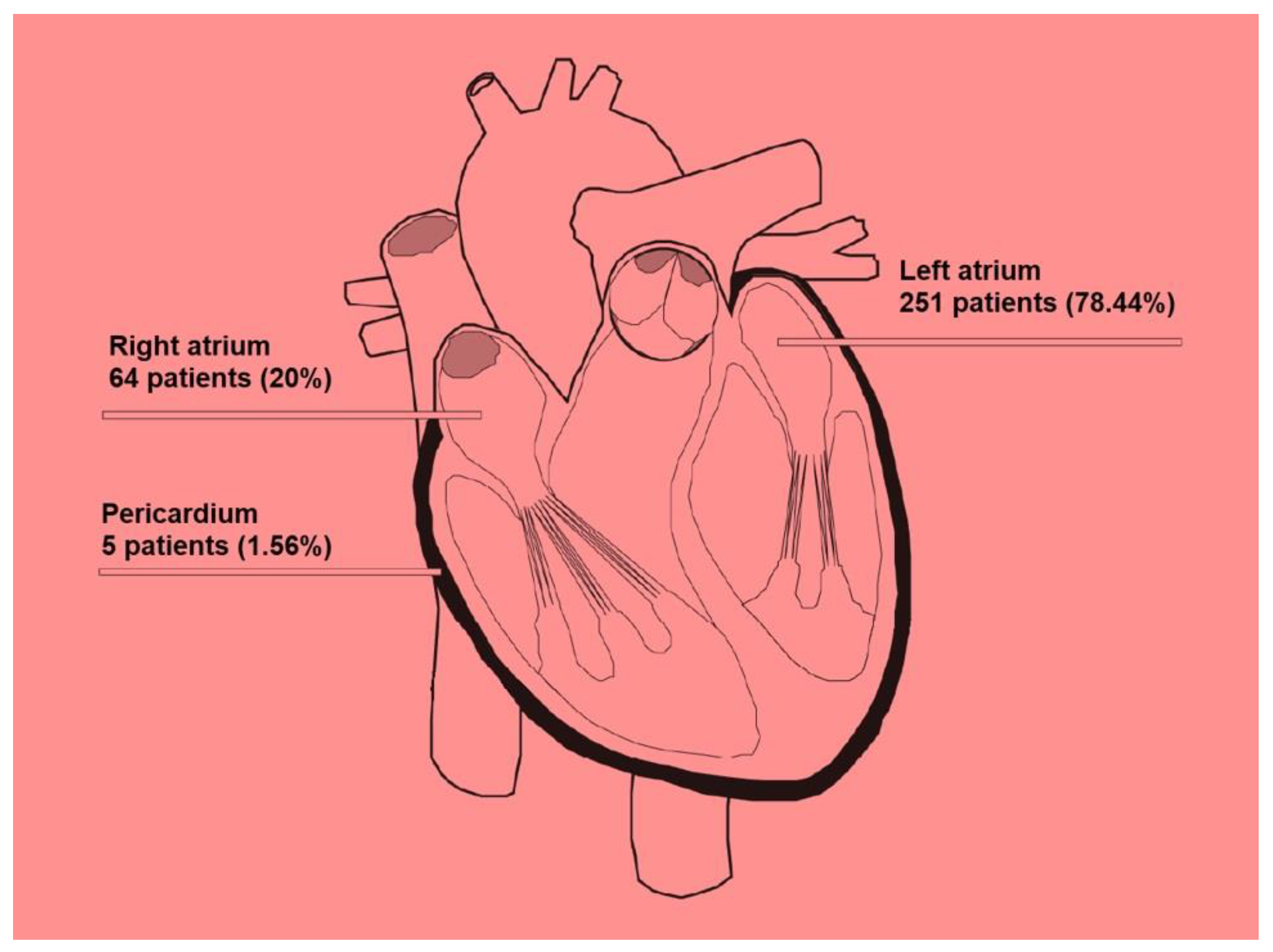
3.4. Location of Cardiac Tumors and Propensity for Metastasis
3.5. Neurological Manifestations and Lesion Characteristics
3.6. Therapeutic Strategies
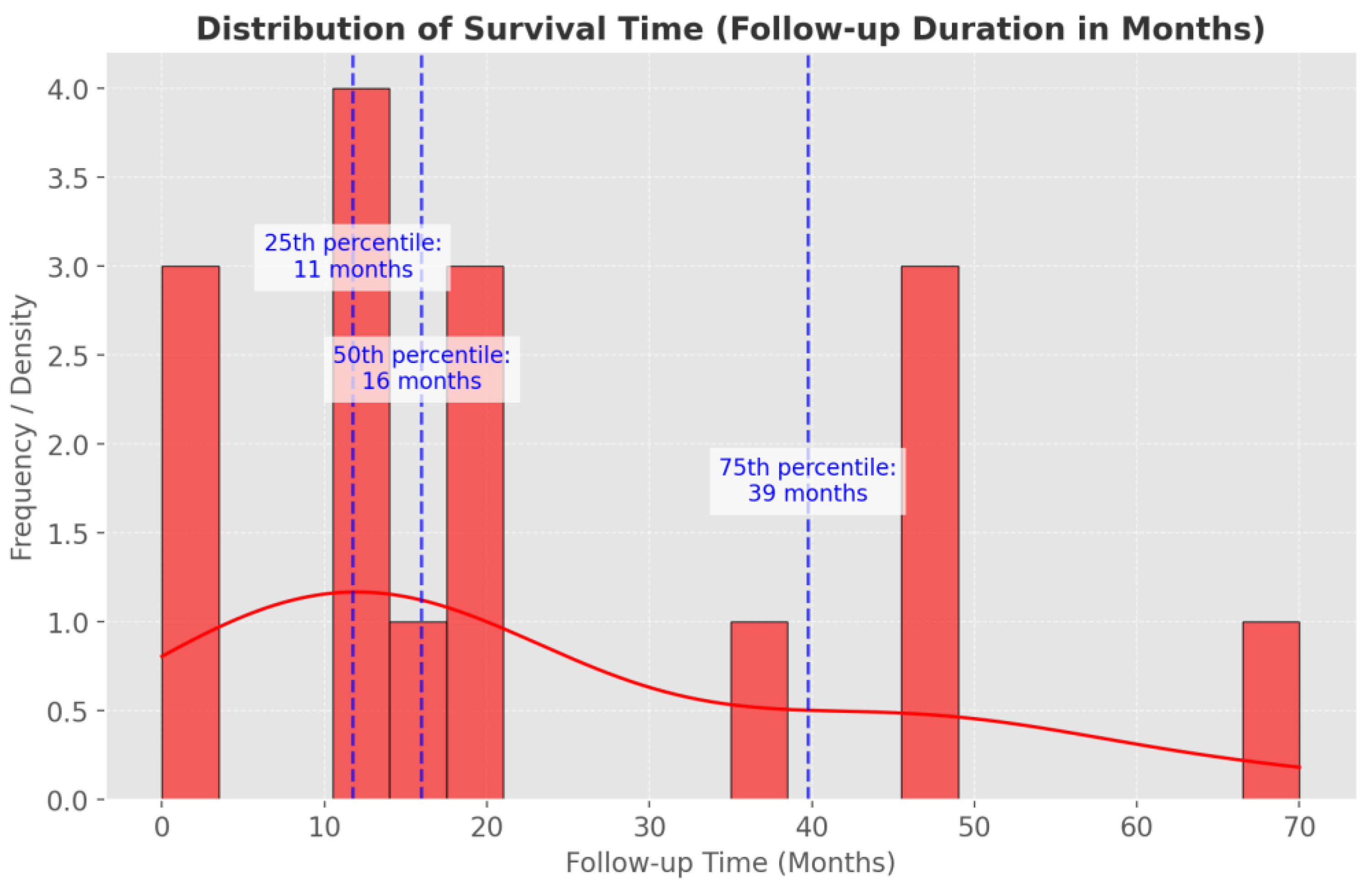
3.7. Prognosis and Follow-Up
4. Discussion
4.1. Pathways of Spread
4.2. Histopathology Relevant to Brain Dissemination
4.3. Heterogeneity in Tumor Histology and Metastatic Patterns
4.4. Imaging Features of Brain Metastases and Cardiac Tumors
4.4.1. Brain Imaging
4.4.2. Cardiac Imaging
4.5. Polyneoplastic Syndromes Involving Both Systems
4.6. Treatment Strategies and Prognostic Considerations
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reynen, K. Frequency of primary tumors of the heart. Am. J. Cardiol. 1996, 77, 107. [Google Scholar] [CrossRef] [PubMed]
- Klatt, E.C.; Heitz, D.R. Cardiac metastases. Cancer 1990, 65, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, F.; Bussani, R.; Pavletic, N.; Mannone, T. Metastases of the heart and pericardium. G. Ital. Cardiol. 1997, 27, 1252–1255. [Google Scholar] [PubMed]
- Butany, J.; Yu, W. Cardiac angiosarcoma: Two cases and a review of the literature. Can. J. Cardiol. 2000, 16, 197–205. [Google Scholar]
- De Rosa, A.F.; Cecchin, G.V.; Kujaruk, M.R.; Gayet, E.G.; Grasso, L.E.; Rigou, D.G. Malignant mesothelioma of the pericardium. Medicina 1994, 54, 49–52. [Google Scholar]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2019, 20, 26–41. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain metastases. Nat. Rev. Dis. Prim. 2019, 5, 1–26. [Google Scholar] [CrossRef]
- Siontis, B.L.; Zhao, L.; Leja, M.; McHugh, J.B.; Shango, M.M.; Baker, L.H.; Schuetze, S.M.; Chugh, R. Primary Cardiac Sarcoma: A Rare, Aggressive Malignancy with a High Propensity for Brain Metastases. Sarcoma 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Lemasle, M.; Badie, Y.L.; Cariou, E.; Fournier, P.; Porterie, J.; Rousseau, H.; Petermann, A.; Hitzel, A.; Carrié, D.; Galinier, M.; et al. Contribution and performance of multimodal imaging in the diagnosis and management of cardiac masses. Int. J. Cardiovasc. Imaging 2020, 36, 971–981. [Google Scholar] [CrossRef]
- Ashraf, M.; Jahangir, A.; Jan, M.F.; Muthukumar, L.; Neitzel, G.; Tajik, A.J. Case report: Metastatic melanoma masquerading as apical hypertrophic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 993631. [Google Scholar] [CrossRef]
- Bishop, A.J.; Zheng, J.; Subramaniam, A.M.; Ghia, A.J.; Wang, C.; McGovern, S.L.; Patel, S.; Guadagnolo, B.A.; Mitra, D.; Farooqi, A.; et al. Cardiac Angiosarcomas. Am. J. Clin. Oncol. 2022, 45, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Badaloni, F.; Pozzati, E.; Marucci, G.; Fiaschi, P.; Fioravanti, A. Primary Cardiac High-grade Myxofibrosarcoma Presenting with Multiple Brain Metastases: A Case Report. Cureus 2017, 9, e1866. [Google Scholar] [CrossRef]
