Circulating Adipocytokines and Insulin Like-Growth Factors and Their Modulation in Obesity-Associated Endometrial Cancer
Abstract
Simple Summary
Abstract
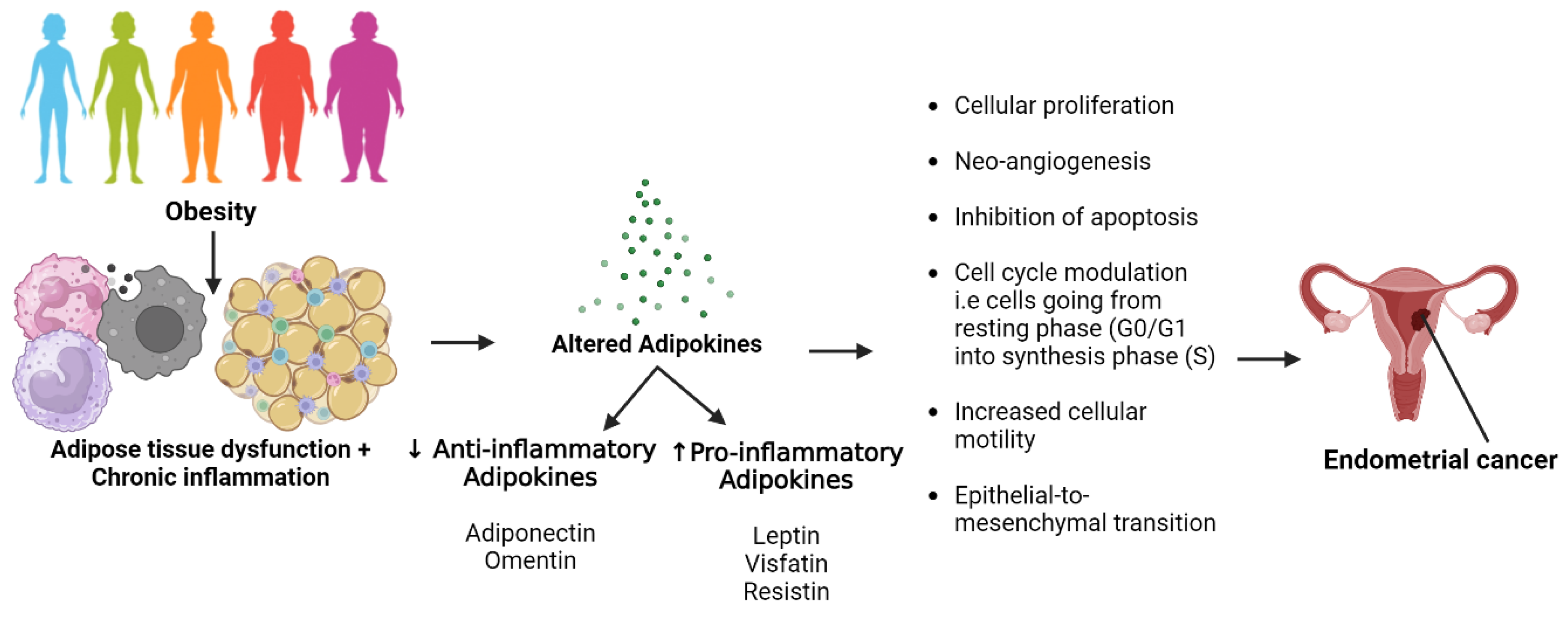
1. Introduction

2. Materials and Methods
2.1. Ethics Approval and Participant Recruitment
2.2. Plasma Preparation and Storage
2.3. Enzyme Linked Immunosorbent Assay (ELISA)
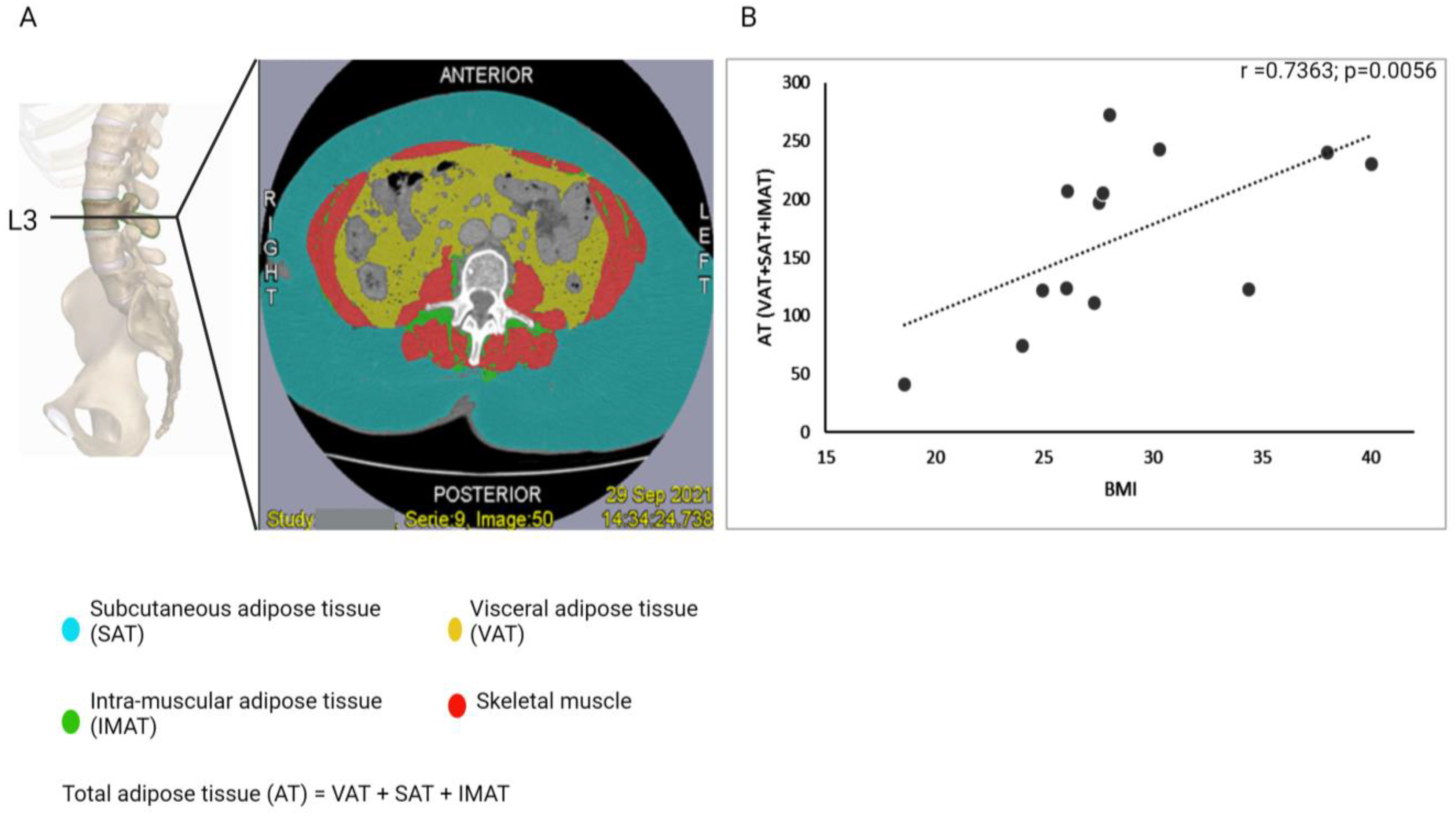
2.4. Assessment of Body Fat Distribution
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. BMI as an Indicator for Obesity
3.3. Endometrial Cancer Tissue Characterisation in Study Patients
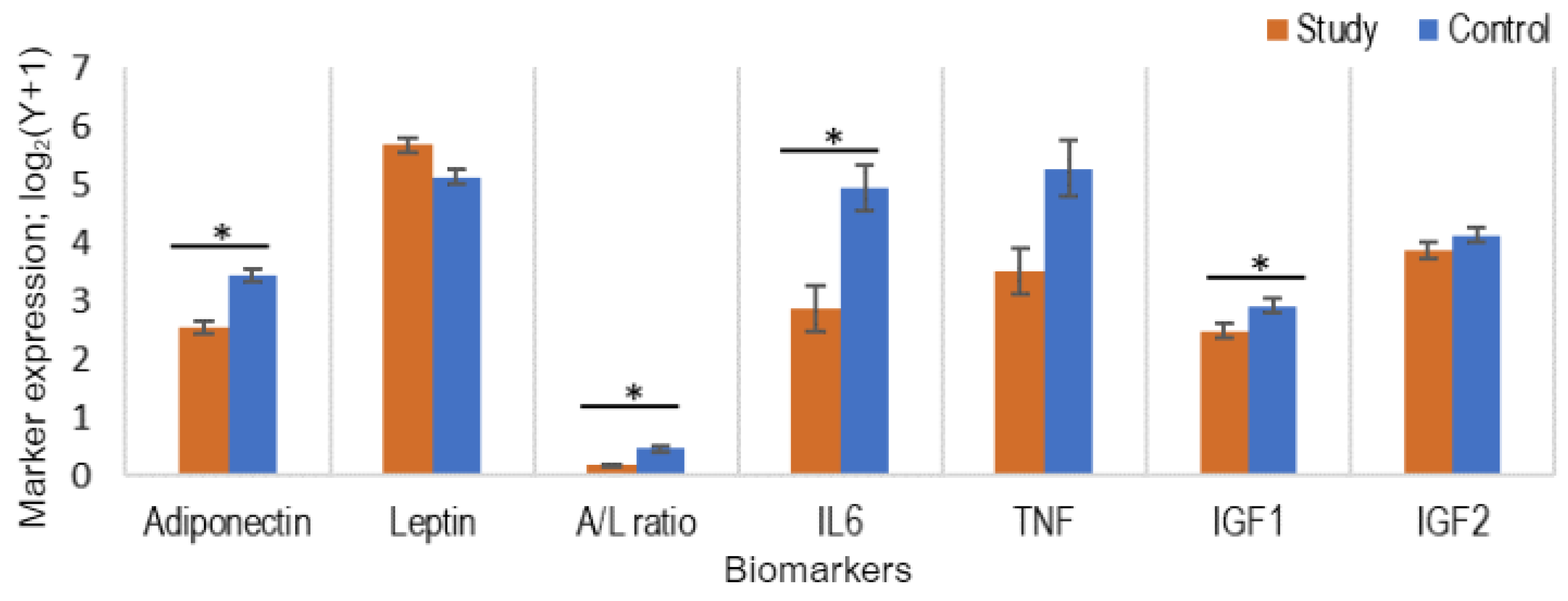
3.4. Comparison of the Levels of Adipocytokines and IGF in Study and Control Populations
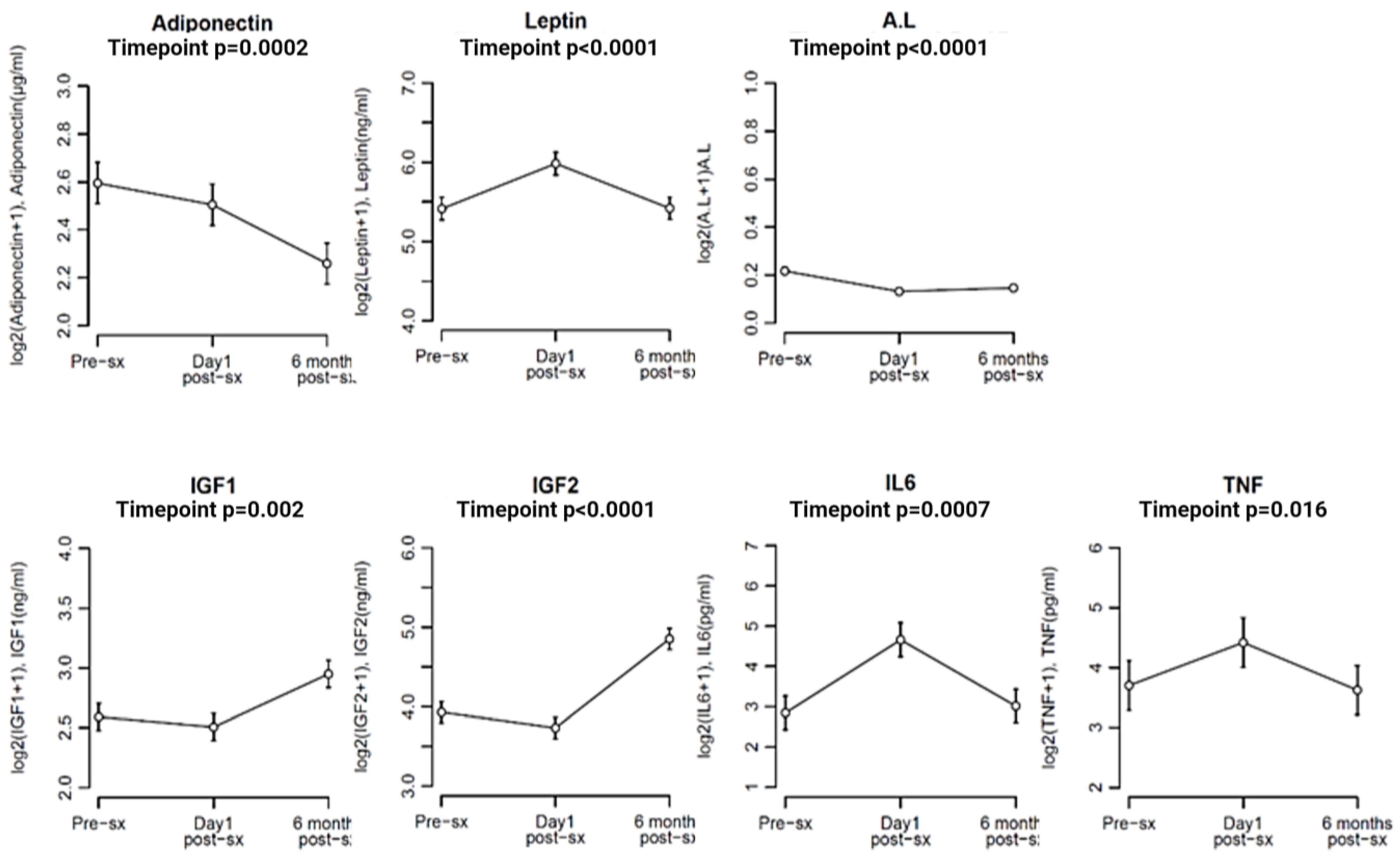
3.5. Comparison of Biomarker Levels at Different Time Points in the Study Population (Pre-Surgery, Day 1 Post-Surgery, and 6 Months Post-Surgery)
3.6. Association between Levels of Biomarkers and Demographic Characteristics of Both Populations
3.7. Associations between Levels of Biomarkers and Cancer Characteristics in the Study Population
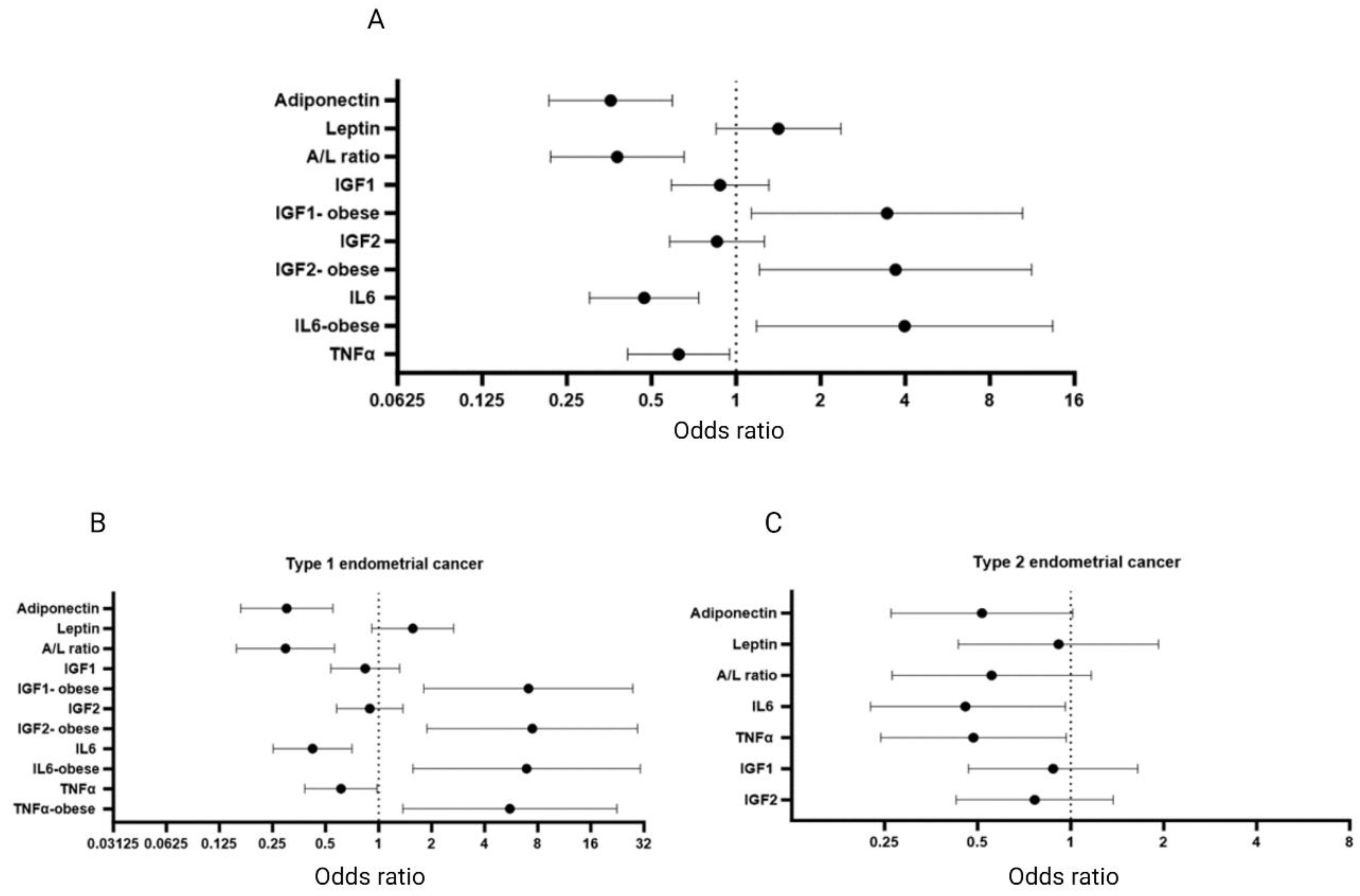
3.8. Endometrial Cancer Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK, Uterine Cancer Incidence Statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/incidence#heading-One (accessed on 22 September 2023).
