Ectopic Cushing’s Syndrome in Advanced Small-Cell Lung Cancer (SCLC): Clinical Challenges and Therapeutic Insights
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Presentation
3.2. Diagnosis of ECS-SCLC
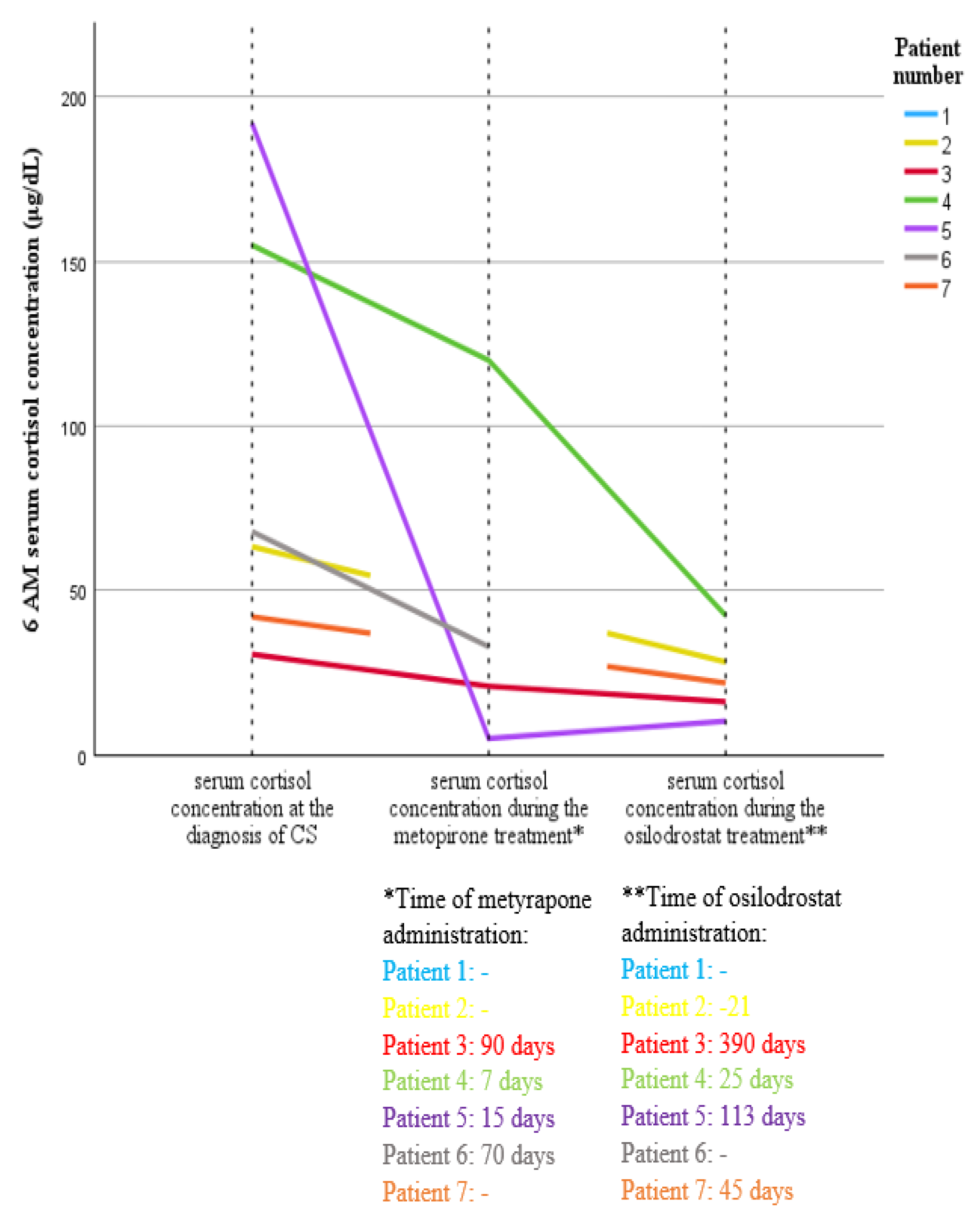
3.3. Treatment Strategy and Results Among Patients with ESC-SCLC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECS | Ectopic Cushing’s Syndrome |
| ACTH | Adrenocorticotropic Hormone |
| SCLC | Small-Cell Lung Cancer |
| GEPNEN | Gastroenteropancreatic neuroendocrine neoplasm |
| CRH | Corticotropin-Releasing Hormone |
| HDDST | High-Dose Dexamethasone Suppression Test |
| BIPSS | Bilateral Inferior Petrosal Sinus Sampling |
| LFT | Liver Function Tests |
| ULN | Upper Limit of Normal |
| MRI | Magnetic Resonance Imaging |
| CT | Computed Tomography |
| EUS | Endoscopic Ultrasound |
| UFC | Urine-Free Cortisol |
| ECOG | Eastern Cooperative Oncology Group Performance Status Scale |
| RTH | Radiotherapy |
| CHT | Chemotherapy |
References
- Gadelha, M.; Gatto, F.; Wildemberg, L.E.; Fleseriu, M. Cushing’s Syndrome. Lancet 2023, 402, 2237–2252. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, O.; Juhlin, C.C.; Torpy, D.J.; Falhammar, H. A Clinical Perspective on Ectopic Cushing’s Syndrome. Trends Endocrinol. Metab. 2024, 35, 347–360. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Qi, X.; Yu, L.; Lin, L. Management of Small Cell Lung Cancer Complicated with Paraneoplastic Cushing’s Syndrome: A Systematic Literature Review. Front. Endocrinol. 2023, 14, 1177125. [Google Scholar] [CrossRef]
- Young, J.; Haissaguerre, M.; Viera-Pinto, O.; Chabre, O.; Baudin, E.; Tabarin, A. Management of Endocrine Disease: Cushing’s Syndrome Due to Ectopic ACTH Secretion: An Expert Operational Opinion. Eur. J. Endocrinol. 2020, 182, R29–R58. [Google Scholar] [CrossRef]
- Hatipoglu, B.A. Cushing’s Syndrome. J. Surg. Oncol. 2012, 106, 565–571. [Google Scholar] [CrossRef]
- Piasecka, M.; Larsson, M.; Papakokkinou, E.; Olsson, L.; Ragnarsson, O. Is Ectopic Cushing’s Syndrome Underdiagnosed in Patients with Small Cell Lung Cancer? Front. Med. 2022, 9, 954033. [Google Scholar] [CrossRef]
