Integrating Muscle Depletion with Barcelona Clinic Liver Cancer Staging to Predict Overall Survival in Hepatocellular Carcinoma
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Study Design and Patient Selection
2.3. Data Collection
2.4. Grading of Noninvasive Liver Functional Reserve Models
2.5. Assessment of Muscle Depletion
2.6. Incorporation of Muscle Depletion and HCC Staging Systems
2.7. Statistical Analysis
2.8. Power Analysis
3. Results
3.1. Baseline Characteristics
3.2. Clinical Predictors of OS in HCC
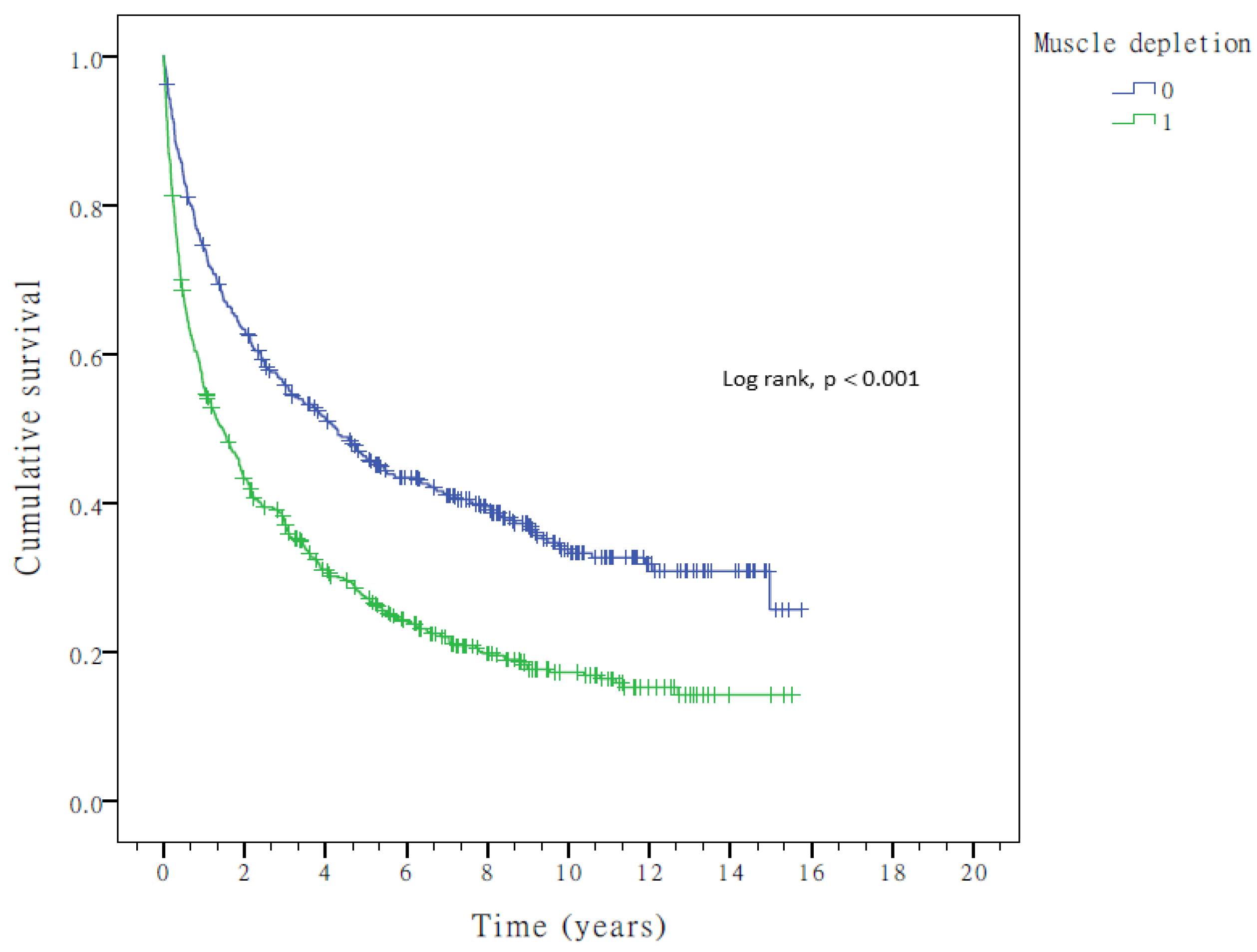
3.3. Noninvasive Liver Functional Reserve Models and OS
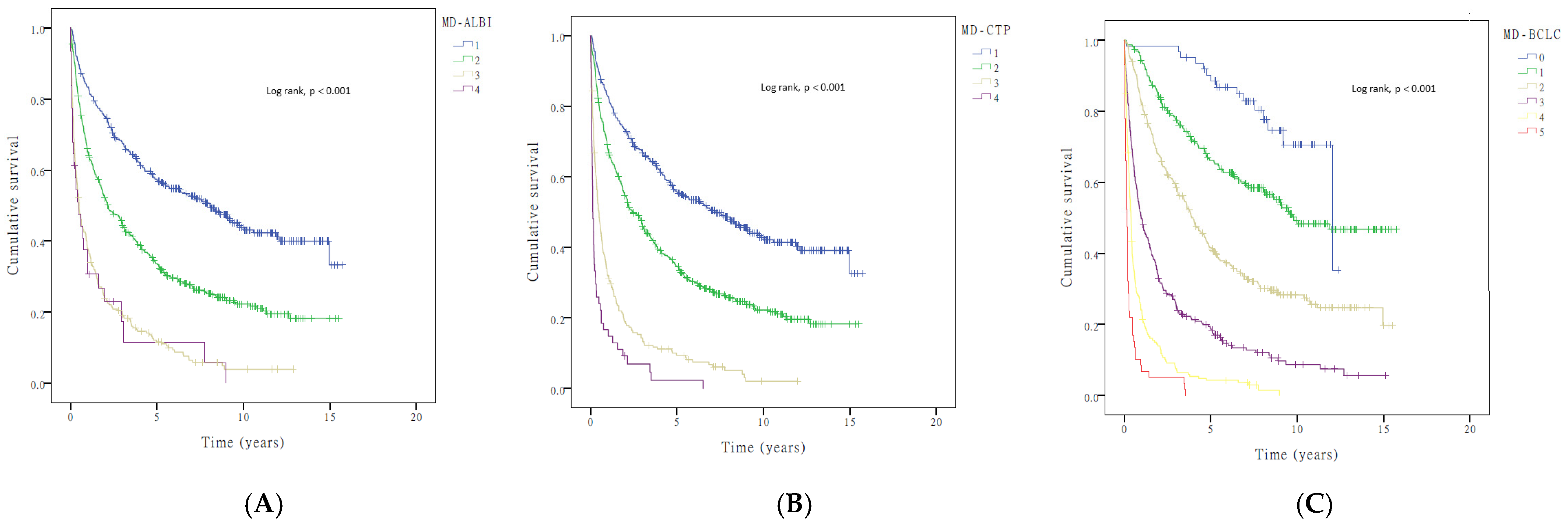
3.4. Incorporation of Muscle Depletion into Current HCC Staging Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLO BOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.X.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016, 10, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Cillo, U.; Vitale, A.; Grigoletto, F.; Farinati, F.; Brolese, A.; Zanus, G.; Neri, D.; Boccagni, P.; Srsen, N.; D’Amico, F.; et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J. Hepatol. 2006, 44, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577, quiz 578. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Cabibbo, G.; Enea, M.; Attanasio, M.; Bruix, J.; Craxì, A.; Cammà, C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology 2010, 51, 1274–1283. [Google Scholar] [CrossRef]
- Llovet, J.M.; Peña, C.E.; Lathia, C.D.; Shan, M.; Meinhardt, G.; Bruix, J. SHARP Investigators Study Group. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2012, 18, 2290–2300. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [PubMed]
- Botta, F.; Giannini, E.; Romagnoli, P.; Fasoli, A.; Malfatti, F.; Chiarbonello, B.; Testa, E.; Risso, D.; Colla, G.; Testa, R. MELD scoring system is useful for predicting prognosis in patients with liver cirrhosis and is correlated with residual liver function: A European study. Gut 2003, 52, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.I.; Lee, P.C.; Huang, Y.H.; Wu, J.C.; Lin, H.C.; Chiang, J.H.; Lee, S.D. The sequential changes of the model for end-stage liver disease score correlate with the severity of liver cirrhosis in patients with hepatocellular carcinoma undergoing locoregional therapy. J. Clin. Gastroenterol. 2006, 40, 543–550. [Google Scholar] [CrossRef]