- Brown, K.F.; Rumgay, H.; Dunlop, C.; Ryan, M.; Quartly, F.; Cox, A.; Deas, A.; Elliss-Brookes, L.; Gavin, A.; Hounsome, L.; et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br. J. Cancer 2018, 118, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef] [PubMed]
- Luhn, P.; Dallal, C.M.; Weiss, J.M.; Black, A.; Huang, W.-Y.; Lacey, J.V.; Hayes, R.B.; Stanczyk, F.Z.; Wentzensen, N.; Brinton, L.A. Circulating adipokine levels and endometrial cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose Tissue, Obesity and Adipokines: Role in Cancer Promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Garikapati, K.K.; Ammu, V.V.V.R.K.; Krishnamurthy, P.T.; Chintamaneni, P.K.; Pindiprolu, S.K.S.S. Type-II Endometrial Cancer: Role of Adipokines. Arch. Gynecol. Obstet. 2019, 300, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Verras, G.-I.; Tchabashvili, L.; Chlorogiannis, D.-D.; Mulita, F.; Argentou, M.-I. Updated Clinical Evidence on the Role of Adipokines and Breast Cancer: A Review. Cancers 2023, 15, 1572. [Google Scholar] [CrossRef]
- Körner, A.; Pazaitou-Panayiotou, K.; Kelesidis, T.; Kelesidis, I.; Williams, C.J.; Kaprara, A.; Bullen, J.; Neuwirth, A.; Tseleni, S.; Mitsiades, N.; et al. Total and High-Molecular-Weight Adiponectin in Breast Cancer: In Vitroandin VivoStudies. J. Clin. Endocrinol. Metab. 2007, 92, 1041–1048. [Google Scholar] [CrossRef]
- Jin, J.H.; Kim, H.-J.; Kim, C.Y.; Kim, Y.H.; Ju, W.; Kim, S.C. Association of Plasma adiponectin and Leptin Levels with the Development and Progression of Ovarian Cancer. Obstet. Gynecol. Sci. 2016, 59, 279–285. [Google Scholar] [CrossRef]
