Current and Future Developments in Radiation Oncology Approach for Rhabdomyosarcoma
Simple Summary
Abstract
1. Introduction
2. Radiotherapy Management
2.1. Radiotherapy Dose Escalation
2.2. Timing of Radiotherapy
2.3. Radiotherapy to Metastatic Sites
2.4. Definition of Radiotherapy Target Volumes and Margins in FaR-RMS
2.4.1. GTV
2.4.2. CTV
- -
- GTVp_pre to CTVp_pre: 1 cm.
- -
- For extremity primary tumor sites, superior and inferior CTV margins of 2 cm are required, with 1 cm expansion circumferentially.
- -
- Skin, scar, drain, or biopsy sites should not be included in the CTVp, except in cases of involvement with gross tumor.
- -
- GTVp_post to CTVp_post: 0.5 cm.
- -
- For tumors arising adjacent to body cavities (e.g., thorax, abdomen, pelvis) that extend or ‘push’ into the cavity but do not infiltrate adjacent organs or tissues, the GTVp should only be expanded, by 1 cm (GTVp_pre) or 0.5 cm (GTVp_post), in the direction of potential infiltration, and there should be no extension of the CTVp into the adjacent, uninvolved body cavity.
- -
- GTVn to CTVn: 3 cm superiorly and inferiorly (or in the direction of nodal drainage), and circumferentially to include adjacent lymph nodes in the anatomically constrained lymph node site. Wherever possible, displaced normal tissue should be excluded from the CTVn. In cases of uncertainty, or particular concern, about the exact extent of nodal involvement at diagnosis, an involved field concept should be used.
- -
- For bulky residual involved lymph nodes, GTVn_post to CTVn_post: 0.5 cm.
2.4.3. ITV
2.4.4. PTV
2.5. Dose Prescription in FaR-RMS
2.5.1. Primary Tumor
2.5.2. Involved Lymph Nodes
2.5.3. Metastases
- -
- Age ≥ 10 y.
- -
- Extremity, other, unidentified primary site.
- -
- Bone and/or bone marrow metastatic involvement.
- -
- ≥3 metastatic organ sites.
2.6. Palliative Radiotherapy
3. Advanced External Beam Radiotherapy Techniques and Other Modalities
3.1. Stereotactic Body Radiotherapy
3.2. Particle Therapy
3.3. Brachytherapy
3.4. Motion Management
3.5. Magnetic Resonance Imaging-Guided Linear Accelerator (MRI-LINAC) Radiotherapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ferrari, A.; Dileo, P.; Casanova, M.; Bertulli, R.; Meazza, C.; Gandola, L.; Navarria, P.; Collini, P.; Gronchi, A.; Olmi, P.; et al. Rhabdomyosarcoma in adults: A retrospective analysis of 171 patients treated at a single institution. Cancer 2003, 98, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Sultan, I.; Qaddoumi, I.; Yaser, S.; Rodriguez-Galindo, C.; Ferrari, A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: An analysis of 2600 patients. J. Clin. Oncol. 2009, 27, 3391–3397. [Google Scholar] [CrossRef]
- Parham, D.M.; Ellison, D.A. Rhabdomyosarcomas in adults and children: An update. Arch. Pathol. Lab. Med. 2006, 130, 1454–1465. [Google Scholar] [CrossRef]
- Rudzinski, E.R.; Anderson, J.R.; Hawkins, D.S.; Skapek, S.X.; Parham, D.M.; Teot, L.A. The World Health Organization Classification of Skeletal Muscle Tumors in Pediatric Rhabdomyosarcoma: A Report from the Children’s Oncology Group. Arch. Pathol. Lab. Med. 2015, 139, 1281–1287. [Google Scholar] [CrossRef]
- Burke, M.; Anderson, J.R.; Kao, S.C.; Rodeberg, D.; Qualman, S.J.; Wolden, S.L.; Meyer, W.H.; Breitfeld, P.P. Assessment of response to induction therapy and its influence on 5-year failure-free survival in group III rhabdomyosarcoma: The Intergroup Rhabdomyosarcoma Study-IV experience—A report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J. Clin. Oncol. 2007, 25, 4909–4913. [Google Scholar] [PubMed]
