Unraveling the Burden of Pancreatic Cancer in the 21st Century: Trends in Incidence, Mortality, Survival, and Key Contributing Factors
Simple Summary
Abstract
1. Introduction
2. Material and Methods
Epidemiology of PC
3. Changes in Incidence
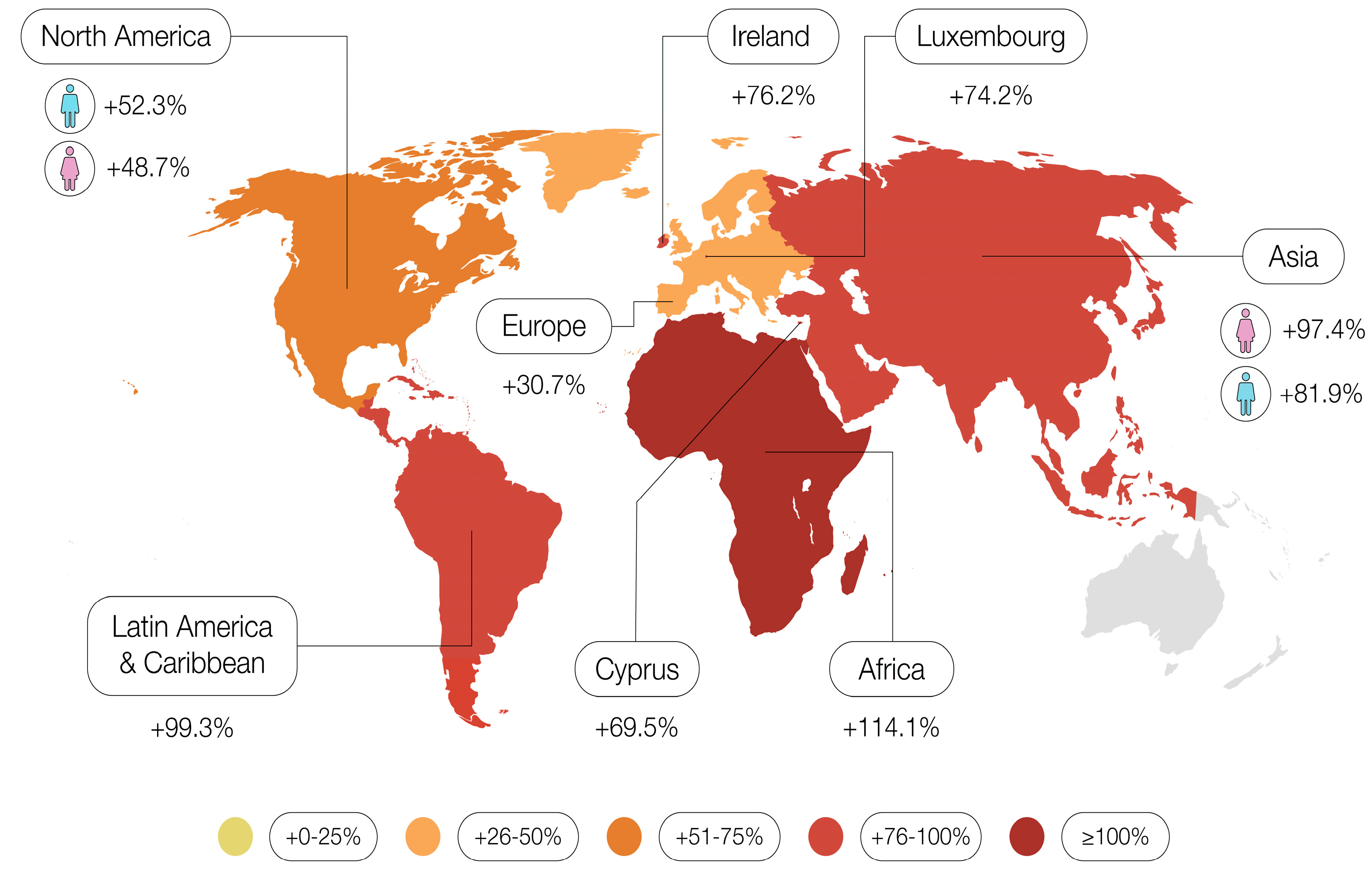
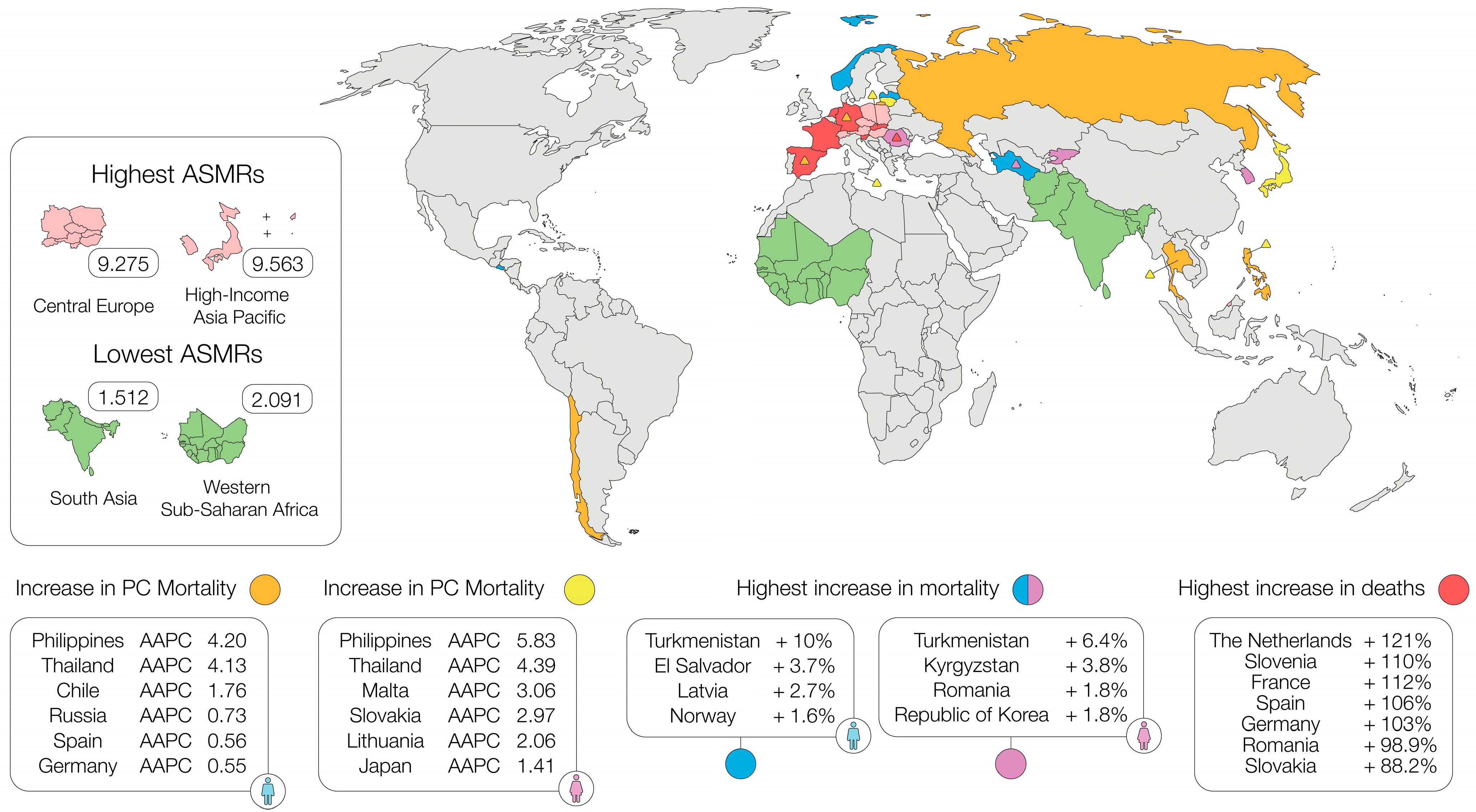
4. Changes in Mortality
- Africa: +82.9% for men, +81.9% for women.
- Asia: +58.2% for men, +62.4% for women.
- Latin America and the Caribbean: +60.8% for men, +56.7% for women.
- Northern America: +52.3% for men, +44% for women.
- Oceania: +65.9% for men, +63.5% for women.
- Europe: +30.1% for men, +21.4% for women.
5. Changes in Survival
6. Reasons for Increasing Numbers
6.1. Demographic Shifts
6.2. Tobacco Smoking and Chronic Pancreatitis
6.3. Impact of the COVID-19 Pandemic
6.4. Diabetes as a Major Risk Factor
6.5. Obesity on the Rise
6.6. Increasing Incidence of PC in Younger Individuals
6.7. Tobacco Smoking and Its Continuing Impact Among Younger People
6.8. Global Trends in Young-Onset PC
6.9. Survival Outcomes in Early-Onset vs. Later-Onset PC
7. Reasons for Improved Survival
7.1. Advancements in Surgery and Centralization
7.2. Perioperative Chemotherapy
7.3. Advancements in Chemotherapy Regimes
8. Limitations and Future Directions
9. Conclusions
9.1. The Role of Modifiable Risk Factors
9.2. Early-Onset Pancreatic Cancer
9.3. The Need for Coordinated Public Health Interventions
9.4. Advances in Therapeutic Strategies Leading to Improved Survival
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 19 November 2024).
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, R.; Lin, D.W.; Montgomery, R.B. Prostate Cancer. JAMA 2025, 333, 1433. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Goggins, W.B.; Wang, H.H.; Fung, F.D.; Leung, C.; Wong, S.Y.; Ng, C.F.; Sung, J.J. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur. Urol. 2016, 70, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Zheng, Y.; Li, N.; Deng, Y.; Zhou, L.; Tian, T.; Yang, S.; Hao, Q.; Song, D.; Wu, Y.; et al. Incidence and disease burden of prostate cancer from 1990 to 2017: Results from the Global Burden of Disease Study 2017. Cancer 2020, 126, 1969–1978. [Google Scholar] [CrossRef]
- Clark, R.; Vesprini, D.; Narod, S.A. The Effect of Age on Prostate Cancer Survival. Cancers 2022, 14, 4149. [Google Scholar] [CrossRef]
- Siegel, D.A.; O’Neil, M.E.; Richards, T.B.; Dowling, N.F.; Weir, H.K. Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity—United States, 2001–2017. MMWR Morb. Mortal Wkly Rep. 2020, 69, 1473–1480. [Google Scholar] [CrossRef]
- Ugai, T.; Sasamoto, N.; Lee, H.Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef]