- Xie, L.; Wang, Y.; Wang, S.; Wu, N.; Chen, Y.; Yan, J. Adiponectin Induces Growth Inhibition and apoptosis in Cervical Cancer HeLa Cells. Biologia 2011, 66, 712–720. [Google Scholar] [CrossRef]
- Cust, A.E.; Kaaks, R.; Friedenreich, C.; Bonnet, F.; Laville, M.; Lukanova, A.; Rinaldi, S.; Dossus, L.; Slimani, N.; Lundin, E.; et al. Plasma Adiponectin Levels and Endometrial Cancer Risk in Pre- and Postmenopausal Women. J. Clin. Endocrinol. Metab. 2007, 92, 255–263. [Google Scholar] [CrossRef]
- Wei, E.K.; Giovannucci, E.; Fuchs, C.S.; Willett, W.C.; Mantzoros, C.S. Low Plasma Adiponectin Levels and Risk of Colorectal Cancer in Men: A Prospective Study. JNCI J. Natl. Cancer Inst. 2005, 97, 1688–1694. [Google Scholar] [CrossRef]
- Goktas, S.; Yilmaz, M.I.; Caglar, K.; Sonmez, A.; Kilic, S.; Bedir, S. Prostate cancer and adiponectin. Urology 2005, 65, 1168–1172. [Google Scholar] [CrossRef]
- Ho, G.Y.; Wang, T.; Gunter, M.J.; Strickler, H.D.; Cushman, M.; Kaplan, R.C.; Wassertheil-Smoller, S.; Xue, X.; Rajpathak, S.N.; Chlebowski, R.T.; et al. Adipokines Linking Obesity with Colorectal Cancer Risk in Postmenopausal Women. Cancer Res. 2012, 72, 3029–3037. [Google Scholar] [CrossRef]
- Song, C.-H.; Liao, J.; Deng, Z.-H.; Zhang, J.-Y.; Xue, H.; Li, Y.-M.; Liang, C.; Han, M.; Zhang, K.; Yan, G.-T. Is Leptin a Predictive Factor in Patients with Lung Cancer? Clin. Biochem. 2014, 47, 230–232. [Google Scholar] [CrossRef]
