Continuing Cyclin-Dependent Kinase 4/6 Inhibitors Beyond Progression in Advanced Breast Cancer: A Meta-Analysis †
Simple Summary
Abstract
1. Introduction
2. Methods
- Literature search and study selection
- 2.
- Data extraction
- 3.
- Data synthesis and statistical analysis
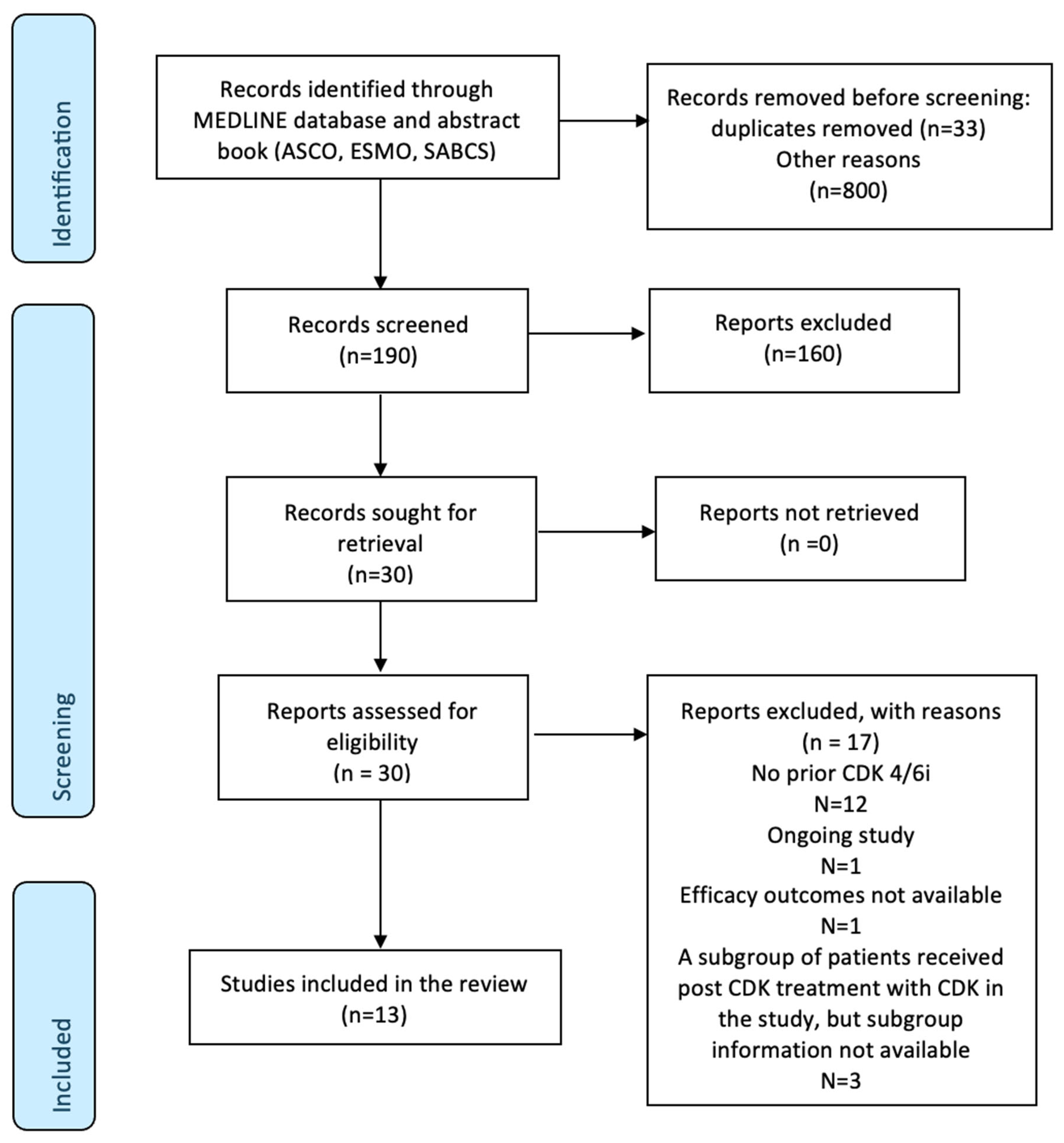
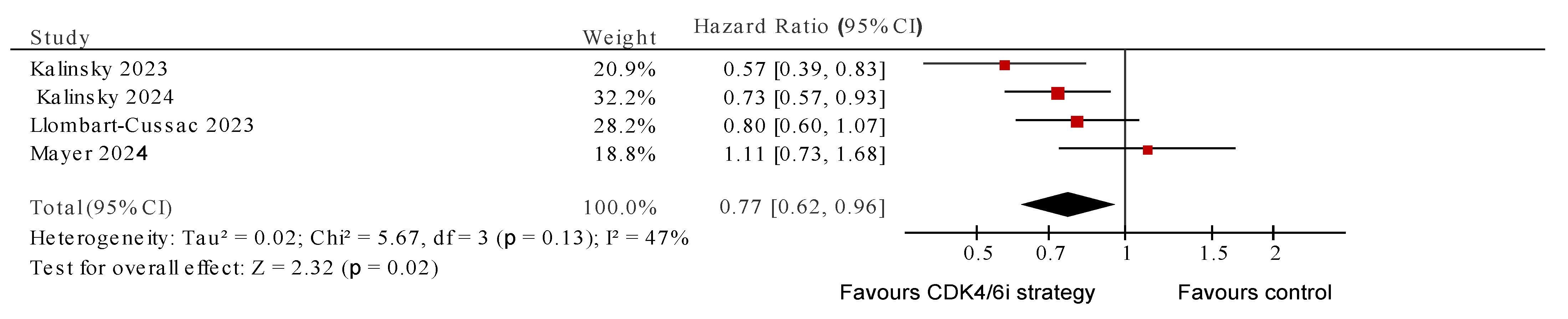
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morrison, L.; Loibl, S.; Turner, N.C. The CDK4/6 Inhibitor Revolution—A Game-Changing Era for Breast Cancer Treatment. Nat. Rev. Clin. Oncol. 2024, 21, 89–105. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; et al. NCCN Guidelines® Insights: Breast Cancer, Version 4.2023. J. Natl. Compr. Cancer Netw. 2023, 21, 594–608. [Google Scholar] [CrossRef]
- Burstein, H.J.; Somerfield, M.R.; Barton, D.L.; Dorris, A.; Fallowfield, L.J.; Jain, D.; Johnston, S.R.D.; Korde, L.A.; Litton, J.K.; Macrae, E.R.; et al. Endocrine Treatment and Targeted Therapy for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 3959–3977. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, S.; Xin, Q.; Zhang, Y.; Wang, K.; Li, M. Recent Progress of CDK4/6 Inhibitors’ Current Practice in Breast Cancer. Cancer Gene Ther. 2024, 31, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Rugo, H.S.; Im, S.-A.; Slamon, D.J.; Harbeck, N.; Bondarenko, I.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2- ABC: Updated Exploratory Analyses of PALOMA-3, a Double-Blind, Phase III Randomized Study. Clin. Cancer Res. 2022, 28, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated Results from MONALEESA-2, a Phase III Trial of First-Line Ribociclib plus Letrozole versus Placebo plus Letrozole in Hormone Receptor-Positive, HER2-Negative Advanced Breast Cancer. Ann. Oncol. 2018, 29, 1541–1547. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2− Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Kappel, C.; Elliott, M.J.; Kumar, V.; Nadler, M.B.; Desnoyers, A.; Amir, E. Comparative Overall Survival of CDK4/6 Inhibitors in Combination with Endocrine Therapy in Advanced Breast Cancer. Sci. Rep. 2024, 14, 3129. [Google Scholar] [CrossRef]
- Ashai, N.; Swain, S.M. Post-CDK 4/6 Inhibitor Therapy: Current Agents and Novel Targets. Cancers 2023, 15, 1855. [Google Scholar] [CrossRef]
- Mittal, A.; Molto Valiente, C.; Tamimi, F.; Schlam, I.; Sammons, S.; Tolaney, S.M.; Tarantino, P. Filling the Gap after CDK4/6 Inhibitors: Novel Endocrine and Biologic Treatment Options for Metastatic Hormone Receptor Positive Breast Cancer. Cancers 2023, 15, 2015. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.-C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.-A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (Oral Selective Estrogen Receptor Degrader) Versus Standard Endocrine Therapy for Estrogen Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, G.J.; Fernando, T.M.; Bowen, R.; Jerzak, K.J.; Song, X.; Decker, T.; Boyle, F.; McCune, S.; Armstrong, A.; Shannon, C.; et al. VERONICA: Randomized Phase II Study of Fulvestrant and Venetoclax in ER-Positive Metastatic Breast Cancer Post-CDK4/6 Inhibitors—Efficacy, Safety, and Biomarker Results. Clin. Cancer Res. 2022, 28, 3256–3267. [Google Scholar] [CrossRef]
