Expression of Potential Antibody–Drug Conjugate Targets in Cervical Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinicopathologic Parameters
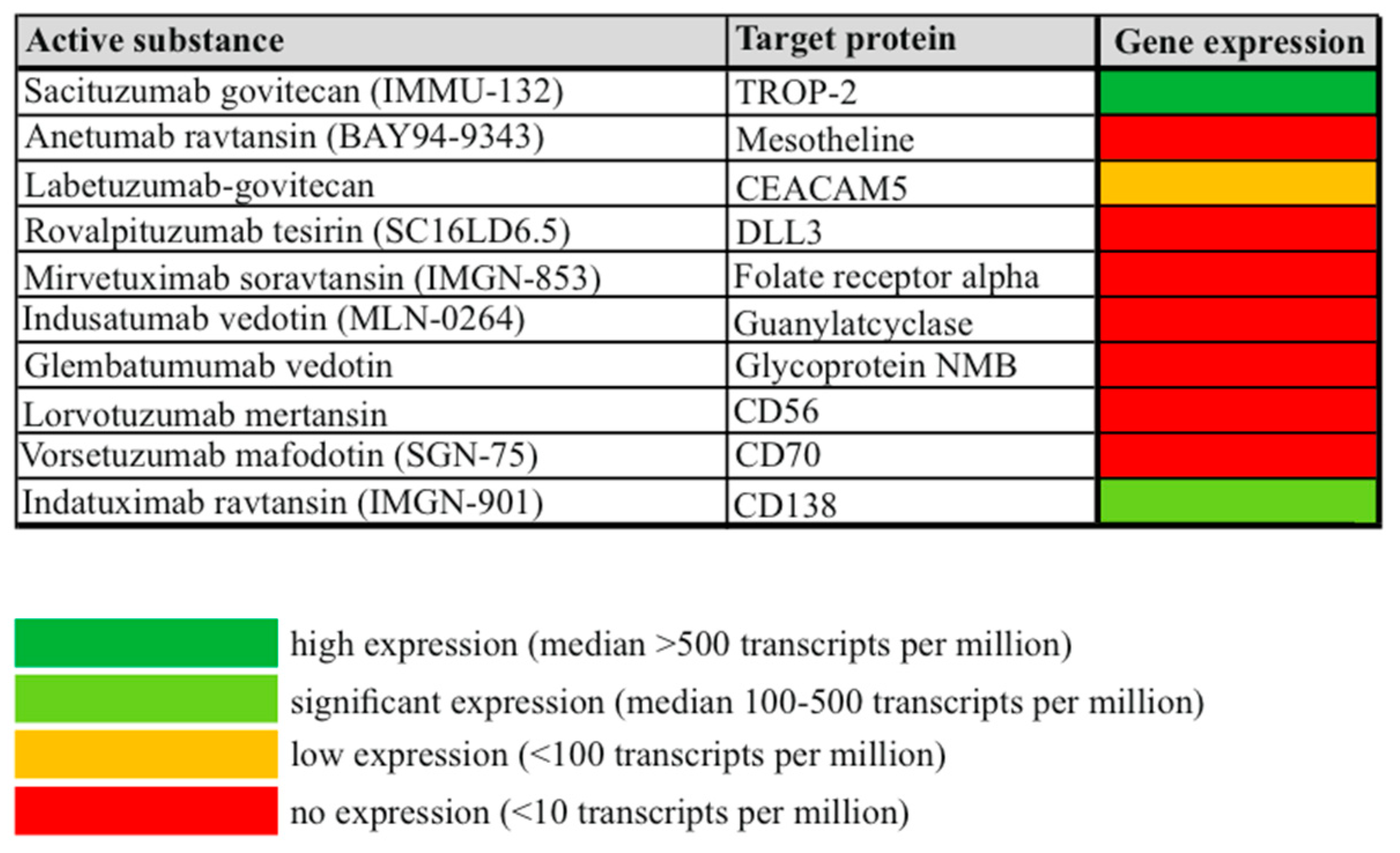
3.2. Overall Gene Expression of Potential ADC Targets in Cervical Cancer
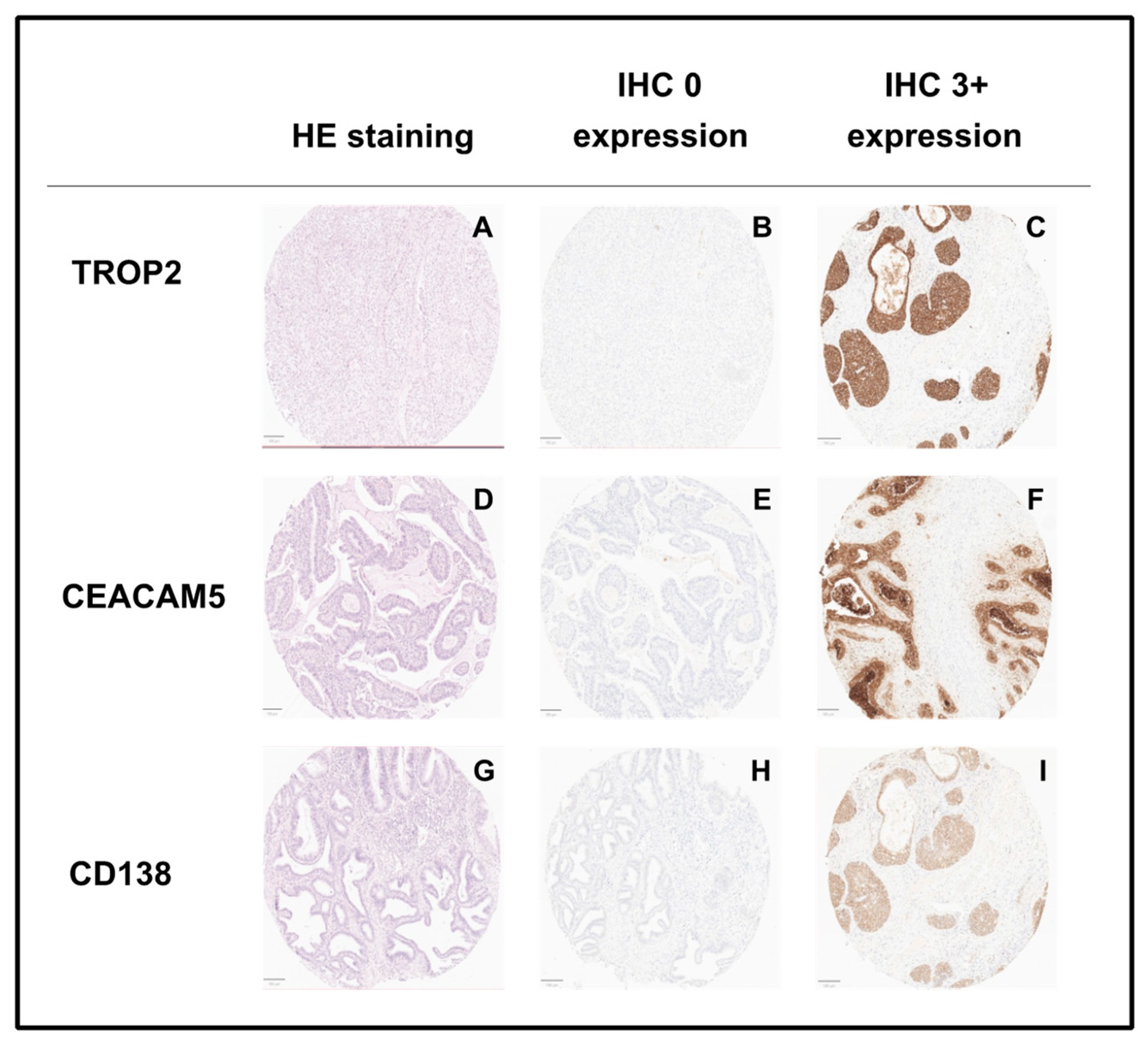
3.3. Gene and Protein Expression of TROP2 According to Tumor Stage in Cervical Cancer
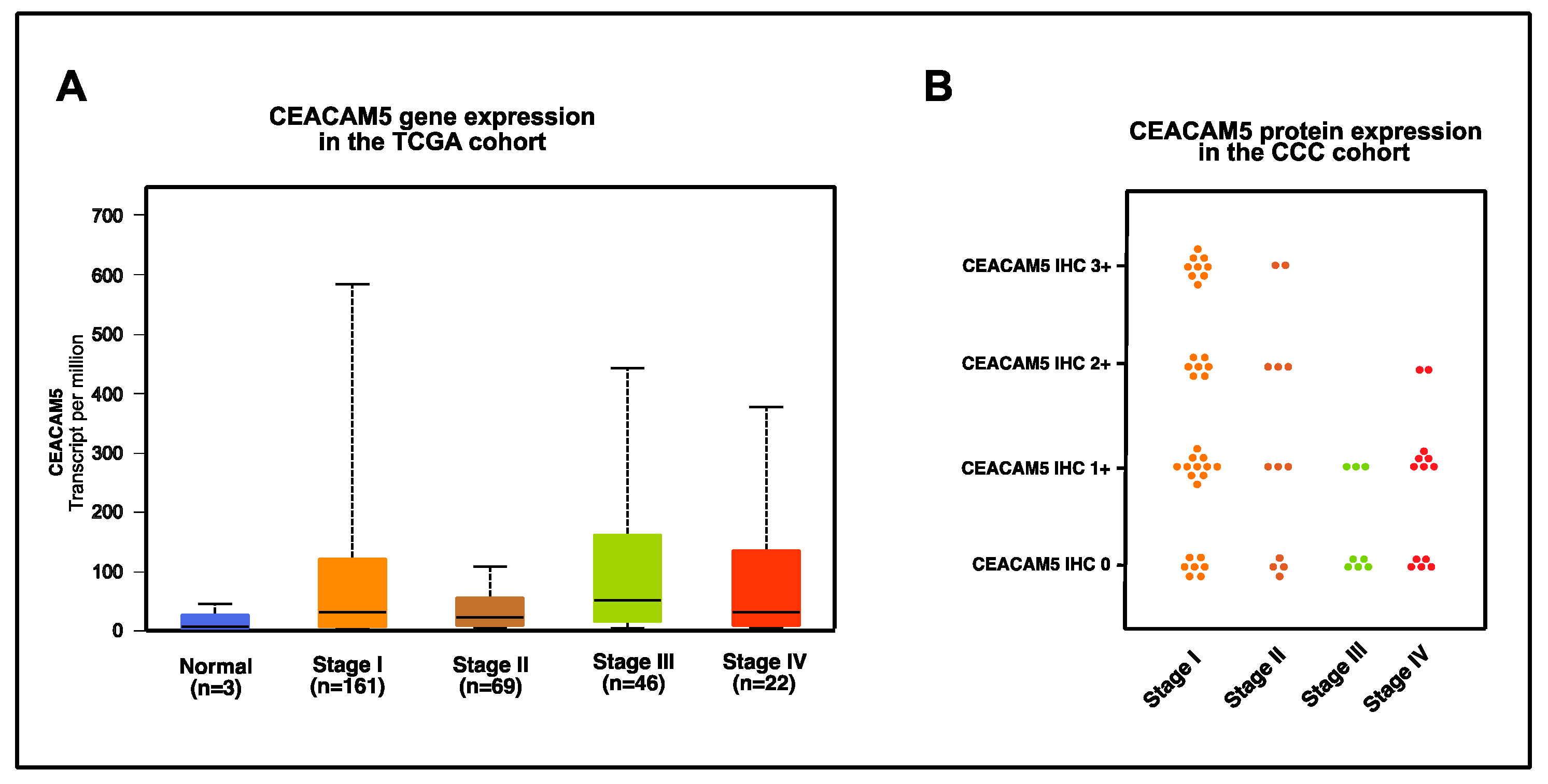
3.4. Gene and Protein Expression of CEACAM5 According to Tumor Stage in Cervical Cancer
3.5. Gene and Protein Expression of CD138 According to Tumor Stage in Cervical Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.S.; Kim, Y.M.; Lisyanskaya, A.; Samouelian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yanez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Antras, J.; Genta, S.; Vijenthira, A.; Siu, L.L. Antibody-drug conjugates: In search of partners of choice. Trends Cancer 2023, 9, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Mirlacher, M.; Sauter, G. Tissue microarrays. Methods Mol. Med. 2005, 114, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Loeser, H.; Kraemer, M.; Gebauer, F.; Bruns, C.; Schroder, W.; Zander, T.; Persa, O.D.; Alakus, H.; Hoelscher, A.; Buettner, R.; et al. The expression of the immune checkpoint regulator VISTA correlates with improved overall survival in pT1/2 tumor stages in esophageal adenocarcinoma. Oncoimmunology 2019, 8, e1581546. [Google Scholar] [CrossRef]
- Coleman, R.L.; Lorusso, D.; Gennigens, C.; Gonzalez-Martin, A.; Randall, L.; Cibula, D.; Lund, B.; Woelber, L.; Pignata, S.; Forget, F.; et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 609–619. [Google Scholar] [CrossRef]
- Vergote, I.; Van Nieuwenhuysen, E.; O’Cearbhaill, R.E.; Westermann, A.; Lorusso, D.; Ghamande, S.; Collins, D.C.; Banerjee, S.; Mathews, C.A.; Gennigens, C.; et al. Tisotumab Vedotin in Combination With Carboplatin, Pembrolizumab, or Bevacizumab in Recurrent or Metastatic Cervical Cancer: Results From the innovaTV 205/GOG-3024/ENGOT-cx8 Study. J. Clin. Oncol. 2023, 41, 5536–5549. [Google Scholar] [CrossRef] [PubMed]