- Rosenberg, A.R.; Anderson, J.R.; Lyden, E.; Rodeberg, D.A.; Wolden, S.L.; Kao, S.C.; Parham, D.M.; Arndt, C.; Hawkins, D.S. Early response as assessed by anatomic imaging does not predict failure-free survival among patients with Group III rhabdomyosarcoma: A report from the Children’s Oncology Group. Eur. J. Cancer 2014, 50, 816–823. [Google Scholar] [CrossRef]
- Oberlin, O.; Rey, A.; de Toledo, J.S.; Martelli, H.; Jenney, M.E.; Scopinaro, M.; Bergeron, C.; Merks, J.H.; Bouvet, N.; Ellershaw, C.; et al. Randomized comparison of intensified six-drug versus standard three-drug chemotherapy for high-risk nonmetastatic rhabdomyosarcoma and other chemotherapy-sensitive childhood soft tissue sarcomas: Long-term results from the International Society of Pediatric Oncology MMT95 study. J. Clin. Oncol. 2012, 30, 2457–2465. [Google Scholar] [PubMed]
- Oberlin, O.; Rey, A.; Anderson, J.; Carli, M.; Raney, R.B.; Treuner, J.; Stevens, M.C. Treatment of orbital rhabdomyosarcoma: Survival and late effects of treatment--results of an international workshop. J. Clin. Oncol. 2001, 19, 197–204. [Google Scholar] [CrossRef]
- Oberlin, O.; Rey, A.; Lyden, E.; Bisogno, G.; Stevens, M.C.; Meyer, W.H.; Carli, M.; Anderson, J.R. Prognostic factors in metastatic rhabdomyosarcomas: Results of a pooled analysis from United States and European cooperative groups. J. Clin. Oncol. 2008, 26, 2384–2389. [Google Scholar] [CrossRef]
- Carli, M.; Colombatti, R.; Oberlin, O.; Stevens, M.; Masiero, L.; Frascella, E.; Koscielniak, E.; Treuner, J.; Pinkerton, C.R. High-dose melphalan with autologous stem-cell rescue in metastatic rhabdomyosarcoma. J. Clin. Oncol. 1999, 17, 2796–2803. [Google Scholar] [CrossRef]
- Pappo, A.S.; Anderson, J.R.; Crist, W.M.; Wharam, M.D.; Breitfeld, P.P.; Hawkins, D.; Raney, R.B.; Womer, R.B.; Parham, D.M.; Qualman, S.J.; et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J. Clin. Oncol. 1999, 17, 3487–3493. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, G.; Jenney, M.; Bergeron, C.; Melcón, S.G.; Ferrari, A.; Oberlin, O.; Carli, M.; Stevens, M.; Kelsey, A.; De Paoli, A.; et al. Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet Oncol. 2018, 19, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Missiaglia, E.; Williamson, D.; Chisholm, J.; Wirapati, P.; Pierron, G.; Petel, F.; Concordet, J.-P.; Thway, K.; Oberlin, O.; Pritchard-Jones, K.; et al. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J. Clin. Oncol. 2012, 30, 1670–1677. [Google Scholar] [CrossRef]
- Skapek, S.X.; Anderson, J.; Barr, F.G.; Bridge, J.A.; Gastier-Foster, J.M.; Parham, D.M.; Rudzinski, E.R.; Triche, T.; Hawkins, D.S. PAX-FOXO1 fusion status drives unfavorable outcome for children with rhabdomyosarcoma: A children’s oncology group report. Pediatr. Blood Cancer 2013, 60, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.; Missiaglia, E.; de Reyniès, A.; Pierron, G.; Thuille, B.; Palenzuela, G.; Thway, K.; Orbach, D.; Laé, M.; Fréneaux, P.; et al. Fusion gene-negative alveolar rhabdomyosarcoma is clinically and molecularly indistinguishable from embryonal rhabdomyosarcoma. J. Clin. Oncol. 2010, 28, 2151–2158. [Google Scholar] [CrossRef]
- FaR-RMS: An Overarching Study for Children and Adults with Frontline and Relapsed RhabdoMyoSarcoma—Full Text View. Available online: https://clinicaltrials.gov/study/NCT04625907 (accessed on 9 May 2025).