- Neupane, N.; Bawek, S.; Gurusinghe, S.; Ghaffary, E.M.; Mirmosayyeb, O.; Thapa, S.; Falkson, C.; O’Regan, R.; Dhakal, A. Oral SERD, a Novel Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer. Cancers 2024, 16, 619. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Chae, Y.S.; Lee, K.S.; et al. Trastuzumab Deruxtecan (T-DXd) versus Treatment of Physician’s Choice (TPC) in Patients (Pts) with HER2-Low Unresectable and/or Metastatic Breast Cancer (mBC): Results of DESTINY-Breast04, a Randomized, Phase 3 Study. J. Clin. Oncol. 2022, 40, LBA3. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Sacituzumab Govitecan in Hormone Receptor–Positive/Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer. J. Clin. Oncol. 2022, 40, 3365–3376. [Google Scholar] [CrossRef]
- Curigliano, G.; Hu, X.; Dent, R.A.; Yonemori, K.; Barrios, C.H.; O’Shaughnessy, J.; Wildiers, H.; Zhang, Q.; Im, S.-A.; Saura, C.; et al. Trastuzumab Deruxtecan (T-DXd) vs Physician’s Choice of Chemotherapy (TPC) in Patients (Pts) with Hormone Receptor-Positive (HR+), Human Epidermal Growth Factor Receptor 2 (HER2)-Low or HER2-Ultralow Metastatic Breast Cancer (mBC) with Prior Endocrine Therapy (ET): Primary Results from DESTINY-Breast06 (DB-06). J. Clin. Oncol. 2024, 42, LBA1000. [Google Scholar] [CrossRef]
- Abelman, R.O.; Spring, L.; Fell, G.G.; Ryan, P.; Vidula, N.; Medford, A.J.; Shin, J.; Abraham, E.; Wander, S.A.; Isakoff, S.J.; et al. Sequential Use of Antibody-Drug Conjugate after Antibody-Drug Conjugate for Patients with Metastatic Breast Cancer: ADC after ADC (A3) Study. J. Clin. Oncol. 2023, 41, 1022. [Google Scholar] [CrossRef]
- Fu, Z.; Liu, J.; Li, S.; Shi, C.; Zhang, Y. Treatment-Related Adverse Events Associated with HER2-Targeted Antibody-Drug Conjugates in Clinical Trials: A Systematic Review and Meta-Analysis. eClinicalMedicine 2023, 55, 101795. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, X.; Chen, Y.; Nian, Q.; Lin, L.; Chen, M. A Real-World Disproportionality Analysis of FDA Adverse Event Reporting System (FAERS) Events for Alpelisib. Heliyon 2024, 10, e27529. [Google Scholar] [CrossRef]
- Ciruelos, E.M.; Rugo, H.S.; Mayer, I.A.; Levy, C.; Forget, F.; Delgado Mingorance, J.I.; Safra, T.; Masuda, N.; Park, Y.H.; Juric, D.; et al. Patient-Reported Outcomes in Patients With PIK3CA-Mutated Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer From SOLAR-1. J. Clin. Oncol. 2021, 39, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Gomez Moreno, H.L.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, E.A.; Sargent, D.J.; Grothey, A.; Kerbel, R.S. Drug Rechallenge and Treatment beyond Progression—Implications for Drug Resistance. Nat. Rev. Clin. Oncol. 2013, 10, 571–587. [Google Scholar] [CrossRef]