- Faust, J.R.; Hamill, D.; Kolb, E.A.; Gopalakrishnapillai, A.; Barwe, S.P. Mesothelin: An Immunotherapeutic Target beyond Solid Tumors. Cancers 2022, 14, 1550. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, S.; Gagelmann, P.; Gorbokon, N.; Lennartz, M.; Menz, A.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Blessin, N.C.; Fraune, C.; et al. Mesothelin Expression in Human Tumors: A Tissue Microarray Study on 12,679 Tumors. Biomedicines 2021, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, S.; Yazaki, S.; Kojima, Y.; Yoshida, H.; Kitadai, R.; Nishikawa, T.; Shimoi, T.; Sudo, K.; Okuma, H.S.; Tanioka, M.; et al. High mesothelin expression is correlated with non-squamous cell histology and poor survival in cervical cancer: A retrospective study. BMC Cancer 2022, 22, 1215. [Google Scholar] [CrossRef]
- Kim, J.W.; Ko, J.H.; Sage, J. DLL3 regulates Notch signaling in small cell lung cancer. iScience 2022, 25, 105603. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E.; Spira, A.; Miguel, M.; Kondo, S.; Gazzah, A.; Millward, M.; Prenen, H.; Rottey, S.; Warburton, L.; Alanko, T.; et al. Safety, pharmacokinetics, and efficacy of budigalimab with rovalpituzumab tesirine in patients with small cell lung cancer. Cancer Treat Res. Commun. 2021, 28, 100405. [Google Scholar] [CrossRef] [PubMed]
- Blackhall, F.; Jao, K.; Greillier, L.; Cho, B.C.; Penkov, K.; Reguart, N.; Majem, M.; Nackaerts, K.; Syrigos, K.; Hansen, K.; et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared With Topotecan as Second-Line Therapy in DLL3-High SCLC: Results From the Phase 3 TAHOE Study. J. Thorac. Oncol. 2021, 16, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; Besse, B.; Greillier, L.; Santana-Davila, R.; Ready, N.; Hann, C.L.; Glisson, B.S.; Farago, A.F.; Dowlati, A.; Rudin, C.M.; et al. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: Results From the Phase II TRINITY Study. Clin. Cancer Res. 2019, 25, 6958–6966. [Google Scholar] [CrossRef]
- Lashari, B.H.; Vallatharasu, Y.; Kolandra, L.; Hamid, M.; Uprety, D. Rovalpituzumab Tesirine: A Novel DLL3-Targeting Antibody-Drug Conjugate. Drugs R&D 2018, 18, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor alpha in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef]