- Araujo Castro, M.; Marazuela Azpiroz, M. Two Types of Ectopic Cushing Syndrome or a Continuum? Pituitary 2018, 21, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Dormoy, A.; Haissaguerre, M.; Vitellius, G.; Do Cao, C.; Geslot, A.; Drui, D.; Lasolle, H.; Vieira-Pinto, O.; Salenave, S.; François, M.; et al. Efficacy and Safety of Osilodrostat in Paraneoplastic Cushing Syndrome: A Real-World Multicenter Study in France. J. Clin. Endocrinol. Metab. 2023, 108, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Gamrat, A.; Rzepka, E.; Ciszek, K.; Wysocki, P.J.; Hubalewska-Dydejczyk, A.; Gilis-Januszewska, A. Complete Remission of Hypercortisolemia in a Patient with Severe Ectopic Cushing’s Syndrome Due to Small Cell Lung Cancer. Pol. Arch. Intern. Med. 2024; in press. [Google Scholar] [CrossRef]
- Paleń-Tytko, J.E.; Przybylik-Mazurek, E.M.; Rzepka, E.J.; Pach, D.M.; Sowa-Staszczak, A.S.; Gilis-Januszewska, A.; Hubalewska-Dydejczyk, A.B. Ectopic ACTH Syndrome of Different Origin—Diagnostic Approach and Clinical Outcome. Experience of One Clinical Center. PLoS ONE 2020, 15, e0242679. [Google Scholar] [CrossRef]
- Srivillibhuthur, M.; Yu, T.; Li, M.; Mader, I.; Arikan, P. Ectopic Cushing’s Syndrome as the First Presenting Sign of Small Cell Lung Carcinoma. J. Brown Hosp. Med. 2023, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A.; Laskey, J.; Evans, W.K.; Goss, P.E.; Johansen, E.; Khamsi, F. Cushing’s Syndrome Associated with Ectopic Corticotropin Production and Small-Cell Lung Cancer. J. Clin. Oncol. 1992, 10, 21. [Google Scholar] [CrossRef]
- Richa, C.G.; Saad, K.J.; Halabi, G.H.; Gharios, E.M.; Nasr, F.L.; Merheb, M.T. Case-Series of Paraneoplastic Cushing Syndrome in Small-Cell Lung Cancer. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 18-0004. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.R.; Grossman, A.B. Distinguishing Cushing’s Disease from the Ectopic ACTH Syndrome: Needles in a Haystack or Hiding in Plain Sight? J. Neuroendocrinol. 2022, 34, e13137. [Google Scholar] [CrossRef]