- Waddell, T.; Kotsori, A.; Constantinidou, A.; Yousaf, N.; Ashley, S.; Parton, M.; Allen, M.; Starling, N.; Papadopoulos, P.; O’Brien, M.; et al. Trastuzumab beyond Progression in HER2-Positive Advanced Breast Cancer: The Royal Marsden Experience. Br. J. Cancer 2011, 104, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Wander, S.A.; Han, H.S.; Zangardi, M.L.; Niemierko, A.; Mariotti, V.; Kim, L.S.L.; Xi, J.; Pandey, A.; Dunne, S.; Nasrazadani, A.; et al. Clinical Outcomes With Abemaciclib After Prior CDK4/6 Inhibitor Progression in Breast Cancer: A Multicenter Experience. J. Natl. Compr. Cancer Netw. 2021, 19, 149–156. [Google Scholar] [CrossRef]
- Eziokwu, A.S.; Varella, L.; Kruse, M.L.; Jia, X.; Moore, H.C.F.; Budd, G.T.; Abraham, J.; Montero, A.J. Real-World Evidence Evaluating Continuation of CDK4/6 Inhibitors beyond First Progression in Hormone Receptor-Positive (HR+) Metastatic Breast Cancer. J. Clin. Oncol. 2019, 37, e12538. [Google Scholar] [CrossRef]
- Martin, J.M.; Handorf, E.A.; Montero, A.J.; Goldstein, L.J. Systemic Therapies Following Progression on First-Line CDK4/6-Inhibitor Treatment: Analysis of Real-World Data. Oncologist 2022, 27, 441–446. [Google Scholar] [CrossRef]
- Kalinsky, K.; Accordino, M.K.; Chiuzan, C.; Mundi, P.S.; Sakach, E.; Sathe, C.; Ahn, H.; Trivedi, M.S.; Novik, Y.; Tiersten, A.; et al. Randomized Phase II Trial of Endocrine Therapy With or Without Ribociclib After Progression on Cyclin-Dependent Kinase 4/6 Inhibition in Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer: MAINTAIN Trial. J. Clin. Oncol. 2023, 41, 4004–4013. [Google Scholar] [CrossRef]
- Mayer, E.L.; Ren, Y.; Wagle, N.; Mahtani, R.; Ma, C.; DeMichele, A.; Cristofanilli, M.; Meisel, J.; Miller, K.D.; Abdou, Y.; et al. PACE: A Randomized Phase II Study of Fulvestrant, Palbociclib, and Avelumab After Progression on Cyclin-Dependent Kinase 4/6 Inhibitor and Aromatase Inhibitor for Hormone Receptor–Positive/Human Epidermal Growth Factor Receptor–Negative Metastatic Breast Cancer. J. Clin. Oncol. 2024, 42, 2050–2060. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Stanley, T.D.; Doucouliagos, H. Neither Fixed nor Random: Weighted Least Squares Meta-Regression. Res. Synth. Methods 2017, 8, 19–42. [Google Scholar] [CrossRef]
- Burnand, B.; Kernan, W.N.; Feinstein, A.R. Indexes and Boundaries for “Quantitative Significance” in Statistical Decisions. J. Clin. Epidemiol. 1990, 43, 1273–1284. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024); Cochrane: London, UK, 2024. [Google Scholar]
- Llombart-Cussac, A.; Harper-Wynne, C.; Perello, A.; Hennequin, A.; Fernandez, A.; Colleoni, M.; Carañana, V.; Quiroga, V.; Medioni, J.; Iranzo, V.; et al. Second-Line Endocrine Therapy (ET) with or without Palbociclib (P) Maintenance in Patients (Pts) with Hormone Receptor-Positive (HR[+])/Human Epidermal Growth Factor Receptor 2-Negative (HER2[-]) Advanced Breast Cancer (ABC): PALMIRA Trial. J. Clin. Oncol. 2023, 41, 1001. [Google Scholar] [CrossRef]
- Kalinsky, K.; Bianchini, G.; Hamilton, E.P.; Graff, S.L.; Park, K.H.; Jeselsohn, R.; Demirci, U.; Martin, M.; Layman, R.M.; Hurvitz, S.A.; et al. Abemaciclib plus Fulvestrant vs Fulvestrant Alone for HR+, HER2- Advanced Breast Cancer Following Progression on a Prior CDK4/6 Inhibitor plus Endocrine Therapy: Primary Outcome of the Phase 3 postMONARCH Trial. J. Clin. Oncol. 2024, 42, LBA1001. [Google Scholar] [CrossRef]