- Rémond, M.; Smolenschi, C.; Tarabay, A.; Gelli, M.; Fernandez-de-Sevilla, E.; Mouawia, A.; Cosconea, S.; Tselikas, L.; Barbe, R.; Fuerea, A.; et al. Clinical and molecular features of early onset pancreatic adenocarcinoma. Int. J. Cancer 2024, 155, 1969–1981. [Google Scholar] [CrossRef]
- Ulanja, M.B.; Moody, A.E.; Beutler, B.D.; Antwi-Amoabeng, D.; Rahman, G.A.; Alese, O.B. Early-onset pancreatic cancer: A review of molecular mechanisms, management, and survival. Oncotarget 2022, 13, 828–841. [Google Scholar] [CrossRef]
- Leonhardt, C.S.; Kinny-Köster, B.; Hank, T.; Habib, J.R.; Shoucair, S.; Klaiber, U.; Cameron, J.L.; Hackert, T.; Wolfgang, C.L.; Büchler, M.W.; et al. Resected Early-Onset Pancreatic Cancer: Practices and Outcomes in an International Dual-Center Study. Ann. Surg. Oncol. 2023, 30, 2433–2443. [Google Scholar] [CrossRef]
- Piciucchi, M.; Capurso, G.; Valente, R.; Larghi, A.; Archibugi, L.; Signoretti, M.; Stigliano, S.; Zerboni, G.; Barucca, V.; La Torre, M.; et al. Early onset pancreatic cancer: Risk factors, presentation and outcome. Pancreatology 2015, 15, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Debernardi, S.; Liszka, L.; Ntala, C.; Steiger, K.; Esposito, I.; Carlotti, E.; Baker, A.M.; McDonald, S.; Graham, T.; Dmitrovic, B.; et al. Molecular characteristics of early-onset pancreatic ductal adenocarcinoma. Mol. Oncol. 2024, 18, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Alese, O.B.; Jiang, R.; Shaib, W.; Wu, C.; Akce, M.; Gaines, T.; Ni, L.; Behera, M.; El-Rayes, B.F. Young Adults with Pancreatic Cancer. Pancreas 2020, 49, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Maisonneuve, P.; Löhr, J.M.; Lowenfels, A.B. Early Onset Pancreatic Cancer: Evidence of a Major Role for Smoking and Genetic Factors. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1894–1897. [Google Scholar] [CrossRef]
- Ilic, I.; Ilic, M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J. Gastroenterol. 2022, 28, 4698–4715. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer: A growing burden. Lancet Gastroenterol. Hepatol. 2019, 4, 895–896. [Google Scholar] [CrossRef]
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef]
- Sebesta, C.; Günther Sebesta, C.; Christine Sebesta, M.; Köcher, M.; Müllner- Ammer, K.; Zottl, J. How the Fight against Stomach Cancer can be won: Decline in Incidence of Gastric Cancer in Europe: A Review of Epidemiologic Trends, Contributing Factors and Recent Treatment Standards. J. Cancer Sci. Clin. Ther. 2024, 8, 295–309. [Google Scholar] [CrossRef]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory Statistic Data. Available online: https://gco.iarc.fr/today/home (accessed on 19 November 2024).