- Torpy, D.J.; Mullen, N.; Ilias, I.; Nieman, L.K. Association of Hypertension and Hypokalemia with Cushing’s Syndrome Caused by Ectopic ACTH Secretion: A Series of 58 Cases. Ann. N. Y. Acad. Sci. 2002, 970, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Delisle, L.; Boyer, M.J.; Warr, D.; Killinger, D.; Payne, D.; Yeoh, J.L.; Feld, R. Ectopic Corticotropin Syndrome and Small-Cell Carcinoma of the Lung: Clinical Features, Outcome, and Complications. Arch. Intern. Med. 1993, 153, 746–752. [Google Scholar] [CrossRef]
- Ghazi, A.A.; Abbasi Dezfooli, A.; Amirbaigloo, A.; Daneshvar Kakhki, A.; Mohammadi, F.; Tirgari, F.; Pourafkari, M. Ectopic Cushing’s Syndrome Secondary to Lung and Mediastinal Tumors—Report from a Tertiary Care Centre in Iran. Endokrynol. Pol. 2015, 66, 2–9. [Google Scholar] [CrossRef][Green Version]
- Zhang, H.Y.; Zhao, J. Ectopic Cushing Syndrome in Small Cell Lung Cancer: A Case Report and Literature Review. Thorac. Cancer 2017, 8, 114–117. [Google Scholar] [CrossRef]
- Tan, M.H.; Iyengar, R.; Mizokami-Stout, K.; Yentz, S.; MacEachern, M.P.; Shen, L.Y.; Redman, B.; Gianchandani, R. Spectrum of Immune Checkpoint Inhibitors-Induced Endocrinopathies in Cancer Patients: A Scoping Review of Case Reports. Clin. Diabetes Endocrinol. 2019, 5, 1. [Google Scholar] [CrossRef]
- Unwin, R.J.; Luft, F.C.; Shirley, D.G. Pathophysiology and Management of Hypokalemia: A Clinical Perspective. Nat. Rev. Nephrol. 2011, 7, 75–84. [Google Scholar] [CrossRef]
- Ito, T.; Jensen, R.T. Perspectives on the current pharmacotherapeutic strategies for management of functional neuroendocrine tumor syndromes. Expert Opin. Pharmacother. 2021, 22, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; Lu, S.J.; Chen, M.S.; Chen, Z.B.; Wang, L.; Wu, P.S. Effects of Hypokalemia on Transmural Dispersion of Ventricular Repolarization in Left Ventricular Myocardium. Asian Pac. J. Trop. Med. 2013, 6, 485–488. [Google Scholar] [CrossRef]
- von Stempel, C.; Perks, C.; Corcoran, J.; Grayez, J. Cardio-respiratory Failure Secondary to Ectopic Cushing’s Syndrome as the Index Presentation of Small-Cell Lung Cancer. BMJ Case Rep. 2013, 2013, bcr2013009974. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.; Lehmann-Horn, F. Hypokalemic Periodic Paralysis. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1338/ (accessed on 16 July 2018).