- Albanell, J.; Pérez-García, J.M.; Gil-Gil, M.; Curigliano, G.; Ruíz-Borrego, M.; Comerma, L.; Gibert, J.; Bellet, M.; Bermejo, B.; Calvo, L.; et al. Palbociclib Rechallenge for Hormone Receptor–Positive/HER-Negative Advanced Breast Cancer: Findings from the Phase II BioPER Trial. Clin. Cancer Res. 2023, 29, 67. [Google Scholar] [CrossRef]
- Mariotti, V.; Khong, H.T.; Soliman, H.H.; Costa, R.L.; Fisher, S.; Boulware, D.; Han, H.S. Efficacy of Abemaciclib (Abema) after Palbociclib (Palbo) in Patients (Pts) with Metastatic Breast Cancer (MBC). J. Clin. Oncol. 2019, 37, e12521. [Google Scholar] [CrossRef]
- Tao, J.J.; Blackford, A.L.; Nunes, R.; Truica, C.I.; Mahosky, J.; Jones, M.K.; Leasure, N.C.; Cescon, T.; Christou, A.; Werner, J.L.; et al. Abstract P3-01-07: Phase II Trial of Palbociclib with Fulvestrant in Individuals with Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer with Disease Progression after Palbociclib with an Aromatase Inhibitor. Cancer Res. 2023, 83, P3-01-07. [Google Scholar] [CrossRef]
- Seki, H.; Nakai, T.; Shimizu, K. Abstract P5-02-49: Efficacy of Subsequent-Abemaciclib Treatment after Disease Progression on Palbociclib Combined with Endocrine Therapy in Patients with ER-Positive HER2-Negative Metastatic Breast Cancer. Cancer Res. 2023, 83, P5-02-49. [Google Scholar] [CrossRef]
- Samuel Eziokwu, A.; Varella, L.; Lynn Kruse, M.; Jia, X.; Moore, H.C.F.; Thomas Budd, G.; Abraham, J.; Montero, A.J. Real-World Outcomes of Cyclin-Dependent Kinase Inhibitors Continued Beyond First Disease Progression in Hormone Receptor-Positive Metastatic Breast Cancer. Clin. Breast Cancer 2021, 21, 205–209. [Google Scholar] [CrossRef]
- Kruse, M.; Smyth, E.N.; Bowman, L.; Gautam, S.; Guimaraes, C.M.; Nisbett, A.R.; Fisher, M.D.; Cui, Z.L.; Sheffield, K.M.; Kalinsky, K. Treatment Patterns and Outcomes Associated with Sequential and Non-Sequential Use of CDK4 & 6 Inhibitors in Patients with HR+, HER2- MBC in the Real World. Breast Cancer Res. Treat. 2023, 201, 105–115. [Google Scholar] [CrossRef]
- Tamragouri, K.; Cobleigh, M.A.; Rao, R.D. Abemaciclib with or without Fulvestrant for the Treatment of Hormone Receptor-Positive and HER2-Negative Metastatic Breast Cancer with Disease Progression Following Prior Treatment with Palbociclib. J. Clin. Oncol. 2019, 37, e12533. [Google Scholar] [CrossRef]
- dos Anjos, C.H.; Razavi, P.; Herbert, J.; Colon, J.; Gill, K.; Modi, S.; Bromberg, J.; Dang, C.T.; Liu, D.; Norton, L.; et al. A Large Retrospective Analysis of CDK 4/6 Inhibitor Retreatment in ER+ Metastatic Breast Cancer (MBC). J. Clin. Oncol. 2019, 37, 1053. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and Misleading Statistical Results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Grosso, V.; Todoerti, M.; Caporali, R. Randomized Controlled Trials and Real-World Data: Differences and Similarities to Untangle Literature Data. Rheumatology 2018, 57, vii54–vii58. [Google Scholar] [CrossRef] [PubMed]