- Liu, P.H.; Hsu, C.Y.; Hsia, C.Y.; Lee, Y.H.; Chiou, Y.Y.; Huang, Y.H.; Lee, F.Y.; Lin, H.C.; Hou, M.C.; Huo, T.I. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J. Gastroenterol. Hepatol. 2017, 32, 879–886. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef]
- Cross, T.J.; Rizzi, P.; Berry, P.A.; Bruce, M.; Portmann, B.; Harrison, P.M. King’s Score: An accurate marker of cirrhosis in chronic hepatitis C. Eur. J. Gastroenterol. Hepatol. 2009, 21, 730–738. [Google Scholar] [CrossRef]
- Lok, A.S.; Ghany, M.G.; Goodman, Z.D.; Wright, E.C.; Everson, G.T.; Sterling, R.K.; Everhart, J.E.; Lindsay, K.L.; Bonkovsky, H.L.; Bisceglie, A.M.D.; et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: Results of the HALT-C cohort. Hepatology 2005, 42, 282–292. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Feinstein, A.R. Clinical biostatistics. XVI. The process of prognostic stratification. 2. Clin. Pharmacol. Ther. 1972, 13, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Hosmer, T.; Le Cessie, S.; Lemeshow, S. A comparison of goodness-of-fit tests for the logistic regression model. Stat. Med. 1997, 16, 965–980. [Google Scholar] [CrossRef]
- Forster, M.R. Key Concepts in Model Selection: Performance and Generalizability. J. Math. Psychol. 2000, 44, 205–231. [Google Scholar] [CrossRef] [PubMed]
- Steck, H.; Krishnapuram, B.; Dehing-Oberije, C.; Lambin, P.; Raykar, V.C. On ranking in survival analysis: Bounds on the concordance index. In Proceedings of the Advances in Neural Information Processing Systems, Vancouver, BC, Canada, 3–6 December 2007; Volume 20. [Google Scholar]
- Lee, K.; Mark, D. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Bagiella, E.; Chang, H. Power analysis and sample size calculation. J. Mol. Cell Cardiol. 2019, 133, 214–216. [Google Scholar] [CrossRef]