- 2018 Statistical Update: Human Development Indices and Indicators. Available online: http://hdr.undp.org/en/2018-update (accessed on 19 November 2024).
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.J.; Wong, M.C.S. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, R.; Li, J.; Zeng, H.; Li, L.; Chen, R.; Sun, K.; Han, B.; Bray, F.; Wei, W.; et al. Global, regional, and national lifetime risks of developing and dying from gastrointestinal cancers in 185 countries: A population-based systematic analysis of GLOBOCAN. Lancet Gastroenterol. Hepatol. 2024, 9, 229–237. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Hur, C.; Tramontano, A.C.; Dowling, E.C.; Brooks, G.A.; Jeon, A.; Brugge, W.R.; Gazelle, G.S.; Kong, C.Y.; Pandharipande, P.V. Early Pancreatic Ductal Adenocarcinoma Survival Is Dependent on Size. Pancreas 2016, 45, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef]
- Calderwood, A.H.; Sawhney, M.S.; Thosani, N.C.; Rebbeck, T.R.; Wani, S.; Canto, M.I.; Fishman, D.S.; Golan, T.; Hidalgo, M.; Kwon, R.S.; et al. American Society for Gastrointestinal Endoscopy guideline on screening for pancreatic cancer in individuals with genetic susceptibility: Methodology and review of evidence. Gastrointest. Endosc. 2022, 95, 827–854.e3. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Li, T.; Lin, C.; Wang, W. Global, regional, and national burden of pancreatic cancer from 1990 to 2021, its attributable risk factors, and projections to 2050: A systematic analysis of the global burden of disease study 2021. BMC Cancer 2025, 25, 189. [Google Scholar] [CrossRef]
- GBD 2021 Forecasting Collaborators. Burden of disease scenarios for 204 countries and territories, 2022–2050: A forecasting analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2204–2256. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, D.; Meng, F.; Wang, J.; Wang, B.; Qiang, J.; Shen, L.; Wang, M.; Fang, H. The global, regional burden of pancreatic cancer and its attributable risk factors from 1990 to 2021. BMC Cancer 2025, 25, 186. [Google Scholar] [CrossRef] [PubMed]
- Koh, B.; Tan, D.J.H.; Ng, C.H.; Fu, C.E.; Lim, W.H.; Zeng, R.W.; Yong, J.N.; Koh, J.H.; Syn, N.; Meng, W.; et al. Patterns in Cancer Incidence Among People Younger Than 50 Years in the US, 2010 to 2019. JAMA Netw. Open 2023, 6, e2328171. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Siegel, R.L.; Rosenberg, P.S.; Jemal, A. Emerging cancer trends among young adults in the USA: Analysis of a population-based cancer registry. Lancet Public Health 2019, 4, e137–e147. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Pancreatic Cancer Incidence Statistics. 2024. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/pancreatic-cancer/incidence#heading-One (accessed on 19 November 2024).