- Webb, S.M.; Valassi, E. Morbidity of Cushing Syndrome and Impact of Treatment. Endocrinol. Metab. Clin. N. Am. 2018, 47, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, M.; Bex, M.; Feelders, R.A.; Heaney, A.P.; Auchus, R.J.; Gilis-Januszewska, A.; Witek, P.; Belaya, Z.; Yu, Y.; Liao, Z.; et al. Randomized Trial of Osilodrostat for the Treatment of Cushing Disease. J. Clin. Endocrinol. Metab. 2022, 107, e2882–e2895. [Google Scholar] [CrossRef]
- Nieman, L.K.; Boscaro, M.; Scaroni, C.M.; Deutschbein, T.; Mezosi, E.; Driessens, N.; Georgescu, C.E.; Hubalewska-Dydejczyk, A.; Berker, D.; Jarzab, B.M.; et al. Metyrapone Treatment in Endogenous Cushing’s Syndrome: Results at Week 12 from PROMPT, a Prospective International Multicenter, Open-Label, Phase III/IV Study. J. Endocr. Soc. 2021, 5, A515. [Google Scholar] [CrossRef]

| ECS Etiology | N = 39 (Percentage) |
|---|---|
| SCLC | 7 (18%) |
| GEPNEN | 9 (23%) |
| -Pancreatic NET | 7 |
| -Gastric NET | 1 |
| -Intestinal NET | 1 |
| Carcinoid | 6 (15.4%) |
| -Lung carcinoid | 5 |
| -Thymic carcinoid | 1 |
| Medullary thyroid cancer | 3 (7.7%) |
| Pheochromocytoma | 2 (5.1%) |
| Other malignant tumors | 7 (18%) |
| -Ovarian cancer | 2 |
| -Gastric adenocarcinoma | 1 |
| -Lung adenocarcionoma | 1 |
| -Uterine clear cell carcinoma | 1 |
| -Pancreatic tumor 1 | 1 |
| -Bladder tumor 1 | 1 |
| Other tumors | 2 (5.1%) |
| -Maxillary sinus papilloma | 1 |
| -Esthesioneneuroblastoma | 1 |
| Occult source 2 | 3 (7.7%) |
| Variable 1 | SCLC N = 7 | Other ECS N = 32 | p-Value 2 |
|---|---|---|---|
| Age (years) | 66 [62–68] | 61 [45.5–70.8] | 0.26 |
| Women | 2 (29%) | 16 (50%) | 0.42 |
| Time to diagnosis (months) | 1 [1–2] | 2 [1.13–4] | 0.03 |
| Death | 6 (86%) | 20 (63%) | 0.39 |
| Time of observation (months) | 3 [2–4] | 12 [2–47.8] | 0.13 |
| ECOG scale 3 at the diagnosis | 4 [4–4] | 3 [1–4] | 0.01 |
| BMI | 27 [22.6–36] | 26.4 [22.2–34.9] | 0.67 |
| Cushing’s syndrome symptoms | |||
| Weight gain | 0 (0%) | 11 (34%) | 0.16 |
| Weight loss | 7 (100%) | 19 (59%) | 0.07 |
| Weight change (kg) | −5 [(−8)–(−5)] | −4 [−7.8–9.3] | 0.16 |
| Fat tissue redistribution | 4 (57%) | 19 (59%) | 0.62 |
| Striae | 1 (14%) | 7 (22%) | 1.00 |
| Oedema | 5 (71%) | 22 (69%) | 1.00 |
| Plethora | 3 (43%) | 18 (56%) | 0.64 |
| Lovett scale | 2 [1–2] | 2 [2–3] | 0.04 |
| Tendency to bruise | 4 (57%) | 22 (69%) | 0.67 |
| Tendency to infection | 5 (71%) | 21 (66%) | 1.00 |
| Electrolyte disturbances | |||
| Na+ concentration (mmol/L) | 144 [138–148] | 144.5 [142–148] | 0.48 |
| K+ concentration (mmol/L) | 2.12 [1.9–3.4] | 2.7 [2.3–3.5] | 0.03 |
| Kalium supplementation (mEq/day) | 200 [150–200] | 120 [65–160] | 0.001 |
| Comorbidities | |||
| Hypertension | 7 (100%) | 29 (91%) | 1.00 |
| Diabetes mellitus | 4 (57%) | 22 (69%) | 0.67 |
| Hyperlipidemia | 3 (43%) | 18 (56%) | 0.38 |
| Initial symptoms 4 | |||
| Hypokalemia | 7 (100%) | 13 (44%) | 0.01 |
| Oedema | 4 (57%) | 9 (28%) | 0.19 |
| Muscle weakness | 3 (43%) | 12 (38%) | 1.00 |
| Weight loss | 1 (14%) | 5 (16%) | 1.00 |