- Hsu, C.S.; Kao, J.H. Sarcopenia and chronic liver diseases. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1229–1244. [Google Scholar] [CrossRef]
- Kuo, M.H.; Tseng, C.W.; Hsu, C.S.; Chen, Y.C.; Kao, I.T.; Wu, C.Y.; Shao, S.C. Prevalence and Effect of Low Skeletal Muscle Mass among Hepatocellular Carcinoma Patients Undergoing Systemic Therapy: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 2426. [Google Scholar] [CrossRef]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Hsu, C.T.; Han, D.S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018, 7, 90–103. [Google Scholar] [CrossRef]

| Noninvasive Blood Testing for Liver Reserve Makers | Formula |
|---|---|
| ALBI, Grade 1/2/3 (<−2.6/−2.6–≤−1.39/>−1.39) | (log(Bilirubin[μmol/L]) × 0.66) + (Albumin[g/L] × −0.085) |
| APRI, Grade 1/2/3 (0.5/0.5–1.5/>1.5) | [(AST/upper limit of normal)/Platelet Count (109/L)] × 100 |
| CDS, Grade 1/2/3 (<4/4–7/>7) | Platelet count (×109/L): >340 = 0; 280–339 = 1; 220–279 = 2; 160–219 = 3; 100–159 = 4; 40–99 = 5; <40 = 6 ALT/AST ratio: >1.7 = 0; 1.2–1.7 = 1; 0.6–1.19 = 2; <0.6 = 3 INR: <1.1 = 0; 1.1–1.4 = 1; >1.4 = 2 CDS is the sum of the above (possible value 0–11). |
| BCLC, Stage 0, A, B, C, D | As reference [21]. |
| CTP scores A, B, C | Encephalopathy: none = 1, grade 1 or 2 = 2, grade 3 or 4 = 3; ascites: none = 1, mild to moderate = 2, severe = 3; bilirubin(mg/dL): <2 = 1, 2–3 = 2, >3 = 3; albumin(g/dL): >3.5 = 1, 2.8–3.5 = 2, <2.8 = 3 PT; sec (INR): <4 (1.7) = 1, 4–6 (1.7–2.3) = 2, >6 (>2.3) = 3 |
| FIB-4 index, Grade 1/2/3 (<1.45/1.45–3.25/>3.25) | (Age[years] × AST[U/L])/(platelet [109] × ALT[U/L]1/2) |
| GUCI, Grade 1/2/3 (<0.5/0.5–1.56/>1.56) | [AST/TOPNORMAL AST] × INR × 100/(Platelets × 109) |
| Lok index, Grade 1/2/3 (<0.5/0.5–0.8/>0.8 | Lok Index = e(LogOddsLok)/(1 + e(LogOddsLok)) Log Odds Lok = (1.26 × AST/ALT) + (5.27 × INR)− (0.0089 × Platelets × 109) − 5.56 |
| MELD, Grade 1/2/3 (<8/8–12/>12) | 10 × ((0.957 × ln(Creatinine)) + (0.378 × ln(Bilirubin)) + (1.12 × ln(INR))) + 6.43 |