- Benz, C.C. Impact of Aging on the Biology of Breast Cancer. Crit. Rev. Oncol. Hematol. 2008, 66, 65–74. [Google Scholar] [CrossRef]
- Yau, C.; Fedele, V.; Roydasgupta, R.; Fridlyand, J.; Hubbard, A.; Gray, J.W.; Chew, K.; Dairkee, S.H.; Moore, D.H.; Schittulli, F.; et al. Aging Impacts Transcriptomes but Not Genomes of Hormone-Dependent Breast Cancers. Breast Cancer Res. 2007, 9, R59. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhu, Y.; Liu, X.; Liao, X.; He, J.; Niu, L. The Clinicopathological Features and Survival Outcomes of Patients with Different Metastatic Sites in Stage IV Breast Cancer. BMC Cancer 2019, 19, 1091. [Google Scholar] [CrossRef]
- Norouzi, S.; Gorgi Valokala, M.; Mosaffa, F.; Zirak, M.R.; Zamani, P.; Behravan, J. Crosstalk in Cancer Resistance and Metastasis. Crit. Rev. Oncol. Hematol. 2018, 132, 145–153. [Google Scholar] [CrossRef]
- Luque-Bolivar, A.; Pérez-Mora, E.; Villegas, V.E.; Rondón-Lagos, M. Resistance and Overcoming Resistance in Breast Cancer. Breast Cancer 2020, 12, 211–229. [Google Scholar] [CrossRef]
- Jacquet, E.; Lardy-Cléaud, A.; Pistilli, B.; Franck, S.; Cottu, P.; Delaloge, S.; Debled, M.; Vanlemmens, L.; Leheurteur, M.; Guizard, A.V.; et al. Endocrine Therapy or Chemotherapy as First-Line Therapy in Hormone Receptor–Positive HER2-Negative Metastatic Breast Cancer Patients. Eur. J. Cancer 2018, 95, 93–101. [Google Scholar] [CrossRef]
- Ravani, L.V.; Calomeni, P.; Vilbert, M.; Madeira, T.; Wang, M.; Deng, D.; Testa, L.; Colombo Bonadio, R.; Clifton, K.; Wander, S.A.; et al. Efficacy of Subsequent Treatments After Disease Progression on CDK4/6 Inhibitors in Patients With Hormone Receptor–Positive Advanced Breast Cancer. JCO Oncol. Pract. 2024, OP-24-00649. [Google Scholar] [CrossRef] [PubMed]
- Dustin, D.; Gu, G.; Fuqua, S.A.W. ESR1 Mutations in Breast Cancer. Cancer 2019, 125, 3714–3728. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Neven, P.; Casalnuovo, M.L.; Kim, S.-B.; Tokunaga, E.; Aftimos, P.; Saura, C.; O’Shaughnessy, J.; Harbeck, N.; Carey, L.A.; et al. Imlunestrant with or without Abemaciclib in Advanced Breast Cancer. N. Engl. J. Med. 2025, 392, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.-C.; Hardy-Bessard, A.-C.; Dalenc, F.; Bachelot, T.; Pierga, J.-Y.; de la Motte Rouge, T.; Sabatier, R.; Dubot, C.; Frenel, J.-S.; Ferrero, J.M.; et al. Switch to Fulvestrant and Palbociclib versus No Switch in Advanced Breast Cancer with Rising ESR1 Mutation during Aromatase Inhibitor and Palbociclib Therapy (PADA-1): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef]
- Nathan, M.R.; Schmid, P. A Review of Fulvestrant in Breast Cancer. Oncol. Ther. 2017, 5, 17–29. [Google Scholar] [CrossRef]
- George, M.A.; Qureshi, S.; Omene, C.; Toppmeyer, D.L.; Ganesan, S. Clinical and Pharmacologic Differences of CDK4/6 Inhibitors in Breast Cancer. Front. Oncol. 2021, 11, 693104. [Google Scholar] [CrossRef] [PubMed]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortés, J.; Diéras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR+/HER2- Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef]