- Komarowska, M.; Chrzanowski, R.; Tylicka, M.; Rutkowski, R.; Mariak, Z.; Zelazowska-Rutkowska, B.; Lyson, T.; Hermanowicz, A. Plasma concentration of Bisphenol A and Leptin in Patients with Meningioma and Glioma: A Pilot Study. Adv. Med. Sci. 2022, 67, 229–233. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, F.; Pérez-Pérez, A.; De La Cruz-Merino, L.; Sánchez-Margalet, V. Obesity and Breast Cancer: Role of Leptin. Front. Oncol. 2019, 9, 596. [Google Scholar] [CrossRef]
- Caruso, A.; Gelsomino, L.; Panza, S.; Accattatis, F.M.; Naimo, G.D.; Barone, I.; Giordano, C.; Catalano, S.; Andò, S. Leptin: A Heavyweight Player in Obesity-Related Cancers. Biomolecules 2023, 13, 1084. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-Leptin Ratio: A Promising Index to Estimate Adipose Tissue Dysfunction. Relation with Obesity-Associated Cardiometabolic Risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- Cleary, M.P.; Ray, A.; Rogozina, O.P.; Dogan, S.; Grossmann, M.E. Targeting the Adiponectin leptin Ratio for Postmenopausal Breast Cancer Prevention. Front. Biosci. 2009, S1, 329–357. [Google Scholar] [CrossRef]
- Landskron, G.; De La Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Dey, G.; Mandal, M. Cancer Development, Chemoresistance, Epithelial to Mesenchymal Transition and Stem Cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016, 375, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Becker, S.; Rinaldi, S.; Lukanova, A.; Tjønneland, A.; Olsen, A.; Overvad, K.; Chabbert-Buffet, N.; Boutron-Ruault, M.; Clavel-Chapelon, F.; et al. Tumor Necrosis Factor (TNF)-α, Soluble TNF Receptors and Endometrial Cancer Risk: The EPIC study. Int. J. Cancer 2010, 129, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Ding, T.V.; Hanningan, E.V. Serum levels of interleukins, growth factors and anglogenin in patients with endometrial cancer. J. Cancer Res. Clin. Oncol. 1997, 123, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Insulin, Insulin-like Growth Factors and Colon Cancer: A Review of the Evidence. J. Nutr. 2001, 131, 3109S–3120S. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-J.; Hao, Q.; Zhang, H.-M.; Wu, Y.-Z.; Wang, J.-D. Insulin-like Growth Factors in Endometrioid Adenocarcinoma: Correlation with Clinico-Pathological Features and Estrogen Receptor Expression. BMC Cancer 2012, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Manson, J.E.; Li, J.; Harris, T.G.; Rohan, T.E.; Xue, X.; Ho, G.Y.F.; et al. A Prospective Evaluation of Insulin and Insulin-like Growth Factor-I as Risk Factors for Endometrial Cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pollak, M.N.; Giovannucci, E.; Chan, J.M.; Tao, Y.; Hennekens, C.H.; Stampfer, M.J. Prospective Study of Colorectal Cancer Risk in Men and Plasma Levels of Insulin-like Growth Factor (IGF)-I and IGF-Binding Protein-3. JNCI J. Natl. Cancer Inst. 1999, 91, 620–625. [Google Scholar] [CrossRef]