- Brinjikji, W.; Morris, J.M.; Brown, R.D.; Thielen, K.R.; Wald, J.T.; Giannini, C.; Cloft, H.J.; Wood, C.P. Neuroimaging Findings in Cardiac Myxoma Patients: A Single-Center Case Series of 47 Patients. Cerebrovasc. Dis. 2015, 40, 35–44. [Google Scholar] [CrossRef]
- Pasalic, D.; Hegerova, L.T.; Gonsalves, W.I.; Robinson, S. An Insidious Cardiac Sarcoma Presenting with Progressive Neurologic Dysfunction. Rare Tumors 2013, 5, 183–184. [Google Scholar] [CrossRef]
- Guan, T.; Wei, Q.; Tang, Y.; Zhao, H.; Lu, Z.; Feng, W.; Teng, Y.; Luo, Z.; Chi, K.; Ou, C.; et al. Metastatic patterns and prognosis of patients with primary malignant cardiac tumor. Front. Cardiovasc. Med. 2022, 9, 1009765. [Google Scholar] [CrossRef] [PubMed]
- Geyer, S.J. Rhabdomyosarcoma of the heart with cerebral metastases. West. J. Med. 1987, 147, 596–597. [Google Scholar] [PubMed]
- Dan, M.S.; Hodge, F.A.J. Osteogenic Sarcoma of the Left Atrium. Ann. Thorac. Surg. 1997, 63, 1766–1768. [Google Scholar] [CrossRef]
- Altundag, M.B.; Ertas, G.; Ucer, A.R.; Durmus, S.; Abanuz, H.; Calikoğlu, T.; Ozbagi, K.; Demirkasimoglu, A.; Kaya, B.; Bakkal, B.H.; et al. Brain Metastasis of Cardiac Myxoma: Case Report and Review of the Literature. J. Neuro-Oncology 2005, 75, 181–184. [Google Scholar] [CrossRef]
- Ikeya, E.; Taguchi, J.; Yamaguchi, M.; Shibuya, M.; Kanabuchi, K. Primary cardiac angiosarcoma: Presenting with cardiac tamponade followed by cerebral hemorrhage with brain metastases. Jpn. J. Thorac. Cardiovasc. Surg. 2006, 54, 528–531. [Google Scholar] [CrossRef]
- Badrisyah, I.; Saiful, R.; Rahmat, H.; Naik, V.R.; Tan, Y.C. Brain Metastasis of Atrial Myxoma: Case report. Med. J. Malays. 2012, 67, 613–615. [Google Scholar]
- Radoi, M.P.; Stefanescu, F.; Arsene, D. Brain metastases and multiple cerebral aneurysms from cardiac myxoma: Case report and review of the literature. Br. J. Neurosurg. 2012, 26, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Jurek, M.; Hoffman-Zacharska, D.; Koziorowski, D.; Mądry, J.; Friedman, A.; Bal, J. Intrafamilial variability of the primary dystonia DYT6 phenotype caused by p.Cys5Trp mutation in THAP1 gene. Neurol. i Neurochir. Polska 2014, 48, 254–257. [Google Scholar] [CrossRef]
- Rose, D.; Papa, A.; Tomao, S.; Greco, E.; Zacharias, J. Cerebral Metastases in Patients with Left Atrial Myxoma. J. Card. Surg. 2016, 31, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Roque, A.; Kimbrough, T.; Traner, C.; Baehring, J.M.; Huttner, A.; Adams, J.; Canosa, S.; Sklar, J.; Madri, J.A. Somatic PRKAR1A mutation in sporadic atrial myxoma with cerebral parenchymal metastases: A case report. J. Med Case Rep. 2019, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Du, H.; Zhang, L.; Guo, S.; Xu, L.; Li, Y.; He, H.; Zhou, L.; Chen, Y.; Mao, L.; et al. Multiple cerebral metastases and metastatic aneurysms in patients with left atrial Myxoma: A case report. BMC Neurol. 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Maas, J.A.; Menes, M.; Siomin, V. Cardiac Myxoma with Cerebral Metastases and Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: A Case Report and Review. J. Neurol. Surg. Rep. 2020, 81, e1–e6. [Google Scholar] [CrossRef]