- O’Brien, N.; Conklin, D.; Beckmann, R.; Luo, T.; Chau, K.; Thomas, J.; Mc Nulty, A.; Marchal, C.; Kalous, O.; von Euw, E.; et al. Preclinical Activity of Abemaciclib Alone or in Combination with Antimitotic and Targeted Therapies in Breast Cancer. Mol. Cancer Ther. 2018, 17, 897–907. [Google Scholar] [CrossRef]
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical Characterization of the CDK4/6 Inhibitor LY2835219: In-Vivo Cell Cycle-Dependent/Independent Anti-Tumor Activities Alone/in Combination with Gemcitabine. Investig. New Drugs 2014, 32, 825–837. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Huober, J.; Sohn, J.; Trédan, O.; Park, I.H.; Campone, M.; Chen, S.-C.; Manso, L.M.; Paluch-Shimon, S.; et al. Abemaciclib plus a Nonsteroidal Aromatase Inhibitor as Initial Therapy for HR+, HER2- Advanced Breast Cancer: Final Overall Survival Results of MONARCH 3. Ann. Oncol. 2024, 35, 718–727. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Diéras, V.; Rugo, H.S.; Harbeck, N.; Im, S.-A.; Gelmon, K.A.; Lipatov, O.N.; Walshe, J.M.; Martin, M.; Chavez-MacGregor, M.; et al. Overall Survival With Palbociclib Plus Letrozole in Advanced Breast Cancer. J. Clin. Oncol. 2024, 42, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Grinshpun, A.; Tolaney, S.M.; Burstein, H.J.; Jeselsohn, R.; Mayer, E.L. The Dilemma of Selecting a First Line CDK4/6 Inhibitor for Hormone Receptor-Positive/HER2-Negative Metastatic Breast Cancer. NPJ Breast Cancer 2023, 9, 15. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Kettner, N.M.; Rao, X.; Bishop, C.S.; Bui, T.N.; Wingate, H.F.; Raghavendra, A.S.; Wang, Y.; Wang, J.; Sahin, A.A.; et al. Abemaciclib Is Effective in Palbociclib-Resistant Hormone Receptor-Positive Metastatic Breast Cancers. Cancer Res. 2023, 83, 3264–3283. [Google Scholar] [CrossRef] [PubMed]
- Zapatero-Solana, E.; Ganado, M.P.; Ortiz-Ruiz, M.J.; Mur, C.; Litchfield, L.; Merzoug, F.; Puig, O.; Lallena, M.J. Abstract 2307: Sequential Treatment with Abemaciclib plus Endocrine Therapy Inhibits Cell Proliferation and Triggers Apoptosis in Cell Lines Resistant to CDK4/6i. Cancer Res. 2022, 82, 2307. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Bardia, A.; Hu, X.; Dent, R.; Yonemori, K.; Barrios, C.H.; O’Shaughnessy, J.A.; Wildiers, H.; Pierga, J.-Y.; Zhang, Q.; Saura, C.; et al. Trastuzumab Deruxtecan after Endocrine Therapy in Metastatic Breast Cancer. N. Engl. J. Med. 2024, 391, 2110–2122. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Sonke, G.S.; van Ommen-Nijhof, A.; Wortelboer, N.; van der Noort, V.; Swinkels, A.C.P.; Blommestein, H.M.; Guerrero Paez, C.; Mol, L.; Beeker, A.; Beelen, K.; et al. Early versus Deferred Use of CDK4/6 Inhibitors in Advanced Breast Cancer. Nature 2024, 636, 474–480. [Google Scholar] [CrossRef]


| Study ID | Study Design | Sample Size and Patient Population | Intervention Arm | Control Arm | Primary Endpoint | |
|---|---|---|---|---|---|---|