- Gaddam, S.; Abboud, Y.; Oh, J.; Samaan, J.S.; Nissen, N.N.; Lu, S.C.; Lo, S.K. Incidence of Pancreatic Cancer by Age and Sex in the US, 2000–2018. JAMA 2021, 326, 2075. [Google Scholar] [CrossRef]
- Abboud, Y.; Samaan, J.S.; Oh, J.; Jiang, Y.; Randhawa, N.; Lew, D.; Ghaith, J.; Pala, P.; Leyson, C.; Watson, R.; et al. Increasing Pancreatic Cancer Incidence in Young Women in the United States: A Population-Based Time-Trend Analysis, 2001–2018. Gastroenterology 2023, 164, 978–989.e6. [Google Scholar] [CrossRef]
- Dahia, S.S.; Konduru, L.; Pandol, S.J.; Barreto, S.G. The burden of young-onset pancreatic cancer and its risk factors from 1990 to 2019: A systematic analysis of the global burden of disease study 2019. Pancreatology 2024, 24, 119–129. [Google Scholar] [CrossRef]
- Li, T.; Qin, C.; Zhao, B.; Li, Z.; Zhao, Y.; Lin, C.; Wang, W. Global and regional burden of pancreatitis: Epidemiological trends, risk factors, and projections to 2050 from the global burden of disease study 2021. BMC Gastroenterol. 2024, 24, 398. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Sun, C.; Li, Z.; Fei, H.; Zhao, D. Global, regional, and national burdens of early onset pancreatic cancer in adolescents and adults aged 15–49 years from 1990 to 2019 based on the Global Burden of Disease Study 2019: A cross-sectional study. Int. J. Surg. 2024, 110, 1929–1940. [Google Scholar] [CrossRef]
- Patel, V.R.; Adamson, A.S.; Liu, J.B.; Welch, H.G. Increasing Incidence and Stable Mortality of Pancreatic Cancer in Young Americans. Ann. Intern. Med. 2024, 178, 142–144. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P. Epidemiology and burden of pancreatic cancer. Presse Med. 2019, 48, e113–e123. [Google Scholar] [CrossRef]
- Didier, A.J.; Nandwani, S.; Fahoury, A.M.; Craig, D.J.; Watkins, D.; Campbell, A.; Spencer, C.T.; Batten, M.; Vijendra, D.; Sutton, J.M. Trends in pancreatic cancer mortality in the United States 1999–2020: A CDC database population-based study. Cancer Causes Control 2024, 35, 1509–1516. [Google Scholar] [CrossRef]
- Yu, J.; Yang, X.; He, W.; Ye, W. Burden of pancreatic cancer along with attributable risk factors in Europe between 1990 and 2019, and projections until 2039. Int. J Cancer 2021, 149, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Kilander, C.; Mattsson, F.; Ljung, R.; Lagergren, J.; Sadr-Azodi, O. Systematic underreporting of the population-based incidence of pancreatic and biliary tract cancers. Acta Oncol. 2014, 53, 822–829. [Google Scholar] [CrossRef]
- Fest, J.; Ruiter, R.; van Rooij, F.J.; van der Geest, L.G.; Lemmens, V.E.; Ikram, M.A.; Coebergh, J.W.; Stricker, B.H.; van Eijck, C.H. Underestimation of pancreatic cancer in the national cancer registry–Reconsidering the incidence and survival rates. Eur. J. Cancer 2017, 72, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.L.; Malvezzi, M.; Carioli, G.; Negri, E.; La Vecchia, C.; Boffetta, P.; Bosetti, C. Global Trends in Pancreatic Cancer Mortality From 1980 Through 2013 and Predictions for 2017. Clin. Gastroenterol. Hepatol. 2016, 14, 1452–1462.e4. [Google Scholar] [CrossRef]
- Ferlay, J.E.M.; Lam, F.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjomataram, I. Global Cancer Observatory: Cancer Tomorrow. Lyon, France: International Agency for Research on Cancer. Available online: http://gco.iarc.fr/tomorrow/graphic-isotype?type=1&population=900&mode=population&sex=0&cancer=39&age_group=value&apc_male=0&apc_female=0 (accessed on 19 January 2025).
- Santucci, C.; Mignozzi, S.; Malvezzi, M.; Boffetta, P.; Collatuzzo, G.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2024 with focus on colorectal cancer. Ann. Oncol. 2024, 35, 308–316. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2024; American Cancer Society: Atlanta, GA, USA, 2024. [Google Scholar]
- Bengtsson, A.; Andersson, R.; Ansari, D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci. Rep. 2020, 10, 16425. [Google Scholar] [CrossRef]
- Khalaf, N.; El-Serag, H.B.; Abrams, H.R.; Thrift, A.P. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clin. Gastroenterol. Hepatol. 2021, 19, 876–884. [Google Scholar] [CrossRef]
- Chen, C.; Yin, L.; Lu, C.; Wang, G.; Li, Z.; Sun, F.; Wang, H.; Li, C.; Dai, S.; Lv, N.; et al. Trends in 5-year cancer survival disparities by race and ethnicity in the US between 2002–2006 and 2015–2019. Sci. Rep. 2024, 14, 22715. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Chen, C.; Guo, J.; Qiao, M.; Lyu, J. Recent estimates and predictions of 5-year survival rate in patients with pancreatic cancer: A model-based period analysis. Front. Med. 2022, 9, 1049136. [Google Scholar] [CrossRef]
- Rossi, S.; Baili, P.; Capocaccia, R.; Caldora, M.; Carrani, E.; Minicozzi, P.; Pierannunzio, D.; Santaquilani, M.; Trama, A.; Allemani, C.; et al. The EUROCARE-5 study on cancer survival in Europe 1999–2007: Database, quality checks and statistical analysis methods. Eur. J. Cancer 2015, 51, 2104–2119. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, K.; Försti, A.; Hemminki, O.; Liska, V.; Hemminki, A. Long-term survival trends for primary liver and pancreatic cancers in the Nordic countries. JHEP Rep. 2022, 4, 100602. [Google Scholar] [CrossRef]