- Casey, D.L.; Chi, Y.-Y.; Donaldson, S.S.; Hawkins, D.S.; Tian, J.; Arndt, C.A.; Rodeberg, D.A.; Routh, J.C.; Lautz, T.B.; Gupta, A.A.; et al. Increased local failure for patients with intermediate-risk rhabdomyosarcoma on ARST0531: A report from the Children’s Oncology Group. Cancer 2019, 125, 3242–3248. [Google Scholar] [CrossRef]
- Réguerre, Y.; Martelli, H.; Rey, A.; Rogers, T.; Gaze, M.; Ben Arush, M.W.; Devalck, C.; Oberlin, O.; Stevens, M.; Orbach, D. Local therapy is critical in localised pelvic rhabdomyosarcoma: Experience of the International Society of Pediatric Oncology Malignant Mesenchymal Tumor (SIOP-MMT) committee. Eur. J. Cancer 2012, 48, 2020–2027. [Google Scholar] [CrossRef]
- Bisogno, G.; De Salvo, G.L.; Bergeron, C.; Carli, M.; Ferrari, A.; Jenney, M.; Mercks, H.; Kelsey, A.; Gallego, S.; Chisholm, J.; et al. The role of doxorubicin in the treatment of rhabdomyosarcoma: Preliminary results from the EpSSG RMS 2005 study. Pediatr. Blood Cancer 2014, 61, S133–S134. [Google Scholar]
- Gallego, S.B.G.; Bergeron, C.; Carli, M.; Ferrari, A.; Jenney, M.; Martelli, H.; Gaze, M.; Oberlin, O.; Chisholm, J.; Merks, J.H.M.; et al. Alveolar rhabdomyosarcoma with nodal involvement, a group with unfavourable outcome: Experience of the European paediatric Soft Tissue Sarcoma Group (EpSSG). In Proceedings of the 46th Congress of the International Society of Paediatric Oncology (SIOP) 2014, Toronto, ON, Canada, 22–25 October 2014. [Google Scholar]
- Kelly, S.M.; Turcas, A.; Corning, C.; Bailey, S.; Cañete, A.; Clementel, E.; di Cataldo, A.; Dieckmann, K.; Gaze, M.N.; Horan, G.; et al. Radiotherapy quality assurance in paediatric clinical trials: First report from six QUARTET-affiliated trials. Radiother. Oncol. 2023, 182, 109549. [Google Scholar] [CrossRef]
- Kelly, S.M.; Effeney, R.; Gaze, M.N.; Bernier-Chastagner, V.; Blondeel, A.; Clementel, E.; Corning, C.; Dieckmann, K.; Essiaf, S.; Gandola, L.; et al. QUARTET: A SIOP Europe project for quality and excellence in radiotherapy and imaging for children and adolescents with cancer. Eur. J. Cancer 2022, 172, 209–220. [Google Scholar] [CrossRef]
- Available online: https://siope.eu/activities/joint-projects/quartet/ (accessed on 10 December 2024).