| PALBI, Grade1/2/3 (≤−2.53, −2.53 and ≤−2.09, >−2.09) | (2.02 × log10 bilirubin) − [0.37 × (log10 bilirubin(umol/L))2] − 0.04 × albumin (g/L) − 3.48 × log10 platelets(109/L) + 1.01 × (log10 platelets(109/L))2 |
| King’s score (<7.6/7.6–16.7/16.7) | Age × AST × INR/[platelets (109/L)] |
| Psoas muscle-to-spine ratio | 4.42 (ROC curve cut point) |
| Psoas muscle-to-vertebra ratio | 2.32 (ROC curve cut point) |
| CT density of Psoas muscle area | 37.2 (ROC curve cut point) |
| Parameters | Scores | |||||
|---|---|---|---|---|---|---|
| CTP score | 1 | 2 | 3 | _ | _ | _ |
| ALBI grade | 1 | 2 | 3 | _ | _ | _ |
| BCLC stage | 0 | 1 | 2 | 3 | 4 | _ |
| Muscle depletion | 0 | 1 | _ | _ | _ | _ |
| MD–CTP score | 1 | 2 | 3 | 4 | _ | _ |
| MD–ALBI grade | 1 | 2 | 3 | 4 | _ | _ |
| MD–BCLC stage | 0 | 1 | 2 | 3 | 4 | 5 |
| Variables | Patients (n = 1072) |
| Age (Mean ± SD) | 63.2 ± 12.8 |
| Sex, n (%) | |
| Male | 759 (70.8) |
| Female | 313 (29.2) |
| HBV infection, n (%) | 354 (33.0) |
| HCV infection, n (%) | 268 (25.0) |
| HBV/HCV co-infection, n (%) | 28 (2.6) |
| Smoking, n (%) | 196 (18.3) |
| Alcohol drinking, n (%) | 65 (6.1) |
| Liver cirrhosis, n (%) | 765 (71.4) |
| Tumor size (≤5 cm/>5 cm), n (%) | 667 (62.2)/405 (37.8) |
| Psoas muscle-to-spine ratio (Mean ± SD) | 4.4 ± 1.5 |
| Psoas muscle-to-vertebra ratio (Mean ± SD) | 2.3 ± 0.8 |
| Laboratory values (Mean ± SD) | |
| AST (IU/L) | 93.0 ± 196.3 |
| ALT (IU/L) | 67.2 ± 122.5 |
| Albumin (g/dL) | 3.3 ± 0.8 |
| Total bilirubin (mg/dL) | 1.5 ± 2.6 |
| Creatinine (mg/dL) | 1.4 ± 5.6 |
| Platelets (1000/μL) | 162.0 ± 99.0 |
| INR | 1.1 ± 0.2 |
| BCLC stage (0/A/B/C/D), n (%) | 113 (10.5)/357 (33.4)/246 (22.9)/280 (26.1)/76 (7.1) |
| CTP score (A/B/C), n (%) | 729 (68.0)/272 (25.4)/71 (6.6) |
| ALBI grade (1/2/3), n (%) | 687(64.1)/333 (31.1)/52 (4.9) |
| APRI grade (1/2/3), n (%) | 223 (20.8)/465 (43.4)/384 (35.8) |
| CDS grade (1/2/3), n (%) | 87 (8.1)/709 (66.1)/276 (25.7) |
| Child–Pugh class (1/2/3), n (%) | 729 (68.0)/272 (25.4)/71 (6.6) |
| FIB-4 grade (1/2/3), n (%) | 134 (12.5)/322 (30.0)/616 (57.5) |