| Patient Number | Gender (F-Female, M-Male) | Age at the Diagnosis (Years) | Initial Symptoms 1 1. Hypokalemia 2. Muscle Weakness 3. Oedema 4. Reistant Hypertension 5. Weight Loss | Weight Changes Before Diagnosis (kg; “-” Means Weight Loss) | Other Symptoms 2. Muscle Weakness 4. Resistant Hypertension 5. Weight Loss 6. Fat Tissue Redistribution 7. Plethora 8. Striae 9. Skin Thinning 10. Tendency to Bruise 11. Tendency to Infection 12. Psychotic Symptoms 13. Depression | Time to Diagnosis of Hypercortisolemia 2 (Months) | Histopathological Diagnosis (Material) | TNM Scale | Size of Primary Lesion (mm) | Site of Metastases 1. Lymph Nodes 2. Liver 3. Bones 4. Adrenal Glands 5. Central Nervous System 6. Subcutaneous Tissue | 6 AM Cortisol Concentration at the Diagnosis (ug/dL) | ACTH Concentration at the Diagnosis (pg/mL; N: 5–56) | Potassium Concentration at the Diagnosis (mmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | M | 55 | 1. Hypokalemia 2. Muscle weakness | −8 | 5. Weight loss 11. Tendency to infection 12. Psychotic symptoms | 1 | SCLC (sample taken during EUS) | IVB-T4N3M1c | 80 × 100 × 120 | 1. thoracic lymph nodes 4. adrenal glands | 84 | 394 | 1.9 |
| 2. | M | 74 | 1. Hypokalemia 4. Resistant hypertension | no information | 2. Muscle weakness 5. Weight loss 11. tendency to infection | 3 | SCLC (sample taken during bronchoscopy) | IVB T4N3M1c | 91 × 56 × 72 | 1. thoracic lymph nodes 4. adrenal glands | 63.4 | 113.7 | 1.4 |
| 3. | M | 65 | 1. Hypokalemia 3. Oedema | −8 | 2. Muscle weakness 4. Resistant hypertension 5. Weight loss 6. Fat tissue redistribution 7. Plethora | 1 | SCLC (sample taken during bronchoscopy) | IIIC-T3N3M0 | 52 × 26 mm | 1. Thoracic lymph nodes | 37.4 | 222 | 2.16 |
| 4. | M | 66 | 1. Hypokalemia 3. Oedema 5. Weight loss | −11 | 2. Muscle weakness 4. Resistant hypertension 5. Weight loss 6. Fat tissue redistribution 7. Plethora 9. Skin thinning 10. Tendency to bruise 11. Tendency to infection 13. Depression | 0.5 | SCLC (sample taken during bronchoscopy) | IVB-T1cN3M1c | 26 × 12 | 1. thoracic lymph nodes 3. bones 5. CNS | 155 | 60 | 2.38 |
| 5. | F | 62 | 1. Hypokalemia 2. Muscle weakness 3. Oedema | −5 | 5. Weight loss 6. Fat tissue redistribution 9. Skin thinning 10. Tendency to bruise 11. Tendency to infection 12. Psychotic symptoms | 1 | SCLC (sample taken during bronchoscopy) | IVB-T1cN3M1c | 28 × 25 | 1. thoracic lymph nodes 2. liver 3. bones 4. adrenal glands | 192 | 1237 | 1.9 |
| 6. | M | 68 | 1. Hypokalemia 3. Oedema | −4 | 2. Muscle weakness 4. Resistant hypertension 5. Weight loss 9. Skin thinning 10. Tendency to bruise 11. Psychotic symptoms | 2 | SCLC (sample taken during bronchoscopy) | IVB-T4N3M1c | 72 × 101 × 122 | 1. thoracic lymph nodes 2. liver 4. adrenal glands | 68 | 223.6 | 2.34 |
| 7. | F | 68 | 1. Hypokalemia 2. Muscle weakness | −5 | 3. Oedema 4. Resistant hypertension 5. Weight loss 6. Fat tissue redistribution 7. Plethora 8. Striae 9. Skin thinning 10. Tendency to bruise 11. Tendency to infection 13. Depression | 1 | SCLC [pleural fluid] | IVB- T?N3M1c | no information | 1. mediastinal, paraaortic lymph nodes 2. liver 7. subcutaneous tissue of the abdomen | 45.9 | 171 | 2.97 |