- Shah, Z.; Shehzad, M.; Saeed, F.; Bakhshi, S.K.; Shamim, M.S. Primary cardiac sarcomas: Risk of brain metastases and management. J. Pak. Med. Assoc. 2023, 73, 939–941. [Google Scholar] [CrossRef]
- Basu, A.; Grindrod, N.; Dar, A.R.; Leung, A.; Cecchini, M.; Lock, M. Left atrial myxoma metastasizing to the brain: A case report and review of literature. Radiat. Oncol. J. 2024, 42, 330–334. [Google Scholar] [CrossRef]
- Solís-Gómez, R.; Dávalos Cabral, N.; Arrieta Limón, G.; Hurtado Presa, B.A.; Salgado Alvear, A.; Reyes Martínez, L.M.; Serrano Arias, F.E. Brain metastases in a patient with antecedent of cardiac myxoma: A case report and review of literature. Rev. Fac. Cien. Med. Univ. Nac. Cordoba 2024, 81, 783–792. [Google Scholar] [CrossRef]
- Hădăreanu, D.R.; Berceanu, M.C.; Stroescu, R.D.; Militaru, S.; Militaru, C.; Hădăreanu, C.D.; Raicea, V.C.; Şoşea, N.I.; Boldu, E.O.; Mirea, O.C.M.; et al. Intracardiac mass presenting as acute myocardial infarction. Romanian J. Morphol. Embryol. 2024, 64, 579–585. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Yin, Z.; Hu, T.; Ren, J.; Wei, J.; Xie, L.; Xiong, J.; Wu, H.; Dai, X.; et al. Right atrial epithelioid angiosarcoma with multiple pulmonary metastasis confirmed by multimodality imaging-guided pulmonary biopsy. Medicine 2018, 97, e11588. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, A.M. Multiple Metastatic Intracranial Lesions Associated with Left Atrial Myxoma. Pol. J. Radiol. 2014, 79, 262–267. [Google Scholar] [CrossRef]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pr. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef]
- Grazzini, I.; Venezia, D.; Del Roscio, D.; Chiarotti, I.; Mazzei, M.A.; Cerase, A. Morphological and Functional Neuroradiology of Brain Metastases. Semin. Ultrasound CT MRI 2023, 44, 170–193. [Google Scholar] [CrossRef] [PubMed]
- Khalafallah, A.M.; Huq, S.; Jimenez, A.E.; Brem, H.; Mukherjee, D. The 5-factor modified frailty index: An effective predictor of mortality in brain tumor patients. J. Neurosurg. 2020, 135, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Hayashida, Y.; Hirai, T.; Morishita, S.; Kitajima, M.; Murakami, R.; Korogi, Y.; Makino, K.; Nakamura, H.; Ikushima, I.; Yamura, M.; et al. Diffusion-weighted Imaging of Metastatic Brain Tumors: Comparison with Histologic Type and Tumor Cellularity. Am. J. Neuroradiol. 2006, 27, 1419–1425. [Google Scholar]
- Sun, Y.-P.; Wang, X.; Gao, Y.-S.; Zhao, S.; Bai, Y. Primary cardiac sarcoma complicated with cerebral infarction and brain metastasis: A case report and literature review. Cancer Biomark. 2017, 21, 247–250. [Google Scholar] [CrossRef]