- Islami, F.; Marlow, E.C.; Thomson, B.; McCullough, M.L.; Rumgay, H.; Gapstur, S.M.; Patel, A.V.; Soerjomataram, I.; Jemal, A. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States, 2019. CA Cancer J. Clin. 2024, 74, 405–432. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xie, Z.; Wang, Q.; Wang, B.; Huang, R.; Xu, W.; Shang, C.; Chen, Y. Epidemiological trends in gastrointestinal cancers and risk factors across U.S. states from 2000 to 2021: A systematic analysis for the global burden of disease study 2021. BMC Public Health 2025, 25, 43. [Google Scholar] [CrossRef]
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef]
- England, K.; Azzopardi-Muscat, N. Demographic trends and public health in Europe. Eur. J. Public Health 2017, 27 (Suppl. 4), 9–13. [Google Scholar] [CrossRef]
- Khan, H.T.A. Population ageing in a globalized world: Risks and dilemmas? J. Eval. Clin. Pract. 2019, 25, 754–760. [Google Scholar] [CrossRef]
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez Robledo, L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The burden of disease in older people and implications for health policy and practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef]
- Vollset, S.E.; Goren, E.; Yuan, C.W.; Cao, J.; Smith, A.E.; Hsiao, T.; Bisignano, C.; Azhar, G.S.; Castro, E.; Chalek, J.; et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: A forecasting analysis for the Global Burden of Disease Study. Lancet 2020, 396, 1285–1306. [Google Scholar] [CrossRef]
- Bhasin, S.; Kerr, C.; Oktay, K.; Racowsky, C. The Implications of Reproductive Aging for the Health, Vitality, and Economic Welfare of Human Societies. J. Clin. Endocrinol. Metab. 2019, 104, 3821–3825. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.E.; Gupta, S.; Kang, J.Y.; Quinn, M.J.; Maxwell, J.D.; Mudan, S.; Majeed, A. Pancreatic cancer in England and Wales 1975–2000: Patterns and trends in incidence, survival and mortality. Aliment. Pharmacol. Ther. 2006, 23, 1205–1214. [Google Scholar] [CrossRef]
- Midha, S.; Chawla, S.; Garg, P.K. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016, 381, 269–277. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, Y.; Schwebel, D.C.; Liu, Z.; Li, L.; Cheng, P.; Ning, P.; Hu, G. Population ageing and mortality during 1990–2017: A global decomposition analysis. PLoS Med. 2020, 17, e1003138. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Smith, B.D.; Smith, G.L.; Hurria, A.; Hortobagyi, G.N.; Buchholz, T.A. Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. J. Clin. Oncol. 2009, 27, 2758–2765. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Lynch, S.M.; Vrieling, A.; Lubin, J.H.; Kraft, P.; Mendelsohn, J.B.; Hartge, P.; Canzian, F.; Steplowski, E.; Arslan, A.A.; Gross, M.; et al. Cigarette Smoking and Pancreatic Cancer: A Pooled Analysis from the Pancreatic Cancer Cohort Consortium. Am. J. Epidemiol. 2009, 170, 403–413. [Google Scholar] [CrossRef]
- Momi, N.; Kaur, S.; Ponnusamy, M.P.; Kumar, S.; Wittel, U.A.; Batra, S.K. Interplay between Smoking-induced Genotoxicity and Altered Signaling in Pancreatic Carcinogenesis. Carcinogenesis 2012, 33, 1617–1628. [Google Scholar] [CrossRef]
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lugo, A.; Peveri, G.; Bosetti, C.; Bagnardi, V.; Crippa, A.; Orsini, N.; Rota, M.; Gallus, S. Strong excess risk of pancreatic cancer for low frequency and duration of cigarette smoking: A comprehensive review and meta-analysis. Eur. J. Cancer 2018, 104, 117–126. [Google Scholar] [CrossRef]
- Flor, L.S.; Reitsma, M.B.; Gupta, V.; Ng, M.; Gakidou, E. The effects of tobacco control policies on global smoking prevalence. Nat. Med. 2021, 27, 239–243. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2030; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Aune, D.; Mahamat-Saleh, Y.; Norat, T.; Riboli, E. Tobacco smoking and the risk of pancreatitis: A systematic review and meta-analysis of prospective studies. Pancreatology 2019, 19, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D. Alcohol Consumption, Cigarette Smoking, and the Risk of Recurrent Acute and Chronic Pancreatitis. Arch. Intern. Med. 2009, 169, 1035. [Google Scholar] [CrossRef]
- Coté, G.A.; Yadav, D.; Slivka, A.; Hawes, R.H.; Anderson, M.A.; Burton, F.R.; Brand, R.E.; Banks, P.A.; Lewis, M.D.; Disario, J.A.; et al. Alcohol and Smoking as Risk Factors in an Epidemiology Study of Patients with Chronic Pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 266–273. [Google Scholar] [CrossRef]
- Nøjgaard, C.; Becker, U.; Matzen, P.; Andersen, J.R.; Holst, C.; Bendtsen, F. Progression from Acute to Chronic Pancreatitis. Pancreas 2011, 40, 1195–1200. [Google Scholar] [CrossRef]
- Whitcomb, D.C.; Yadav, D.; Adam, S.; Hawes, R.H.; Brand, R.E.; Anderson, M.A.; Money, M.E.; Banks, P.A.; Bishop, M.D.; Baillie, J.; et al. Multicenter Approach to Recurrent Acute and Chronic Pancreatitis in the United States: The North American Pancreatitis Study 2 (NAPS2). Pancreatology 2008, 8, 520–531. [Google Scholar] [CrossRef]