- Donaldson, S.S.; Meza, J.; Breneman, J.C.; Crist, W.M.; Laurie, F.; Qualman, S.J.; Wharam, M. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma—A report from the IRSG. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 718–728. [Google Scholar] [CrossRef]
- Michalski, J.M.; Meza, J.; Breneman, J.C.; Wolden, S.L.; Laurie, F.; Jodoin, M.; Raney, B.; Wharam, M.D.; Donaldson, S.S. Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in Intergroup Rhabdomyosarcoma Study Group trials II through IV. Int. J. Radiat. Oncol. 2004, 59, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, J.; Mandeville, H.; Adams, M.; Minard-Collin, V.; Rogers, T.; Kelsey, A.; Shipley, J.; van Rijn, R.R.; de Vries, I.; van Ewijk, R.; et al. Frontline and Relapsed Rhabdomyosarcoma (FaR-RMS) Clinical Trial: A Report from the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG). Cancers 2024, 16, 998. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Chisholm, J.C.; Jenney, M.; Minard-Colin, V.; Orbach, D.; Casanova, M.; Guillen, G.; Glosli, H.; van Rijn, R.R.; Schoot, R.A.; et al. Adolescents and young adults with rhabdomyosarcoma treated in the European paediatric Soft tissue sarcoma Study Group (EpSSG) protocols: A cohort study. Lancet Child. Adolesc. Health 2022, 6, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Weiss, A.R.; Chen, Y.-L.; Scharschmidt, T.J.; Chi, Y.-Y.; Tian, J.; Black, J.O.; Davis, J.L.; Fanburg-Smith, J.C.; Zambrano, E.; Anderson, J.; et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): A multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 1110–1122. [Google Scholar] [CrossRef]
- Seitz, G.; Dantonello, T.M.; Inform, C.I.D.; Blumenstock, G.; Godzinski, J.; Klingebiel, T.; Schuck, A.; Leuschner, I.; Koscielniak, E.; Fuchs, J.; et al. Treatment efficiency, outcome and surgical treatment problems in patients suffering from localized embryonal bladder/prostate rhabdomyosarcoma: A report from the Cooperative Soft Tissue Sarcoma trial CWS-96. Pediatr. Blood Cancer 2011, 56, 718–724. [Google Scholar] [CrossRef]
- Schoot, R.A.; Chisholm, J.C.; Casanova, M.; Minard-Colin, V.; Geoerger, B.; Cameron, A.L.; Coppadoro, B.; Zanetti, I.; Orbach, D.; Kelsey, A.; et al. Merks Metastatic Rhabdomyosarcoma: Results of the European Paediatric Soft Tissue Sarcoma Study Group MTS 2008 Study and Pooled Analysis with the Concurrent BERNIE Study. J. Clin. Oncol. 2022, 40, 3730–3740. [Google Scholar] [CrossRef]
- Cameron, A.; Chisholm, J.; Elze, M.C.; Casanova, M.; Geoerger, B.; Gaze, M.; Oberlin, O.; Bachir, J.; Fürst-Recktenwald, S.; Merks, J.H. Role of radiotherapy to primary/metastatic sites in pediatric patients with metastatic rhabdomyosarcoma in the BERNIE study. J. Clin. Oncol. 2017, 35, 10541. [Google Scholar] [CrossRef]