- Zhang, E.; Farag, S.; Dietz, H.; Wang, D.; Hirbe, A.; Ganjoo, K.; Van Tine, B.; Zaid, S.; Miah, A.; Keedy, V.; et al. Brain Metastases in Sarcomas: A Multicenter Retrospective Cohort Study. Cancers 2024, 16, 3760. [Google Scholar] [CrossRef]
- Huffaker, T.; Pak, S.; Asif, A.; Otchere, P. Tricuspid mass-curious case of Li-Fraumeni syndrome: A case report. World J. Clin. Cases 2024, 12, 1936–1939. [Google Scholar] [CrossRef]
- Jünger, S.T.; Pennig, L.; Schödel, P.; Goldbrunner, R.; Friker, L.; Kocher, M.; Proescholdt, M.; Grau, S. The Debatable Benefit of Gross-Total Resection of Brain Metastases in a Comprehensive Treatment Setting. Cancers 2021, 13, 1435. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, A.M.; Moschos, S.J.; Anders, C.K.; Glaubiger, S.B.; Collichio, F.A.; Lee, C.B.; Castillo, M.; Lee, Y.Z. Use of Susceptibility-Weighted Imaging (SWI) in the Detection of Brain Hemorrhagic Metastases from Breast Cancer and Melanoma. J. Comput. Assist. Tomogr. 2016, 40, 803–805. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; MacDonald, R.J.; Sorajja, P.; Edwards, W.D.; Ommen, S.R.; Klarich, K.W. Papillary Fibroelastomas in 19 Patients with Hypertrophic Cardiomyopathy Undergoing Septal Myectomy. J. Am. Soc. Echocardiogr. 2010, 23, 595–598. [Google Scholar] [CrossRef]
- Soon, G.; Ow, G.W.; Chan, H.L.; Ng, S.B.; Wang, S. Primary cardiac diffuse large B-cell lymphoma in immunocompetent patients: Clinical, histologic, immunophenotypic, and genotypic features of 3 cases. Ann. Diagn. Pathol. 2016, 24, 40–46. [Google Scholar] [CrossRef]
- Laura, D.M.; Donnino, R.; Kim, E.E.; Benenstein, R.; Freedberg, R.S.; Saric, M. Lipomatous Atrial Septal Hypertrophy: A Review of Its Anatomy, Pathophysiology, Multimodality Imaging, and Relevance to Percutaneous Interventions. J. Am. Soc. Echocardiogr. 2016, 29, 717–723. [Google Scholar] [CrossRef]
- Derks, S.H.A.E.; van der Veldt, A.A.M.; Smits, M. Brain metastases: The role of clinical imaging. Br. J. Radiol. 2021, 95, 20210944. [Google Scholar] [CrossRef]
- Wu, Z.; Mittal, S.; Kish, K.; Yu, Y.; Hu, J.; Haacke, E.M. Identification of calcification with MRI using susceptibility-weighted imaging: A case study. J. Magn. Reson. Imaging 2008, 29, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Tyebally, S.; Chen, D.; Bhattacharyya, S.; Mughrabi, A.; Hussain, Z.; Manisty, C.; Westwood, M.; Ghosh, A.K.; Guha, A. Cardiac Tumors. JACC: CardioOncology 2020, 2, 293–311. [Google Scholar] [CrossRef]
- Meng, J.; Zhao, H.; Liu, Y.; Chen, D.; Hacker, M.; Wei, Y.; Li, X.; Zhang, X.; Kreissl, M.C. Assessment of cardiac tumors by 18F-FDG PET/CT imaging: Histological correlation and clinical outcomes. J. Nucl. Cardiol. 2020, 28, 2233–2243. [Google Scholar] [CrossRef]
- Morris, S.M.; Gupta, A.; Kim, S.; Foraker, R.E.; Gutmann, D.H.; Payne, P.R.O. Predictive Modeling for Clinical Features Associated With Neurofibromatosis Type 1. Neurol. Clin. Pr. 2021, 11, 497–505. [Google Scholar] [CrossRef]