- Löhr, J.M.; Dominguez-Munoz, E.; Rosendahl, J.; Besselink, M.; Mayerle, J.; Lerch, M.M.; Haas, S.; Akisik, F.; Kartalis, N.; Iglesias-Garcia, J.; et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United Eur. Gastroenterol. J. 2017, 5, 153–199. [Google Scholar] [CrossRef]
- Gardner, T.B.; Adler, D.G.; Forsmark, C.E.; Sauer, B.G.; Taylor, J.R.; Whitcomb, D.C. ACG Clinical Guideline: Chronic Pancreatitis. Am. J. Gastroenterol. 2020, 115, 322–339. [Google Scholar] [CrossRef]
- Lowenfels, A.B.; Maisonneuve, P.; Cavallini, G.; Ammann, R.W.; Lankisch, P.G.; Andersen, J.R.; Dimagno, E.P.; Andrén-Sandberg, A.; Domellöf, L. Pancreatitis and the Risk of Pancreatic Cancer. N. Engl. J. Med. 1993, 328, 1433–1437. [Google Scholar] [CrossRef]
- Korpela, T.; Udd, M.; Mustonen, H.; Ristimäki, A.; Haglund, C.; Seppänen, H.; Kylänpää, L. Association between chronic pancreatitis and pancreatic cancer: A 10-year retrospective study of endoscopically treated and surgical patients. Int. J. Cancer 2020, 147, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Munigala, S.; Subramaniam, D.S.; Subramaniam, D.P.; Burroughs, T.E.; Conwell, D.L.; Sheth, S.G. Incidence and Risk of Pancreatic Cancer in Patients with a New Diagnosis of Chronic Pancreatitis. Dig. Dis. Sci. 2022, 67, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Tran, T.P.T.; Oh, J.K. Chronic pancreatitis and cancer risk in a matched cohort study using national claims data in South Korea. Sci. Rep. 2022, 12, 5545. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Gweon, T.G.; Park, S.H.; Kim, T.H.; Kim, C.W.; Chang, J.H. Incidence and risk of pancreatic cancer in patients with chronic pancreatitis: Defining the optimal subgroup for surveillance. Sci. Rep. 2023, 13, 106. [Google Scholar] [CrossRef]
- Yadav, D.; Lowenfels, A.B. The Epidemiology of Pancreatitis and Pancreatic Cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Tan, K.; Zhang, X.L.; Han, X.; Pan, J.P.; Huang, Z.Y.; Tang, C.W.; Li, J. Incidence, prevalence, and comorbidities of chronic pancreatitis: A 7-year population-based study. World J. Gastroenterol. 2023, 29, 4671–4684. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar]
- Ali, J.K.; Riches, J.C. The Impact of the COVID-19 Pandemic on Oncology Care and Clinical Trials. Cancers 2021, 13, 5924. [Google Scholar] [CrossRef] [PubMed]
- Manso, L.; De Velasco, G.; Paz-Ares, L. Impact of the COVID-19 outbreak on cancer patient flow and management: Experience from a large university hospital in Spain. ESMO Open. 2020, 5, e000828. [Google Scholar] [CrossRef]
- Brugel, M.; Carlier, C.; Essner, C.; Debreuve-Theresette, A.; Beck, M.F.; Merrouche, Y.; Bouché, O. Dramatic Changes in Oncology Care Pathways During the COVID-19 Pandemic: The French ONCOCARE-COV Study. Oncologist 2021, 26, e338–e341. [Google Scholar] [CrossRef] [PubMed]
- Lièvre, A.; Turpin, A.; Ray-Coquard, I.; Le Malicot, K.; Thariat, J.; Ahle, G.; Neuzillet, C.; Paoletti, X.; Bouché, O.; Aldabbagh, K.; et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19). Eur. J. Cancer 2020, 141, 62–81. [Google Scholar] [CrossRef]
- Assaad, S.; Avrillon, V.; Fournier, M.L.; Mastroianni, B.; Russias, B.; Swalduz, A.; Cassier, P.; Eberst, L.; Steineur, M.P.; Kazes, M.; et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-COV-2 on RT-PCR. Eur. J. Cancer 2020, 135, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Albiges, L.; Foulon, S.; Bayle, A.; Gachot, B.; Pommeret, F.; Willekens, C.; Stoclin, A.; Merad, M.; Griscelli, F.; Lacroix, L.; et al. Determinants of the outcomes of patients with cancer infected with SARS-CoV-2: Results from the Gustave Roussy cohort. Nat. Cancer 2020, 1, 965–975. [Google Scholar] [CrossRef]
- Brugel, M.; Letrillart, L.; Evrard, C.; Thierry, A.; Tougeron, D.; El Amrani, M.; Piessen, G.; Truant, S.; Turpin, A.; d’Engremont, C.; et al. Impact of the COVID-19 pandemic on disease stage and treatment for patients with pancreatic adenocarcinoma: A French comprehensive multicentre ambispective observational cohort study (CAPANCOVID). Eur. J. Cancer 2022, 166, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Salirrosas, O.; Vega, E.A.; Panettieri, E.; Salehi, O.; Kozyreva, O.; Harandi, H.; Ganta, S.; Conrad, C. The impact of the COVID-19 pandemic on patients with pancreatic cancer. J. Gastrointest. Surg. 2024, 28, 830–835. [Google Scholar] [CrossRef]
- Hall, L.A.; McKay, S.C.; Halle-Smith, J.; Soane, J.; Osei-Bordom, D.C.; Goodburn, L.; Magill, L.; Pinkney, T.; Radhakrishna, G.; Valle, J.W.; et al. The impact of the COVID-19 pandemic upon pancreatic cancer treatment (CONTACT Study): A UK national observational cohort study. Br. J. Cancer 2023, 128, 1922–1932. [Google Scholar] [CrossRef]