- Cameron, A.L.; Elze, M.C.; Casanova, M.; Geoerger, B.; Gaze, M.N.; Minard-Colin, V.; McHugh, K.; van Rijn, R.R.; Kelsey, A.; Martelli, H.; et al. The Impact of Radiation Therapy in Children and Adolescents with Metastatic Rhabdomyosarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 968–978. [Google Scholar] [CrossRef]
- Ferrari, A.; Bergamaschi, L.; Chiaravalli, S.; Livellara, V.; Sironi, G.; Nigro, O.; Puma, N.; Gattuso, G.; Morosi, C.; Gasparini, P.; et al. Metastatic rhabdomyosarcoma: Evidence of the impact of radiotherapy on survival. A retrospective single-centre experience. Pediatr. Blood Cancer 2022, 69, e29853. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.K.; Stinauer, M.; Albano, E.; Greffe, B.; Tello, T.; Maloney, K. Local control of metastatic sites with radiation therapy in metastatic Ewing sarcoma and rhabdomyosarcoma. Pediatr. Blood Cancer 2011, 57, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Skamene, S.; Abish, S.; Mitchell, D.; Freeman, C. Radiotherapy Is Important for Local Control at Primary and Metastatic Sites in Pediatric Rhabdomyosarcoma. Cureus 2015, 7, e388. [Google Scholar] [CrossRef]
- Rodeberg, D.; Arndt, C.; Breneman, J.; Lyden, E.; Donaldson, S.; Paidas, C.; Andrassy, R.; Meyer, W.; Wiener, E. Characteristics and outcomes of rhabdomyosarcoma patients with isolated lung metastases from IRS-IV. J. Pediatr. Surg. 2005, 40, 256–262. [Google Scholar] [CrossRef]
- Chisholm, J.C.; Schoot, R.A.; Cameron, A.L.; Casanova, M.; Minard-Colin, V.; Coppadoro, B.; Garrido, M.; Rogers, T.; Orbach, D.; Glosli, H.; et al. Outcomes in lung-only metastatic rhabdomyosarcoma: An analysis of data from the European paediatric Soft tissue sarcoma Study Group MTS 2008 study. EJC Paediatr. Oncol. 2023, 2, 100018. [Google Scholar] [CrossRef]
- Dantonello, T.M.; Winkler, P.; Boelling, T.; Friedel, G.; Schmid, I.; Mattke, A.C.; Ljungman, G.; Bielack, S.S.; Klingebiel, T.; Koscielniak, E.; et al. Embryonal rhabdomyosarcoma with metastases confined to the lungs: Report from the CWS Study Group. Pediatr. Blood Cancer 2011, 56, 725–732. [Google Scholar] [CrossRef]
- Rich, S.E.; Chow, R.; Raman, S.; Zeng, K.L.; Lutz, S.; Lam, H.; Silva, M.F.; Chow, E. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother. Oncol. 2018, 126, 547–557. [Google Scholar] [CrossRef]
- Yarnold, J. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: Randomised comparison with a multifraction schedule over 12 months of patient follow-up. On behalf of the Bone Pain Trial Working Party. Radiother. Oncol. 1999, 52, 111–121. [Google Scholar] [CrossRef]