- Muto, M.; Frauenfelder, G.; Senese, R.; Zeccolini, F.; Schena, E.; Giurazza, F.; Jäger, H.R. Dynamic susceptibility contrast (DSC) perfusion MRI in differential diagnosis between radionecrosis and neoangiogenesis in cerebral metastases using rCBV, rCBF and K2. La Radiol. Med. 2018, 123, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Palaskas, N.; Thompson, K.; Gladish, G.; Agha, A.M.; Hassan, S.; Iliescu, C.; Kim, P.; Durand, J.B.; Lopez-Mattei, J.C. Evaluation and Management of Cardiac Tumors. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, B.; Li, J.; Sang, S.; Zhang, W. Identification of multiple cardiac metastases from nonsmall-cell lung cancer by 18F-FDG PET/CT. Medicine 2018, 97, e12868. [Google Scholar] [CrossRef]
- Noda, R.; Akabane, A.; Kawashima, M.; Oshima, A.; Tsunoda, S.; Segawa, M.; Inoue, T. Fractionated Gamma Knife radiosurgery after cyst aspiration for large cystic brain metastases: Case series and literature review. Neurosurg. Rev. 2022, 45, 3457–3465. [Google Scholar] [CrossRef]
- Stratakis, C.A.; Kirschner, L.S.; Carney, J.A. Clinical and Molecular Features of the Carney Complex: Diagnostic Criteria and Recommendations for Patient Evaluation. J. Clin. Endocrinol. Metab. 2001, 86, 4041–4046. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Singh, A.K. Neurofibromatosis Type 2. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, 2025. PMID: 292. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Jozwiak, S.; Curatolo, P. Editorial: Tuberous Sclerosis Complex—Diagnosis and Management. Front. Neurol. 2021, 12, 755868. [Google Scholar] [CrossRef]
- Pope, W.B. Brain metastases: Neuroimaging. In Brain Metastases; Graff, J., Ed.; Springer: Berlin/Heilderberg, Germany, 2018; pp. 89–112. [Google Scholar]
- Proescholdt, M.A.; Schödel, P.; Doenitz, C.; Pukrop, T.; Höhne, J.; Schmidt, N.O.; Schebesch, K.-M. The Management of Brain Metastases—Systematic Review of Neurosurgical Aspects. Cancers 2021, 13, 1616. [Google Scholar] [CrossRef]
- Ramos, A.; Giantini-Larsen, A.; Pannullo, S.C.; Brandmaier, A.; Knisely, J.; Magge, R.; A Wilcox, J.; Pavlick, A.C.; Ma, B.; Pisapia, D.; et al. A multidisciplinary management algorithm for brain metastases. Neuro-Oncol. Adv. 2022, 4, vdac176. [Google Scholar] [CrossRef]
- Qureshi, H.M.; Tabor, J.K.; Pickens, K.; Lei, H.; Vasandani, S.; Jalal, M.I.; Vetsa, S.; Elsamadicy, A.; Marianayagam, N.; Theriault, B.C.; et al. Frailty and postoperative outcomes in brain tumor patients: A systematic review subdivided by tumor etiology. J. Neuro-Oncol. 2023, 164, 299–308. [Google Scholar] [CrossRef]
- Frappaz, D.; Bonneville-Levard, A.; Ricard, D.; Carrie, S.; Schiffler, C.; Xuan, K.H.; Weller, M. Assessment of Karnofsky (KPS) and WHO (WHO-PS) performance scores in brain tumour patients: The role of clinician bias. Support. Care Cancer 2020, 29, 1883–1891. [Google Scholar] [CrossRef]