| Patient Number | ECOG 1 scale Assessment at ECS Diagnosis | Treatment of Hypercortisolemia (Maximal Dose Used) | Time from ECS Diagnosis to Hypercortisolemia Treatment Introduction (Days) | Total Duration of Treatment for HYPERCORTISOLISM (Days) | Time to Improve Muscle Strength 2 | Time to Kalemia Response 3 | Oncological Treatment | ECOG Scale Assessment Before Oncological Treatment | Time from ECS Diagnosis to Oncological Treatment Introduction | Total Duration of Oncological Treatment | RECIST 1.1 CR-Complete Response PR-Partial Response (% Reduction in Tumor Size) SD-Stable Disease PD-PROGRESSIVE disease (What Kind) | ECOG Scale Assessment During Oncological Treatment (Assessment Time 4) | Death (D), Survival Time/Follow-Up Time (Months) | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 4 | - | - | - | - | - | - | - | - | - | - | D, 3 | 1. progression of the primary disease 2. infection | |
| 2. | 4 | 1. Osilodrostat (4 mg) | 4 | 1.21 | 10 | 5 | RTH | 4 | 20 | 2 days | no imaging tests during therapy | 5 (2 days) | D, 1 | 1. progression of the disease 2. infection—sepsis |
| 3. | 4 | 1. Metyrapone (3250 mg) 2. Osilodrostat (5 mg) | 6 | 1. 90 2. 390 | 18 | 21 | CHT—carboplatin, etoposide, atezolizumab | 3 | 49 | 31 months, 30 cycles, ongoing | PR (71%) | 1 (4 months) | 33, ongoing | - |
| 4. | 4 | 1. Metyrapone (1750 mg) 2. Osilodrostat (8 mg) | 7 | 1. 7 2.25 | 15 | 29 | Brain RTH-20 Gy in 5 fractions | 3 | 21 | 8 days, death 2 weeks after RTH | no imaging tests during therapy | 5 (2 weeks) | D, 1.5 | 1. progression of the primary disease |
| 5. | 4 | 1. Metyrapone (3000 mg) 2. Osilodrostat (10 mg) | 1 | 1.15 2.113 | 11 | 17 | CHT—cisplatine, etoposide | 3 | 24 | 2.5 months, 4 cycles | PD (increased number and size of metastatic lesions in the liver) | 2 (3 weeks); 5 (2.5 months) | D, 4 | 1. progression of the primary disease 2. infection—sepsis |
| 6. | 4 | 1. Metyrapone (1000 mg) | 3 | 1.70 | 7 | 5 | Mediastinal RTH | 3 | 8 | 2.5 months | no imaging tests during therapy | 3 (3 weeks); 5 (2.5 months) | D, 3 | 1. progression of the primary disease |
| 7. | 4 | 1. Osilodrostat (40 mg) | 1 | 7.45 | - | 6 | - | - | - | - | - | - | D, 1.5 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamrat-Żmuda, A.; Minasyan, M.; Wysocki, P.J.; Hubalewska-Dydejczyk, A.; Gilis-Januszewska, A. Ectopic Cushing’s Syndrome in Advanced Small-Cell Lung Cancer (SCLC): Clinical Challenges and Therapeutic Insights. Cancers 2025, 17, 1611. https://doi.org/10.3390/cancers17101611
Gamrat-Żmuda A, Minasyan M, Wysocki PJ, Hubalewska-Dydejczyk A, Gilis-Januszewska A. Ectopic Cushing’s Syndrome in Advanced Small-Cell Lung Cancer (SCLC): Clinical Challenges and Therapeutic Insights. Cancers. 2025; 17(10):1611. https://doi.org/10.3390/cancers17101611
Chicago/Turabian StyleGamrat-Żmuda, Aleksandra, Mari Minasyan, Piotr J. Wysocki, Alicja Hubalewska-Dydejczyk, and Aleksandra Gilis-Januszewska. 2025. "Ectopic Cushing’s Syndrome in Advanced Small-Cell Lung Cancer (SCLC): Clinical Challenges and Therapeutic Insights" Cancers 17, no. 10: 1611. https://doi.org/10.3390/cancers17101611
APA StyleGamrat-Żmuda, A., Minasyan, M., Wysocki, P. J., Hubalewska-Dydejczyk, A., & Gilis-Januszewska, A. (2025). Ectopic Cushing’s Syndrome in Advanced Small-Cell Lung Cancer (SCLC): Clinical Challenges and Therapeutic Insights. Cancers, 17(10), 1611. https://doi.org/10.3390/cancers17101611