- Tinkle, C.L.; Singh, C.; Lloyd, S.; Guo, Y.; Li, Y.; Pappo, A.S.; DuBois, S.G.; Lucas, J.T.; Haas-Kogan, D.A.; Terezakis, S.A.; et al. Stereotactic body radiotherapy for metastatic and recurrent solid tumors in children and young adults. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wu, J.; Wu, R.; Wang, D.; Liu, R.; Luo, H.; Wang, Y.; Chen, J.; Ou, Y.; Zhang, Q.; et al. Efficacy and safety of proton beam therapy for rhabdomyosarcoma: A systematic review and meta-analysis. Radiat. Oncol. 2023, 18, 31. [Google Scholar] [CrossRef]
- Gaze, M.N.; Smeulders, N.; Ackwerh, R.; Allen, C.; Bal, N.; Boutros, M.; Cho, A.; Eminowicz, G.; Gill, E.; Fittall, M.W.; et al. A national referral service for paediatric brachytherapy: An evolving practice and outcomes over thirteen years. Clin. Oncol. 2023, 35, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Ellerkamp, V.; Schmidt, A.; Warmann, S.W.; Eckert, F.; Schaefer, J.; Paulsen, F.; Fuchs, J. Detailed functional results after bladder-preserving surgery and high-dose rate brachytherapy in pediatric bladder/prostate rhabdomyosarcoma. J. Cancer Res. Clin. Oncol. 2022, 149, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
- Lobo, S.; Gaze, M.N.; Slater, O.; Hoskin, P.; Sands, G.; Sullivan, T.; Cho, A.; Eminowicz, G.; Smeulders, N. Bladder function after conservative surgery and high-dose rate brachytherapy for bladder-prostate rhabdomyosarcoma. Pediatr. Blood Cancer 2022, 69, e29574. [Google Scholar] [CrossRef]
- Stenman, J.; Wickart-Johansson, G.; Sundquist, F.; Nilsson, J.; Ljungman, G.; Österlundh, G.; Jalnäs, M.; Pal, N.; Mercke, C. Five-Year Follow-Up After Multimodal Treatment Incorporating HDR Brachytherapy for Bladder Prostate Rhabdomyosarcoma in Children. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 355–359. [Google Scholar] [CrossRef]
- Chargari, C.; Haie-Meder, C.; Espenel, S.; Garcia, M.A.; Ben-Arush, M.; Bolle, S.; Borjesson, A.; Cesen, M.; Lago, R.C.; Defachelles, A.S.; et al. Brachytherapy for Pediatric Patients at Gustave Roussy Cancer Campus: A Model of International Cooperation for Highly Specialized Treatments. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 602–613. [Google Scholar] [CrossRef]
- Schmidt, A.; Warmann, S.W.; Eckert, F.; Ellerkamp, V.; Schaefer, J.; Blumenstock, G.; Paulsen, F.; Fuchs, J. The role of reconstructive surgery and brachytherapy in pediatric bladder/prostate rhabdomyosarcoma. J. Urol. 2020, 204, 825–834. [Google Scholar] [CrossRef]