| 1. | Mayer 2024 [29] * (PACE) | Phase II RCT | Ni = 111 Nc = 55 ER+ HER2- aBC, PD on AI + CDK4/6i (at least 6 m of therapy in the metastatic setting or ≤12 m of CDK4/6i as adjuvant); 1 CT and ≤2 ET allowed | Palbociclib + fulvestrant | Fulvestrant | PFS |
| 2. | Kalinsky 2023 [28] (MAINTAIN) | Phase II RCT | Ni = 60 Nc = 59 ER+ HER2- aBC, PD on ET + CDK4/6i, ≤1 CT allowed | Ribociclib + fulvestrant/exemestane | Placebo + fulvestrant/exemestane | PFS |
| 3. | Kalinsky 2024 [35] (postMONARCH) | Phase III RCT | Ni = 182 Nc = 186 ER+ HER2- aBC, PD on CDK4/6i + AI as 1st-line therapy or relapse on/after a CDK4/6i + ET as adjuvant therapy for eBC. No other prior treatment for aBC was permitted. | Abemaciclib + fulvestrant | Placebo + fulvestrant | PFS |
| 4. | Llombart-Cussac 2023 [34] (PALMIRA) | Phase II RCT | Ni = 136 Nc = 62 women with ER+ HER2- aBC who had PD on 1st-line palbociclib plus ET with benefit (SD or better response for ≥24 weeks) or in eBC adjuvant P + ET for ≥12 m, and within 12 m of completion | Palbociclib + 2nd-line ET | 2nd-line ET alone | PFS |
| 5. | Albanell 2023 [36] (BioPER) | Phase II single arm | N = 33 Women with ER+ HER2- aBC, PD on palbociclib + ET with documented benefit (SD or better response for ≥24 weeks), intervening treatment not allowed | Palbociclib + ET of physician’s choice | - | CBR and % of tumors with baseline loss of Rb protein expression |
| 6. | Tao 2022 [38] | Phase II single arm | N = 58 ER+ HER2- aBC, PD on palbociclib + AI (after 1st-line palbociclib + AI at least for ≥24 weeks) | Palbociclib + fulvestrant | - | PFS |
| 7. | Mariotti 2019 [37] | Retrospective | N = 19 ER+ HER2- aBC, PD on palbociclib + ET | Abemaciclib +/− ET | - | - |
| 8. | Wander 2021 [25] | Retrospective | N = 87 ER+ HER2- aBC, PD on palbociclib/ribociclib plus ET 71.3% received some intervening therapy | Abemaciclib +/− ET | - | - |
| 9. | Eziokwu 2021 [40] | Retrospective | N = 30 women with ER+ HER2- aBC, PD on CDK4/6i where CDK4/6i was continued beyond PD, intervening treatment not allowed | CDK4/6i + ET b | - | PFS |
| 10. | Tamragouri 2019 [42] | Retrospective | N = 21 ER+ HER2- aBC, PD on palbociclib + ET | Abemaciclib +/− fulvestrant | - | - |
| 11. | dos Anjos 2019 [43], cohorts 2 and 3 | Retrospective | N = 43 in cohort 2 N = 84 in cohort 3 ER+ HER2- aBC, PD on CDK4/6i + ET | Cohort 2: same CDK4/6i + change in ET Cohort 3: different CDK4/6i +/− ET | - | Time to subsequent therapy TTST |
| 12. | Seki 2022 [39] | Retrospective | N = 25 ER+ HER2- aBC, PD on palbociclib + ET | Abemaciclib +/− fulvestrant | - | - |
| 13. | Kruse 2023 [41] | Retrospective | N (cohort 1): 165 N (cohort 2): 115 ER+, HER2-, and documentation of an abemaciclib- containing regimen and at least 1 other anti-cancer systemic therapy | Cohort 1: subsequent CDK4/6i after 1st-line CDK4/6i Cohort 2: subsequent CDK4/6i after 2nd-line CDK4/6i | - | PFS |
| Study ID | Age (Median Years) | Visceral Disease | mESR1 | Prior Palbociclib | Prior CT | CDK 4/6i ≥ 12 Months | PFS (Months); HR, (CI) | OS, Median (Months); HR (95% CI) | ORR (%) | CBR (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Mayer 2024 * [29] (PACE) | 57 | 59.6% | 54% | 92.8% | 16.3% | 77.7% | 4.6 vs. 4.8; HR, 1.11 (0.79 to 1.55) | 24.6 vs. 27.5; HR 1.02 (0.67 to 1.56) | 9% vs. 7% a | 32.4% vs. 29.1 a % |