- Kempf, E.; Priou, S.; Lamé, G.; Laurent, A.; Guével, E.; Tzedakis, S.; Bey, R.; Fuks, D.; Chatellier, G.; Tannier, X.; et al. No changes in clinical presentation, treatment strategies and survival of pancreatic cancer cases during the SARS-COV-2 outbreak: A retrospective multicenter cohort study on real-world data. Int. J. Cancer 2023, 153, 1988–1996. [Google Scholar] [CrossRef]
- Madge, O.; Brodey, A.; Bowen, J.; Nicholson, G.; Sivakumar, S.; Bottomley, M.J. The COVID-19 Pandemic Is Associated with Reduced Survival after Pancreatic Ductal Adenocarcinoma Diagnosis: A Single-Centre Retrospective Analysis. J. Clin. Med. 2022, 11, 2574. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Pyun, W.Y.; Park, H.W. Cancer Metabolism: Phenotype, Signaling and Therapeutic Targets. Cells 2020, 9, 2308. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Kartsonaki, C.; Guo, Y.; Bragg, F.; Yang, L.; Bian, Z.; Chen, Y.; Iona, A.; Millwood, I.Y.; Lv, J.; et al. Diabetes, plasma glucose and incidence of pancreatic cancer: A prospective study of 0.5 million Chinese adults and a meta-analysis of 22 cohort studies. Int. J. Cancer 2017, 140, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.E.; Davis, W.A. The relationship between pancreatic cancer and type 2 diabetes: The Fremantle Diabetes Study Phase I. Intern. Med. J. 2022, 52, 1258–1262. [Google Scholar] [CrossRef]
- Xia, B.; He, Q.; Pan, Y.; Gao, F.; Liu, A.; Tang, Y.; Chong, C.; Teoh, A.Y.B.; Li, F.; He, Y.; et al. Metabolic syndrome and risk of pancreatic cancer: A population-based prospective cohort study. Int. J. Cancer 2020, 147, 3384–3393. [Google Scholar] [CrossRef]
- Levi, Z.; Rottenberg, Y.; Twig, G.; Katz, L.; Leiba, A.; Derazne, E.; Tzur, D.; Eizenstein, S.; Keinan-Boker, L.; Afek, A.; et al. Adolescent overweight and obesity and the risk for pancreatic cancer among men and women: A nationwide study of 1.79 million Israeli adolescents. Cancer 2019, 125, 118–126. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, Z.; Bancks, M.P.; Carnethon, M.R.; Greenland, P.; Feng, Y.Q.; Wang, H.; Zhong, V.W. Trends in Prevalence of Diabetes and Control of Risk Factors in Diabetes Among US Adults, 1999–2018. JAMA 2021, 326, 704. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 US Obesity Forecasting Collaborators. National-level and state-level prevalence of overweight and obesity among children, adolescents, and adults in the USA, 1990–2021, and forecasts up to 2050. Lancet 2024, 404, 2278–2298. [Google Scholar] [CrossRef]
- Chandana, S.R.; Woods, L.M.; Maxwell, F.; Gandolfo, R.; Bekaii-Saab, T. Risk factors for early-onset pancreatic ductal adenocarcinoma: A systematic literature review. Eur. J. Cancer 2024, 198, 113471. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, M.B.; Flor, L.S.; Mullany, E.C.; Gupta, V.; Hay, S.I.; Gakidou, E. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990–2019. Lancet Public Health 2021, 6, e472–e481. [Google Scholar] [CrossRef]
- Nodari, Y.; Gentiluomo, M.; Mohelnikova-Duchonova, B.; Kreivenaite, E.; Milanetto, A.C.; Skieceviciene, J.; Landi, S.; Lawlor, R.T.; Petrone, M.C.; Arcidiacono, P.G.; et al. Genetic and non-genetic risk factors for early-onset pancreatic cancer. Dig. Liver Dis. 2023, 55, 1417–1425. [Google Scholar] [CrossRef]
- Cai, J.; Lu, B.; Chen, H.; Lu, M.; Zhang, Y.; Luo, C.; You, L.; Dai, M.; Zhao, Y. The impacts of exposure to risk factors during youth on the increasing global trend of early-onset pancreatic cancer. Public Health 2024, 229, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Althini, C.; Ohlsson, H.; Andersson, R. Early-onset pancreatic cancer: A population-based study using the SEER registry. Langenbecks Arch. Surg. 2019, 404, 565–571. [Google Scholar] [CrossRef]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Baxter, N.N.; Whitson, B.A.; Tuttle, T.M. Trends in the Treatment and Outcome of Pancreatic Cancer in the United States. Ann. Surg. Oncol. 2007, 14, 1320–1326. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Warshaw, A.L.; Allen, J.N.; Blaszkowsky, L.S.; Castillo, C.F.; Deshpande, V.; Hong, T.S.; Kwak, E.L.; Lauwers, G.Y.; Ryan, D.P.; et al. Pancreatic Ductal Adenocarcinoma. Ann. Surg. 2013, 257, 731–736. [Google Scholar] [CrossRef]
- Riall, T.S.; Sheffield, K.M.; Kuo, Y.; Townsend, C.M.; Goodwin, J.S. Resection Benefits Older Adults with Locoregional Pancreatic Cancer Despite Greater Short-Term Morbidity and Mortality. J. Am. Geriatr. Soc. 2011, 59, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Hackert, T.; Strobel, O.; Büchler, M.W. Technical advances in surgery for pancreatic cancer. Br. J. Surg. 2021, 108, 777–785. [Google Scholar] [CrossRef]
- Sharon, C.E.; Thaler, A.S.; Straker RJ3rd Kelz, R.R.; Raper, S.E.; Vollmer, C.M.; DeMatteo, R.P.; Miura, J.T.; Karakousis, G.C. Fourteen years of pancreatic surgery for malignancy among ACS-NSQIP centers: Trends in major morbidity and mortality. Surgery 2022, 172, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Pastrana Del Valle, J.; Mahvi, D.A.; Fairweather, M.; Wang, J.; Clancy, T.E.; Ashley, S.W.; Urman, R.D.; Whang, E.E.; Gold, J.S. The improvement in post-operative mortality following pancreaticoduodenectomy between 2006 and 2016 is associated with an improvement in the ability to rescue patients after major morbidity, not in the rate of major morbidity. HPB 2021, 23, 434–443. [Google Scholar] [CrossRef]