- Chargari, C.; Haie-Meder, C.; Guérin, F.; Minard-Colin, V.; de Lambert, G.; Mazeron, R.; Escande, A.; Marsolat, F.; Dumas, I.; Deutsch, E.; et al. Brachytherapy Combined with Surgery for Conservative Treatment of Children with Bladder Neck and/or Prostate Rhabdomyosarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 352–359. [Google Scholar] [CrossRef]
- Fuchs, J.; Paulsen, F.; Bleif, M.; Lamprecht, U.; Weidner, N.; Zips, D.; Neunhoeffer, F.; Seitz, G. Conservative surgery with combined high dose rate brachytherapy for patients suffering from genitourinary and perianal rhabdomyosarcoma. Radiother. Oncol. 2016, 121, 262–267. [Google Scholar] [CrossRef]
- Blank, L.E.; Koedooder, K.; van der Grient, H.N.; Wolffs, N.A.; van de Kar, M.; Merks, J.H.; Pieters, B.R.; Saeed, P.; Baldeschi, L.; Freling, N.J.; et al. Brachytherapy as part of the multidisciplinary treatment of childhood rhabdomyosarcomas of the orbit. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Blank, L.E.; Koedooder, K.; Pieters, B.R.; van der Grient, H.N.; van de Kar, M.; Buwalda, J.; Balm, A.J.; Merks, J.H.; Strackee, S.D.; Freling, N.J.; et al. The AMORE protocol for advanced-stage and recurrent non-orbital rhabdomyosarcoma in the head-and-neck region of children: A radiation oncology view. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1555–1562. [Google Scholar] [CrossRef]
- Magné, N.N.; Oberlin, O.; Martelli, H.; Gerbaulet, A.; Chassagne, D.; Haie-Meder, C. Vulval and vaginal rhabdomyosarcoma in children: Update and reappraisal of Institut Gustave Roussy brachytherapy experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Tippin, D.B. Brachytherapy for pediatric tumors. Brachytherapy 2003, 2, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chargari, C.; Martelli, H.; Guérin, F.; Bacorro, W.; de Lambert, G.; Escande, A.; Minard-Colin, V.; Dumas, I.; Deutsch, E.; Haie-Meder, C. Pulsed-dose rate brachytherapy for pediatric bladder prostate rhabdomyosarcoma: Compliance and early clinical results. Radiother. Oncol. 2017, 124, 285–290. [Google Scholar] [CrossRef]
- Demanes, D.J.; JJMazeron Martinez, A.A.; Rivard, M.J. American Brachytherapy Society-Groupe Europeen de Curietherapie-European Society of Therapeutic Radiation Oncology (ABS-GEC-ESTRO) consensus statement for penile brachytherapy. Brachytherapy 2013, 12, 191–198. [Google Scholar] [CrossRef]
- Fajardo, R.D.; Scarzello, G.; Gaze, M.N.; Boterberg, T.; Cameron, A.; Fuchs, J.; Guérin, F.; Hoskin, P.; Krasin, M.J.; Kroon, P.; et al. Brachytherapy for rhabdomyosarcoma: Survey of international clinical practice and development of guidelines. Radiother. Oncol. 2024, 195, 110273. [Google Scholar] [CrossRef]