| GUCI grade (1/2/3), n (%) | 193 (18.0)/457 (42.6)/422 (39.4) |
| King’s score (1/2/3), n (%) | 91 (8.5)/218 (20.3)/763 (71.2) |
| Lok index grade (1/2/3), n (%) | 381 (35.5)/351 (32.7)/340 (31.7) |
| MELD score (1/2/3), n (%) | 4 (0.4)/54 (5.0)/1014 (94.6) |
| PALBI grade (1/2/3), n (%) | 25 (2.3)/50 (4.7)/997 (93.0) |
| Psoas muscle-to-spine ratio (>4.42/≤4.42), n (%) | 463 (43.2)/609 (56.8) |
| Psoas muscle-to-vertebra ratio (>2.32/≤2.32), n (%) | 473 (44.1)/599 (55.9) |
| CT density of psoas muscle area (>37.2/≤37.2), n (%) | 487 (45.4)/585 (54.6) |
| Overall Survival | N | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | ||
| Age (>65/≤65) | 467/605 | 1.207 | 1.047–1.392 | 0.009 | |||
| Sex (male/female) | 759/313 | 0.996 | 0.853–1.164 | 0.963 | |||
| HBsAg (positive/negative) | 354/718 | 0.591 | 0.505–0.691 | <0.001 | 0.674 | 0.571–0.795 | <0.001 |
| Anti-HCV (positive/negative) | 268/804 | 0.750 | 0.632–0.889 | 0.001 | 0.809 | 0.676–0.970 | 0.022 |
| Alcohol (yes/no) | 65/1007 | 1.120 | 0.831–1.510 | 0.456 | |||
| Smoke (yes/no) | 196/876 | 0.925 | 0.765–1.118 | 0.419 | |||
| DM (yes/no) | 195/877 | 0.916 | 0.763–1.100 | 0.349 | |||
| Liver cirrhosis (yes/no) | 765/307 | 1.444 | 1.226–1.702 | <0.001 | 1.543 | 1.303–1.827 | <0.001 |
| Tumor size (>5 cm/≤5 cm) | 405/667 | 3.502 | 3.022–4.058 | <0.001 | 1.533 | 1.288–1.825 | <0.001 |
| Alpha-fetoprotein (>20/≤20 ng/mL) | 594/478 | 2.043 | 1.763–2.367 | <0.001 | 1.704 | 1.465–1.982 | <0.001 |
| Muscle depletion (MD: psoas muscle-to-spine ratio > 4.42 vs. ≤4.42) | 609/463 | 1.737 | 1.500–2.011 | <0.001 | 1.566 | 1.351–1.814 | <0.001 |
| MD–BCLC stage | |||||||
| 0 | 62 | 1 | 1 | ||||
| 1 | 232 | 2.178 | 1.267–3.743 | <0.001 | 2.007 | 1.167–3.454 | <0.001 |
| 2 | 278 | 4.305 | 2.543–7.286 | <0.001 | 3.461 | 2.038–5.877 | <0.001 |
| 3 | 245 | 9.807 | 5.797–16.592 | <0.001 | 6.238 | 3.638–10.696 | <0.001 |
| 4 | 196 | 20.938 | 12.303–35.632 | <0.001 | 12.881 | 7.450–22.269 | <0.001 |
| 5 | 59 | 43.949 | 24.696–78.213 | <0.001 | 29.553 | 16.349–53.421 | <0.001 |