- Gong, W.; Jiang, T.; Zuo, D. Recurrence benefit from supramarginal resection in brain metastases of lung adenocarcinoma. Heliyon 2022, 8, e10109. [Google Scholar] [CrossRef]
- Shiue, K.; Sahgal, A.; Lo, S.S. Precision Radiation for Brain Metastases with a Focus on Hypofractionated Stereotactic Radiosurgery. Semin. Radiat. Oncol. 2023, 33, 114–128. [Google Scholar] [CrossRef]
- Singh, S.K.; Agris, J.M.; Leeds, N.E.; Ginsberg, L.E. Intracranial Leptomeningeal Metastases: Comparison of Depiction at FLAIR and Contrast-enhanced MR Imaging. Radiology 2000, 217, 50–53. [Google Scholar] [CrossRef]
- Soffietti, R.; Ahluwalia, M.; Lin, N.; Rudà, R. Management of brain metastases according to molecular subtypes. Nat. Rev. Neurol. 2020, 16, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Giammalva, G.R.; Gagliardo, C.; Marrone, S.; Paolini, F.; Gerardi, R.M.; Umana, G.E.; Yağmurlu, K.; Chaurasia, B.; Scalia, G.; Midiri, F.; et al. Focused Ultrasound in Neuroscience. State of the Art and Future Perspectives. Brain Sci. 2021, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.P.; Paleologos, N.A.; Mikkelsen, T.; Robinson, P.D.; Ammirati, M.; Andrews, D.W.; Asher, A.L.; Burri, S.H.; Cobbs, C.S.; Gaspar, L.E.; et al. The role of chemotherapy in the management of newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neuro-Oncol. 2009, 96, 71–83. [Google Scholar] [CrossRef]
- Peereboom, D.M. Chemotherapy in Brain Metastases. Neurosurgery 2005, 57, S4–S54. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current approaches to the management of brain metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef]
- Petit, C.; Tallet, A. Brain metastases reirradiation. Cancer Radiother. 2024, 28, 538–546. [Google Scholar] [CrossRef]
- Sung, K.S. Clinical Practice Guidelines for Brain Metastasis from Solid Tumors. Brain Tumor Res. Treat. 2024, 12, 14–22. [Google Scholar] [CrossRef]
- Pibarot, P.; Dumesnil, J.G. Prosthetic Heart Valves. Circulation 2009, 119, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Brozzi, N.; Cifuentes, R.; Ghodsizad, A.; Loebe, M. Application of total artificial heart in patients with primary malignant cardiac tumors—current treatment strategies. Ann. Cardiothorac. Surg. 2020, 9, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Frappaz, D.; Taillandier, L.; Levard-Bonneville, A.; Sore, J.; Ricard, D.; Schiffler, C.; Weller, M. P05.28 Karnofsky performance score of brain tumor patients depends on clinician status. Neuro-Oncology 2018, 20, iii309. [Google Scholar] [CrossRef]
- Totaro, P.; Musto, M. Overall approaches to cardiac tumors: Still an unsolved enigma? World J. Clin. Cases 2024, 12, 3654–3656. [Google Scholar] [CrossRef]
| Author | Year | Patient Number | Gender/ Age | Clinical Presentation | Histological Type | Cardiac Topography | Brain Symptoms | Brain Topography | Treatment | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Geyer SJ et al. [16] | 1987 | 1 | M/23 | Cough; pericardial effusion | RMS | RA | Pulmonary sx | NS | NS | NS |