- Young, O.; Ngo, N.; Lin, L.; Stanbery, L.; Creeden, J.F.; Hamouda, D.; Nemunaitis, J. Folate Receptor as a Biomarker and Therapeutic Target in Solid Tumors. Curr. Probl. Cancer 2023, 47, 100917. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, S.; Kojima, Y.; Yoshida, H.; Takamizawa, S.; Kitadai, R.; Nishikawa, T.; Shimoi, T.; Sudo, K.; Saito, A.; Okuma, H.S.; et al. High expression of folate receptor alpha is associated with poor prognosis in patients with cervical cancer. J. Gynecol. Oncol. 2022, 33, e82. [Google Scholar] [CrossRef]
- Liu, C.; Ding, L.; Bai, L.; Chen, X.; Kang, H.; Hou, L.; Wang, J. Folate receptor alpha is associated with cervical carcinogenesis and regulates cervical cancer cells growth by activating ERK1/2/c-Fos/c-Jun. Biochem. Biophys. Res. Commun. 2017, 491, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Almhanna, K.; Prithviraj, G.K.; Veiby, P.; Kalebic, T. Antibody-drug conjugate directed against the guanylyl cyclase antigen for the treatment of gastrointestinal malignancies. Pharmacol. Ther. 2017, 170, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Almhanna, K.; Wright, D.; Mercade, T.M.; Van Laethem, J.L.; Gracian, A.C.; Guillen-Ponce, C.; Faris, J.; Lopez, C.M.; Hubner, R.A.; Bendell, J.; et al. A phase II study of antibody-drug conjugate, TAK-264 (MLN0264) in previously treated patients with advanced or metastatic pancreatic adenocarcinoma expressing guanylyl cyclase C. Investig. New Drugs 2017, 35, 634–641. [Google Scholar] [CrossRef]
- Almhanna, K.; Miron, M.L.; Wright, D.; Gracian, A.C.; Hubner, R.A.; Van Laethem, J.L.; Lopez, C.M.; Alsina, M.; Munoz, F.L.; Bendell, J.; et al. Phase II study of the antibody-drug conjugate TAK-264 (MLN0264) in patients with metastatic or recurrent adenocarcinoma of the stomach or gastroesophageal junction expressing guanylyl cyclase C. Investig. New Drugs 2017, 35, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.T.; Wakiya, K.; Dowell, J.M.; Herndon, J.E., 2nd; Reardon, D.A.; Graner, M.W.; Riggins, G.J.; Wikstrand, C.J.; Bigner, D.D. Glycoprotein nonmetastatic melanoma protein B, a potential molecular therapeutic target in patients with glioblastoma multiforme. Clin. Cancer Res. 2006, 12, 1970–1982. [Google Scholar] [CrossRef]