| Kalinsky 2023 [28] (MAINTAIN) | 57 | 59.7% | 42.3% | 86.6% | 9.2% | 67% | 5.3 vs. 2.8; HR, 0.57 (0.39–0.85 | - | 20% vs. 11% p 0.51 | 43% vs. 25% p 0.06 |
| Kalinsky 2024 [35] (postMONARCH) | 61 | 60.5% | 46.8% | 59% | 0 | 76.1% | 6 vs. 5.3; HR, 0.73 (0.57–0.95) | immature | 17% vs. 7% p 0.01 | - |
| Lombart-Cussac 2023 [34] (PALMIRA) | 59 | 61.1% | - | 100% | 0 | 85.9% | 4.2 vs. 3.6; HR, 0.8 (0.6–1.1) | 28.3 vs. 28.8; HR, 1.06 (0.75–1.51) | 6.4% vs. 2.3% | 33% vs. 29.5% |
| Albanell 2023 [36] (BioPER) | 59.5 | 78.1% | 52% | 100% | 12.5% | - | 2.6 (95%CI 1.8–6.7) | 23.9 (95% CI 16.4-NE) | 6.3% | 34.4% |
| Tao 2022 [38] | 57.9 | - | - | - | - | - | 3.7 | - | - | - |
| Mariotti 2019 [37] | 57 | 73.8% | - | 100% | - | - | 7.0 (95%CI 1.8–12.1) | - | 0 | 33% |
| Wander 2021 [25] | 52 | - | - | 100% | - | - | 5.3 (95% CI 3.5–7.8) | 17.2 (13.2-NR) | - | - |
| Eziokwu 2021 [40] | 47.5 | 43.3% | - | 100% | - | - | 11.8 (95%CI 5.34–13.13) | 45.4 | - | - |
| Tamragouri 2019 [42] | 57.8 | - | - | 100% | - | - | - | - | - | 29% |
| dos Anjos 2019 [43] Cohort 2 | - | - | - | - | - | - | 4.5 (95%CI 3.3–7.6) | - | - | - |
| dos Anjos 2019 [43] Cohort 3 | - | - | - | - | - | - | 4.4 (95%CI 3.8–5.9) | - | 29% | - |
| Seki 2022 [39] | 69 | 68% | - | - | - | - | 5.3 (95%CI 3.08–7.52) | - | 16% | 44% |
| Kruse 2023 [41] | cohort 1: 63.3 cohort 2: 66.5 | - | - | - | - | - | cohort 1: 12.7 (95%Ci 9.4, 20) cohort 2: 9.1 (95%CI 5.2, 12.1) | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathak, N.; Kumar, S.; Gimenez, D.M.; Di Iorio, M.; Savill, J.; Berner-Wygoda, Y.; Li, M.; Valiente, C.M.; Cuthbert, D.; Gupta, A.; et al. Continuing Cyclin-Dependent Kinase 4/6 Inhibitors Beyond Progression in Advanced Breast Cancer: A Meta-Analysis. Cancers 2025, 17, 1609. https://doi.org/10.3390/cancers17101609
Pathak N, Kumar S, Gimenez DM, Di Iorio M, Savill J, Berner-Wygoda Y, Li M, Valiente CM, Cuthbert D, Gupta A, et al. Continuing Cyclin-Dependent Kinase 4/6 Inhibitors Beyond Progression in Advanced Breast Cancer: A Meta-Analysis. Cancers. 2025; 17(10):1609. https://doi.org/10.3390/cancers17101609
Chicago/Turabian StylePathak, Neha, Sudhir Kumar, Diego Malon Gimenez, Massimo Di Iorio, Jacqueline Savill, Yael Berner-Wygoda, Meredith Li, Consolacion Molto Valiente, Danielle Cuthbert, Aarushi Gupta, and et al. 2025. "Continuing Cyclin-Dependent Kinase 4/6 Inhibitors Beyond Progression in Advanced Breast Cancer: A Meta-Analysis" Cancers 17, no. 10: 1609. https://doi.org/10.3390/cancers17101609
APA StylePathak, N., Kumar, S., Gimenez, D. M., Di Iorio, M., Savill, J., Berner-Wygoda, Y., Li, M., Valiente, C. M., Cuthbert, D., Gupta, A., Arteaga, D. P., Batra, A., Amir, E., & Mittal, A. (2025). Continuing Cyclin-Dependent Kinase 4/6 Inhibitors Beyond Progression in Advanced Breast Cancer: A Meta-Analysis. Cancers, 17(10), 1609. https://doi.org/10.3390/cancers17101609