| Dan S et al. [17] | 1997 | 1 | F/32 | Right hemiparesis; TIA | OsteoS | LA | Generalized | Brain | Atrial recon; valve repl | 18 mo |
| Altundag MB et al. [18] | 2005 | 1 | F/41 | Palpitations; HA; vertigo; seizures | Myx | LA | HA; vertigo; seizures | Cerebrum; cerebellum | Surg; WBRT (30 Gy) | 48 mo, NED |
| Ikeya E et al. [19] | 2006 | 1 | M/49 | SOB; tamponade | AS | RA | Hemorrhage; hemiparesis | RT lobe | Pericardiotomy; symptomatic | Died, 37 days |
| Idris B et al. [20] | 2012 | 1 | F/15 | HA; nausea; vomiting | Myx | LA | HA; vomiting | R parietal; occipital | Craniotomy | 12 mo, NED |
| Radoi MP et al. [21] | 2012 | 1 | M/45 | HA; gait issues; seizures | Myx | LA | Intra-cr. Tumors; aneurysms | Parietal; frontal | Surgery | 12 mo; NED |
| Pasalic D et al. [14] | 2013 | 1 | F/45 | HA; mental changes; somnolence | UPS | LA | Multiple lesions | Frontal; parietal | Radiosurg; atrial surg | Progression |
| Kierdaszuk B et al. [22] | 2014 | 1 | F/41 | Limb weakness | Myx | LA | Hemorrhagic lesions | Parietal; occipital | Surgery | 18 mo; stable |
| Brinjikji W et al. [13] | 2015 | 47 | Mixed/50 | Arm weakness; visual changes | Myx | LA | Infarcts; aneurysms | Multifocal cortical | Surg; conservative | Long-term; mild deficits |
| Rose D et al. [23] | 2016 | 1 | M/44 | Light-headed; speech disturbance | Myx | LA | Aneurysms | 4th ventricle | WBRT; steroids | 14 mo, died |
| Badaloni F et al. [12] | 2017 | 1 | M/41 | Partial seizures; hemiparesis | MyxoS | Mitral valve; LA | Seizures; aphasia | Parietal, rolandic; cerebellum | Craniotomy | Died; 70 days |
| Roque A et al. [24] | 2019 | 1 | F/48 | HA; fatigue; numbness | Myx | LA | Hemorrhage; pseudoaneurysms | Parietal, frontal, occipital | WBRT; HS | 18 mo; stable |
| Siontis BL et al. [8] | 2019 | 39 | Mixed/41 | Dyspnea; CP; edema | AS, others | LA (46%), RA (41%) | Brain mets (31%) | Multifocal | Surg; CT; RT | Median OS 12.1 mo |
| Wan Y et al. [25] | 2019 | 1 | F/39 | HA; blurred vision; vomiting | Myx | LA | Hemorrhage; aneurysms | MCA, ACA terr. | Craniotomy, clip | 11 mo; recovery |
| Maas JA et al. [26] | 2020 | 1 | M/62 | TIA; arm numbness; vision | Myx | LA | Hemorrhage; edema | R parieto-occipital | Brain surg | 48 mo; NED |
| Guan T et al. [15] | 2022 | 218 | Mixed/Var. | Cardiac mass sx | Sarcoma | LA/RA | Rare | Parenchyma | Surg (some) | Poor if brain mets |
| Shah Z et al. [27] | 2023 | 1 | M/37 | Neuro sx; hemorrhage | AS | RA | Hemorrhagic lesions | R frontal lobe | Radiosurg; systemic | 12 mo; died |
| Basu A et al. [28] | 2024 | 1 | F/56 | HA; arm weakness; vision | Myx | LA | Hemorrhagic lesions | Multiple sites | WBRT (20 Gy/5 fx) | 48 mo; recovery |
| Solís-Gómez R et al. [29] | 2024 | 1 | F/54 | Dizziness; seizures | Myx | LA | Visual sx; seizures | Occipital; multiple | Surg; valve repl; craniotomy | 3 yr; NED |