- Rose, A.A.; Pepin, F.; Russo, C.; Abou Khalil, J.E.; Hallett, M.; Siegel, P.M. Osteoactivin promotes breast cancer metastasis to bone. Mol. Cancer Res. 2007, 5, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, L.; Ke, S.; Liu, T.; Hao, L.; Zhao, P.; Tu, W.; Cang, S. High expression of GPNMB indicates an unfavorable prognosis in glioma: Combination of data from the GEO and CGGA databases and validation in tissue microarray. Oncol. Lett. 2020, 20, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.H.; Wergedal, J.E.; Mohan, S.; Lau, K.H. Osteoactivin is a novel osteoclastic protein and plays a key role in osteoclast differentiation and activity. FEBS Lett. 2008, 582, 1451–1458. [Google Scholar] [CrossRef]
- Vahdat, L.T.; Schmid, P.; Forero-Torres, A.; Blackwell, K.; Telli, M.L.; Melisko, M.; Mobus, V.; Cortes, J.; Montero, A.J.; Ma, C.; et al. Glembatumumab vedotin for patients with metastatic, gpNMB overexpressing, triple-negative breast cancer (“METRIC”): A randomized multicenter study. NPJ Breast Cancer 2021, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Bosi, S.; Bassi, C.; Ferracin, M.; Lanza, G.; Gafa, R.; Magri, E.; Selvatici, R.; Torresani, S.; Marci, R.; et al. Gene expression changes in progression of cervical neoplasia revealed by microarray analysis of cervical neoplastic keratinocytes. J. Cell. Physiol. 2015, 230, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, K.; Jimi, S.; Goldmacher, V.S.; Ab, O.; Tamura, K. Targeting CD56 by the maytansinoid immunoconjugate IMGN901 (huN901-DM1): A potential therapeutic modality implication against natural killer/T cell malignancy. Br. J. Haematol. 2008, 141, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.I.; Pressey, J.G.; Smith, M.A.; Kudgus, R.A.; Cajaiba, M.; Reid, J.M.; Hall, D.; Barkauskas, D.A.; Voss, S.D.; Cho, S.Y.; et al. ADVL1522: A phase 2 study of lorvotuzumab mertansine (IMGN901) in children with relapsed or refractory wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, malignant peripheral nerve sheath tumor, or synovial sarcoma-A Children’s Oncology Group study. Cancer 2020, 126, 5303–5310. [Google Scholar] [CrossRef] [PubMed]