| Overall Survival | Corrected Akaike Information Criteria (AIC) | Homogeneity (Wald x2) | Concordance Index (C-Index) |
|---|---|---|---|
| ALBI grade | 5557.464 | 97.244 | 0.749 |
| APRI grade | 5621.803 | 32.905 | 0.733 |
| BCLC stage | 5466.796 | 192.023 | 0.782 |
| CDS grade | 5618.206 | 36.503 | 0.726 |
| CTP score | 5542.581 | 112.127 | 0.762 |
| FIB-4 grade | 5627.292 | 27.416 | 0.729 |
| GUCI grade | 5608.640 | 46.069 | 0.737 |
| King’s score | 5628.484 | 26.224 | 0.730 |
| Lok index grade | 5568.016 | 86.692 | 0.743 |
| MELD score | 5652.365 | 2.344 | 0.720 |
| PALBI grade | 5653.887 | 0.822 | 0.720 |
| Psoas muscle-to-spine ratio | 5628.330 | 24.329 | 0.726 |
| Psoas muscle-to-vertebra ratio | 5645.909 | 6.750 | 0.724 |
| CT density of psoas muscle area | 5648.150 | 4.509 | 0.721 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.-R.; Wu, C.-C.; Hsiao, J.-K.; Chou, Y.-C.; Liao, Y.-L.; Chen, Y.-C.; Shao, P.-J.; Wu, T.-W.; Hsu, C.-S. Integrating Muscle Depletion with Barcelona Clinic Liver Cancer Staging to Predict Overall Survival in Hepatocellular Carcinoma. Cancers 2025, 17, 24. https://doi.org/10.3390/cancers17010024
Peng T-R, Wu C-C, Hsiao J-K, Chou Y-C, Liao Y-L, Chen Y-C, Shao P-J, Wu T-W, Hsu C-S. Integrating Muscle Depletion with Barcelona Clinic Liver Cancer Staging to Predict Overall Survival in Hepatocellular Carcinoma. Cancers. 2025; 17(1):24. https://doi.org/10.3390/cancers17010024
Chicago/Turabian StylePeng, Tzu-Rong, Chao-Chuan Wu, Jong-Kai Hsiao, Yi-Chun Chou, Yuan-Ling Liao, Yen-Chih Chen, Pei-Jung Shao, Ta-Wei Wu, and Ching-Sheng Hsu. 2025. "Integrating Muscle Depletion with Barcelona Clinic Liver Cancer Staging to Predict Overall Survival in Hepatocellular Carcinoma" Cancers 17, no. 1: 24. https://doi.org/10.3390/cancers17010024
APA StylePeng, T.-R., Wu, C.-C., Hsiao, J.-K., Chou, Y.-C., Liao, Y.-L., Chen, Y.-C., Shao, P.-J., Wu, T.-W., & Hsu, C.-S. (2025). Integrating Muscle Depletion with Barcelona Clinic Liver Cancer Staging to Predict Overall Survival in Hepatocellular Carcinoma. Cancers, 17(1), 24. https://doi.org/10.3390/cancers17010024