| Tumor Type | Echocardiography (TTE/TEE) | Cardiac Magnetic Resonance (CMR) | Computed Tomography (CT) | Positron Emission Tomography/CT (PET/CT) |
|---|---|---|---|---|
| Myx | Mobile mass LA (fossa ovalis); isoechoic | Hyperintense SSFP/T2; iso T1; hetero CE | Well-defined; ±calcifications; no CE | Mild/absent uptake |
| Rhabdo | Hyperechoic; intramural or protruding | Iso/hyper SSFP/T2; iso T1; no CE | Homogeneous; hypodense | Mild/absent |
| Lip | Hyperechoic (intracav); hypoechoic (pericard) | Hyper SSFP/T1; hypo T2; no CE | Homogeneous; hypodense | Mild/absent |
| Fibro | Small, mobile, and valve-attached mass | Hypo SSFP/T1/T2; no CE | Hypodense mass | None |
| Hema | Mass from myocardium or pericardium | Hyper SSFP/T2; iso/hetero T1; CE+ | Well-defined margins | Mild/absent |
| AS | Irreg., immobile, infiltrative RA mass, and PEff | Iso/hyper SSFP; hetero T1; hyper T2; strong CE | Infiltrative; into pericardium/cavity | High |
| RMS | Immobile and infiltrative mass | Iso/hyper SSFP; hetero T1; hyper T2; CE+ | Infiltrative/protruding mass | High |
| DLBCL | ‘Cauliflower-like’ and all chambers | Iso/hyper SSFP; hetero T1; hyper T2; CE+ | Hypodense; irreg/well-defined | High |
| Met | Multiple lesions and often PEff | Iso/hyper SSFP; hetero T1; hyper T2; CE+ | Hypodense; CE varies | High (aggressive dep.) |
| Thrombus | Hyperechoic, akinetic zones, and no CE | Hypo SSFP/T1; hyper T2; no CE | Filling defect; no CE | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrone, S.; Gueli, I.A.; Lo Coco, R.; Scalia, L.; Rizzica, S.; Baiamonte, G.; Costanzo, R.; Rubino, A.S.; Ferini, G.; Umana, G.E.; et al. Brain Metastases from Primary Cardiac Tumors: A Systematic Review of Diagnosis, Treatment, and Prognosis. Cancers 2025, 17, 1621. https://doi.org/10.3390/cancers17101621
Marrone S, Gueli IA, Lo Coco R, Scalia L, Rizzica S, Baiamonte G, Costanzo R, Rubino AS, Ferini G, Umana GE, et al. Brain Metastases from Primary Cardiac Tumors: A Systematic Review of Diagnosis, Treatment, and Prognosis. Cancers. 2025; 17(10):1621. https://doi.org/10.3390/cancers17101621
Chicago/Turabian StyleMarrone, Salvatore, Ignazio Alessio Gueli, Roberta Lo Coco, Lorenzo Scalia, Salvatore Rizzica, Giuliana Baiamonte, Roberta Costanzo, Antonino Salvatore Rubino, Gianluca Ferini, Giuseppe Emmanuele Umana, and et al. 2025. "Brain Metastases from Primary Cardiac Tumors: A Systematic Review of Diagnosis, Treatment, and Prognosis" Cancers 17, no. 10: 1621. https://doi.org/10.3390/cancers17101621
APA StyleMarrone, S., Gueli, I. A., Lo Coco, R., Scalia, L., Rizzica, S., Baiamonte, G., Costanzo, R., Rubino, A. S., Ferini, G., Umana, G. E., & Scalia, G. (2025). Brain Metastases from Primary Cardiac Tumors: A Systematic Review of Diagnosis, Treatment, and Prognosis. Cancers, 17(10), 1621. https://doi.org/10.3390/cancers17101621