- Arens, R.; Nolte, M.A.; Tesselaar, K.; Heemskerk, B.; Reedquist, K.A.; van Lier, R.A.; van Oers, M.H. Signaling through CD70 regulates B cell activation and IgG production. J. Immunol. 2004, 173, 3901–3908. [Google Scholar] [CrossRef] [PubMed]
- Junker, K.; Hindermann, W.; von Eggeling, F.; Diegmann, J.; Haessler, K.; Schubert, J. CD70: A new tumor specific biomarker for renal cell carcinoma. J. Urol. 2005, 173, 2150–2153. [Google Scholar] [CrossRef] [PubMed]
- Israel, B.F.; Gulley, M.; Elmore, S.; Ferrini, S.; Feng, W.H.; Kenney, S.C. Anti-CD70 antibodies: A potential treatment for EBV+ CD70-expressing lymphomas. Mol. Cancer Ther. 2005, 4, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Hishima, T.; Fukayama, M.; Hayashi, Y.; Fujii, T.; Ooba, T.; Funata, N.; Koike, M. CD70 expression in thymic carcinoma. Am. J. Surg. Pathol. 2000, 24, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Williams, R. Discontinued in 2013: Oncology drugs. Expert. Opin. Investig. Drugs 2015, 24, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.R.; Ailawadhi, S.; Siegel, D.S.; Heffner, L.T.; Somlo, G.; Jagannath, S.; Zimmerman, T.M.; Munshi, N.C.; Madan, S.; Chanan-Khan, A.; et al. Indatuximab ravtansine plus dexamethasone with lenalidomide or pomalidomide in relapsed or refractory multiple myeloma: A multicentre, phase 1/2a study. Lancet Haematol. 2021, 8, e794–e807. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Ouyang, D.; Zou, Q.; Chen, Q.; Luo, N.; He, H.; Anwar, M.; Yi, W. A literature review of the promising future of TROP2: A potential drug therapy target. Ann. Transl. Med. 2022, 10, 1403. [Google Scholar] [CrossRef]