- PancreasGroup.org Collaborative. Pancreatic surgery outcomes: Multicentre prospective snapshot study in 67 countries. Br. J. Surg. 2024, 111, znad330. [Google Scholar] [CrossRef] [PubMed]
- Lidsky, M.E.; Sun, Z.; Nussbaum, D.P.; Adam, M.A.; Speicher, P.J.; Blazer, D.G. Going the Extra Mile. Ann. Surg. 2017, 266, 333–338. [Google Scholar] [CrossRef]
- Fisher, A.V.; Ma, Y.; Wang, X.; Campbell-Flohr, S.A.; Rathouz, P.J.; Ronnekleiv-Kelly, S.M.; Abbott, D.E.; Weber, S.M. National Trends in Centralization of Surgical Care and Multimodality Therapy for Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2020, 24, 2021–2029. [Google Scholar] [CrossRef]
- Hoehn, R.S.; Zenati, M.; Rieser, C.J.; Stitt, L.; Winters, S.; Paniccia, A.; Zureikat, A.H. Pancreatic Cancer Multidisciplinary Clinic is Associated with Improved Treatment and Elimination of Socioeconomic Disparities. Ann. Surg. Oncol. 2024, 31, 1906–1915. [Google Scholar] [CrossRef]
- Roessler, M.; Schmitt, J.; Bobeth, C.; Gerken, M.; Kleihues-van Tol, K.; Reissfelder, C.; Rau, B.M.; Distler, M.; Piso, P.; Günster, C.; et al. Is treatment in certified cancer centers related to better survival in patients with pancreatic cancer? Evidence from a large German cohort study. BMC Cancer 2022, 22, 621. [Google Scholar] [CrossRef]
- Hsu, D.S.; Kumar, N.S.; Le, S.T.; Chang, A.L.; Kazantsev, G.; Spitzer, A.L.; Peng, P.D.; Chang, C.K. Centralization of pancreatic cancer treatment within an integrated healthcare system improves overall survival. Am. J. Surg. 2022, 223, 1035–1039. [Google Scholar] [CrossRef]
- Strobel, O.; Neoptolemos, J.; Jäger, D.; Büchler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef]
- Mohammed, S.; Van Buren, G.; Fisher, W.E. Pancreatic cancer: Advances in treatment. World J. Gastroenterol. 2014, 20, 9354–9360. [Google Scholar] [PubMed]
- Jang, J.Y.; Han, Y.; Lee, H.; Kim, S.W.; Kwon, W.; Lee, K.H.; Oh, D.Y.; Chie, E.K.; Lee, J.M.; Heo, J.S.; et al. Oncological Benefits of Neoadjuvant Chemoradiation with Gemcitabine Versus Upfront Surgery in Patients with Borderline Resectable Pancreatic Cancer. Ann. Surg. 2018, 268, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Versteijne, E.; van Dam, J.L.; Suker, M.; Janssen, Q.P.; Groothuis, K.; Akkermans-Vogelaar, J.M.; Besselink, M.G.; Bonsing, B.A.; Buijsen, J.; Busch, O.R.; et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J. Clin. Oncol. 2022, 40, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, L.; Chan, W.Y.; Nagrial, A.; Chantrill, L.A.; Sim, H.W.; Yip, D.; Chin, V. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database Syst. Rev. 2024, 2024, CD011044. [Google Scholar]
- Zhang, K.J.; Dyson, G.; Gatz, J.L.; Silverman, M.E.; Tesfaye, A.A.; Shields, A.F.; Philip, P.A. Outcomes in Patients with Metastatic Pancreatic Adenocarcinoma with the Introduction of New Chemotherapeutic Drugs. Am. J. Clin. Oncol. 2019, 42, 243–246. [Google Scholar] [CrossRef]
- Kelly, B.N.; Nicolais, L.; Mohamed, A.; Fitzgerald, T.L. Contemporary Treatment Paradigms are Associated with Improved Survival in Pancreatic Cancer. Am. Surg. 2023, 89, 3390–3398. [Google Scholar] [CrossRef]
- Waugh, E.; Glinka, J.; Breadner, D.; Liu, R.; Tang, E.; Allen, L.; Welch, S.; Leslie, K.; Skaro, A. Survival benefit of neoadjuvant FOLFIRINOX for patients with borderline resectable pancreatic cancer. Ann. Hepatobiliary Pancreat. Surg. 2024, 28, 229–237. [Google Scholar] [CrossRef]
- Petrelli, F.; Coinu, A.; Borgonovo, K.; Cabiddu, M.; Ghilardi, M.; Lonati, V.; Aitini, E.; Barni, S. FOLFIRINOX-Based Neoadjuvant Therapy in Borderline Resectable or Unresectable Pancreatic Cancer. Pancreas 2015, 44, 515–521. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zottl, J.; Sebesta, C.G.; Tomosel, E.; Sebesta, M.-C.; Sebesta, C. Unraveling the Burden of Pancreatic Cancer in the 21st Century: Trends in Incidence, Mortality, Survival, and Key Contributing Factors. Cancers 2025, 17, 1607. https://doi.org/10.3390/cancers17101607
Zottl J, Sebesta CG, Tomosel E, Sebesta M-C, Sebesta C. Unraveling the Burden of Pancreatic Cancer in the 21st Century: Trends in Incidence, Mortality, Survival, and Key Contributing Factors. Cancers. 2025; 17(10):1607. https://doi.org/10.3390/cancers17101607
Chicago/Turabian StyleZottl, Jakob, Christian Günther Sebesta, Elena Tomosel, Marie-Christine Sebesta, and Christian Sebesta. 2025. "Unraveling the Burden of Pancreatic Cancer in the 21st Century: Trends in Incidence, Mortality, Survival, and Key Contributing Factors" Cancers 17, no. 10: 1607. https://doi.org/10.3390/cancers17101607
APA StyleZottl, J., Sebesta, C. G., Tomosel, E., Sebesta, M.-C., & Sebesta, C. (2025). Unraveling the Burden of Pancreatic Cancer in the 21st Century: Trends in Incidence, Mortality, Survival, and Key Contributing Factors. Cancers, 17(10), 1607. https://doi.org/10.3390/cancers17101607