- Seravalli, E.; Kroon, P.S.; Buatti, J.M.; Hall, M.D.; Mandeville, H.C.; Marcus, K.J.; Onal, C.; Ozyar, E.; Paulino, A.C.; Paulsen, F.; et al. The potential role of MR-guided adaptive radiotherapy in pediatric oncology: Results from a SIOPE-COG survey. Clin. Transl. Radiat. Oncol. 2021, 29, 71–78. [Google Scholar] [CrossRef]
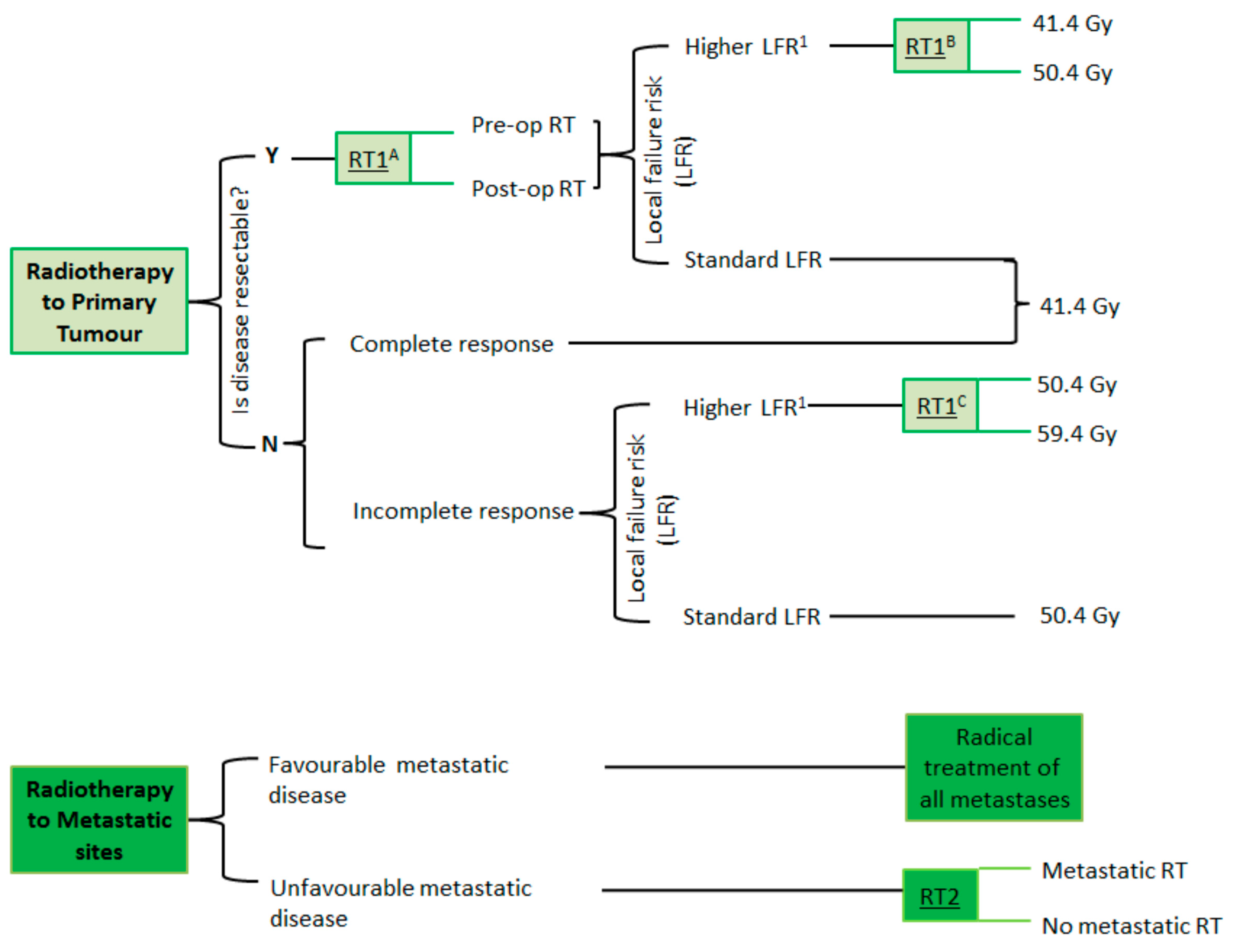

| Primary Tumor | Standard Fractionation | Simultaneous Integrated Boost (SIB) |
|---|---|---|
| Resectable pre- or post-op radiotherapy HLFR Standard dose | 41.4 Gy in 23 fractions over 4.5 weeks to PTVp_pre. | NA |
| Resectable pre- or post-op radiotherapy HLFR Escalated dose | 41.4 Gy in 23 fr over 4.5 weeks to PTVp_Pre 9 Gy in 5 fr to PTVp_Post | 42.5 Gy in 28 fr to PTVp_Pre 50.4 Gy in 28 fr to PTVp_post |
| Resectable pre- or post-op radiotherapy SLFR Standard dose | 41.4 Gy in 23 fr over 4.5 weeks to PTVp_pre | NA |
| Unresectable complete response (to induction chemotherapy) Standard dose | 41.4 Gy in 23 fr over 4.5 weeks to PTVp_pre | NA |
| Unresectable incomplete response (to induction chemotherapy) HLFR Standard dose | 41.4 Gy in 23 f over 4.5 weeks to PTVp_Pre 9 Gy in 5 fr to PTVp_Post | 42.5 Gy in 28 fr to PTVp_Pre 50.4 Gy in 28 fr to PTVp_post |
| Unresectable incomplete response (to induction chemotherapy) HLFR Escalated dose | 41.4 Gy in 23 fr over 4.5 weeks to PTVp_Pre 18 Gy in 10 fr to PTVp_Post | 42.5 Gy in 28 fr to PTVp_Pre 58.1 Gy in 28 fr to PTVp_post |
| Unresectable incomplete response (to induction chemotherapy) SLFR Standard dose | 41.4 Gy in 23 fr over 4.5 weeks to PTVp_Pre 9 Gy in 5 fr to PTVp_Post | 42.5 Gy in 28 fr to PTVp_Pre 50.4 Gy in 28 fr to PTVp_post |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dávila Fajardo, R.; Magelssen, H.; Cameron, A.L.; Boterberg, T.; Mandeville, H.C. Current and Future Developments in Radiation Oncology Approach for Rhabdomyosarcoma. Cancers 2025, 17, 1618. https://doi.org/10.3390/cancers17101618
Dávila Fajardo R, Magelssen H, Cameron AL, Boterberg T, Mandeville HC. Current and Future Developments in Radiation Oncology Approach for Rhabdomyosarcoma. Cancers. 2025; 17(10):1618. https://doi.org/10.3390/cancers17101618
Chicago/Turabian StyleDávila Fajardo, Raquel, Henriette Magelssen, Alison L. Cameron, Tom Boterberg, and Henry C. Mandeville. 2025. "Current and Future Developments in Radiation Oncology Approach for Rhabdomyosarcoma" Cancers 17, no. 10: 1618. https://doi.org/10.3390/cancers17101618
APA StyleDávila Fajardo, R., Magelssen, H., Cameron, A. L., Boterberg, T., & Mandeville, H. C. (2025). Current and Future Developments in Radiation Oncology Approach for Rhabdomyosarcoma. Cancers, 17(10), 1618. https://doi.org/10.3390/cancers17101618