- Trerotola, M.; Cantanelli, P.; Guerra, E.; Tripaldi, R.; Aloisi, A.L.; Bonasera, V.; Lattanzio, R.; de Lange, R.; Weidle, U.H.; Piantelli, M.; et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene 2013, 32, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Deng, J.; Yuan, Y.; Chen, W.; Sun, W.; Wang, Y.; Huang, H.; Liang, B.; Ming, T.; Wen, J.; et al. Advances in Trop2-targeted therapy: Novel agents and opportunities beyond breast cancer. Pharmacol. Ther. 2022, 239, 108296. [Google Scholar] [CrossRef] [PubMed]
- Zeybek, B.; Manzano, A.; Bianchi, A.; Bonazzoli, E.; Bellone, S.; Buza, N.; Hui, P.; Lopez, S.; Perrone, E.; Manara, P.; et al. Cervical carcinomas that overexpress human trophoblast cell-surface marker (Trop-2) are highly sensitive to the antibody-drug conjugate sacituzumab govitecan. Sci. Rep. 2020, 10, 973. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Starodub, A.N.; Ocean, A.J.; Shah, M.A.; Guarino, M.J.; Picozzi, V.J., Jr.; Vahdat, L.T.; Thomas, S.S.; Govindan, S.V.; Maliakal, P.P.; Wegener, W.A.; et al. First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors. Clin. Cancer Res. 2015, 21, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, G.A.; Moroose, R.; Santin, A.D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef]
- Blumenthal, R.D.; Leon, E.; Hansen, H.J.; Goldenberg, D.M. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer 2007, 7, 2. [Google Scholar] [CrossRef]
- Gazzah, A.; Bedard, P.L.; Hierro, C.; Kang, Y.K.; Abdul Razak, A.; Ryu, M.H.; Demers, B.; Fagniez, N.; Henry, C.; Hospitel, M.; et al. Safety, pharmacokinetics, and antitumor activity of the anti-CEACAM5-DM4 antibody-drug conjugate tusamitamab ravtansine (SAR408701) in patients with advanced solid tumors: First-in-human dose-escalation study. Ann. Oncol. 2022, 33, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Dotan, E.; Cohen, S.J.; Starodub, A.N.; Lieu, C.H.; Messersmith, W.A.; Simpson, P.S.; Guarino, M.J.; Marshall, J.L.; Goldberg, R.M.; Hecht, J.R.; et al. Phase I/II Trial of Labetuzumab Govitecan (Anti-CEACAM5/SN-38 Antibody-Drug Conjugate) in Patients With Refractory or Relapsing Metastatic Colorectal Cancer. J. Clin. Oncol. 2017, 35, 3338–3346. [Google Scholar] [CrossRef] [PubMed]


| Clinicopathologic Characteristics | Title 3 | |
|---|---|---|
| Mean age at diagnosis (years) | 47.5 (range, 18–84) | |
| Tumor stage (FIGO classification) | ||
| Stage I | 34/67 (50.7%) | |
| Stage II | 12/67 (17.9%) | |
| Stage III | 8/67 (11.9%) | |
| Stage IV | 13/67 (19.4%) | |
| Histologic subtype | ||
| squamous | 45/67 (67.2%) | |
| adenocarcinoma | 16/67 (23.8%) | |
| adenosquamous | 6/67 (9%) | |
| Grading | ||
| G1 | 2/67 (3%) | |
| G2 | 34/67 (50.7%) | |
| G3 | 31/67 (46.3%) | |
| Lymphovascular space invasion | ||
| L0 | 34/67 (50.7%) | |
| L1 | 33/67 (49.3%) | |
| Vascular space invasion | ||
| V0 | 54/67 (80.6%) | |
| V1 | 13/67 (19.4%) | |
| Protein Expression | ||
|---|---|---|
| TROP2 | ||
| TROP2 IHC 0 | 1.5% (1/67) | |
| TROP2 IHC 1+ | 4.5% (3/67) | |
| TROP2 IHC 2+ | 1.5% (1/67) | |
| TROP2 IHC 3+ | 92.5% (62/67) | |
| CEACAM5 | ||
| CEACAM5 IHC 0 | 31.3% (21/67) | |
| CEACAM5 IHC 1+ | 34.3% (23/67) | |
| CEACAM5 IHC 2+ | 17.9% (12/67) | |
| CEACAM5 IHC 3+ | 16.4% (11/67) | |
| CD138 | ||
| CD138 IHC 0 | 26.9% (18/67) | |
| CD138 IHC 1+ | 34.3% (23/67) | |
| CD138 IHC 2+ | 13.4% (9/67) | |
| CD138 IHC 3+ | 25.4% (17/67) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallmann, M.R.; Tamir, S.; Alfter, K.; Ratiu, D.; Quaas, A.; Domroese, C.M. Expression of Potential Antibody–Drug Conjugate Targets in Cervical Cancer. Cancers 2024, 16, 1787. https://doi.org/10.3390/cancers16091787
Mallmann MR, Tamir S, Alfter K, Ratiu D, Quaas A, Domroese CM. Expression of Potential Antibody–Drug Conjugate Targets in Cervical Cancer. Cancers. 2024; 16(9):1787. https://doi.org/10.3390/cancers16091787
Chicago/Turabian StyleMallmann, Michael R., Sina Tamir, Katharina Alfter, Dominik Ratiu, Alexander Quaas, and Christian M. Domroese. 2024. "Expression of Potential Antibody–Drug Conjugate Targets in Cervical Cancer" Cancers 16, no. 9: 1787. https://doi.org/10.3390/cancers16091787
APA StyleMallmann, M. R., Tamir, S., Alfter, K., Ratiu, D., Quaas, A., & Domroese, C. M. (2024). Expression of Potential Antibody–Drug Conjugate Targets in Cervical Cancer. Cancers, 16(9), 1787. https://doi.org/10.3390/cancers16091787