- Watts, E.L.; Perez-Cornago, A.; Fensom, G.K.; Smith-Byrne, K.; Noor, U.; Andrews, C.D.; Gunter, M.J.; Holmes, M.V.; Martin, R.M.; Tsilidis, K.K.; et al. Circulating insulin-like Growth Factors and Risks of Overall, Aggressive and Early-Onset Prostate Cancer: A Collaborative Analysis of 20 Prospective Studies and Mendelian Randomization Analysis. Leuk. Res. 2023, 52, 71–86. [Google Scholar] [CrossRef]
- Hankinson, S.E.; Willett, W.C.; Colditz, G.A.; Hunter, D.J.; Michaud, D.S.; Deroo, B.; Rosner, B.; Speizer, F.E.; Pollak, M. Circulating Concentrations of Insulin-like Growth Factor I and Risk of Breast Cancer. Lancet 1998, 351, 1393–1396. [Google Scholar] [CrossRef]
- Dziadziuszko, R.; Camidge, D.R.; Hirsch, F.R. The Insulin-like Growth Factor Pathway in Lung Cancer. J. Thorac. Oncol. 2008, 3, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Hunt, K.J.; Toniolo, P.; Akhmedkhanov, A.; Lukanova, A.; Dechaud, H.; Rinaldi, S.; Zeleniuch-Jacquotte, A.; E Shore, R.; Riboli, E.; Kaaks, R. Insulin-like Growth Factor II and Colorectal Cancer Risk in Women. Cancer Epidemiol. Biomark. Prev. 2002, 11, 901–905. [Google Scholar]
- Gao, Y.; Katki, H.; Graubard, B.; Pollak, M.; Martin, M.; Tao, Y.; Schoen, R.E.; Church, T.; Hayes, R.B.; Greene, M.H.; et al. Serum IGF1, IGF2 and IGFBP3 and Risk of Advanced Colorectal Adenoma. Int. J. Cancer 2012, 131, E105–E113. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Kondo, M.; Takeda, S.; Osada, H.; Takahashi, T.; Nakao, A.; Takahashi, T. Altered Transcriptional Regulation of the Insulin-like Growth Factor 2 Gene in Human Hepatocellular Carcinoma. Mol. Carcinog. 1997, 18, 193–198. [Google Scholar] [CrossRef]
- Petridou, E.; Koukoulomatis, P.; Alexe, D.M.; Voulgaris, Z.; Spanos, E.; Trichopoulos, D. Endometrial Cancer and the IGF System: A Case-Control Study in Greece. Oncology 2003, 64, 341–345. [Google Scholar] [CrossRef]
- Ray, I.; Meira, L.B.; Michael, A.; Ellis, P.E. Adipocytokines and Disease Progression in Endometrial Cancer: A Systematic Review. Cancer Metastasis Rev. 2022, 41, 211–242. [Google Scholar] [CrossRef]
- Masaki, T.; Chiba, S.; Tatsukawa, H.; Yasuda, T.; Noguchi, H.; Seike, M.; Yoshimatsu, H. Adiponectin Protects LPS-Induced Liver Injury through Modulation of TNF-Alpha in KK-Ay Obese Mice. Hepatology 2004, 40, 177–184. [Google Scholar] [CrossRef]
- Hector, J.; Schwarzloh, B.; Goehring, J.; Strate, T.G.; Hess, U.F.; Deuretzbacher, G.; Hansen-Algenstaedt, N.; Beil, F.-U.; Algenstaedt, P. TNF-α Alters Visfatin and Adiponectin Levels in Human Fat. Horm. Metab. Res. 2007, 39, 250–255. [Google Scholar] [CrossRef]
- Simons, P.J.; Pangaart, P.S.V.D.; Aerts, J.M.F.G.; Boon, L. Pro-inflammatory Delipidizing Cytokines Reduce Adiponectin Secretion from Human Adipocytes without Affecting Adiponectin Oligomerization. J. Endocrinol. 2007, 192, 289–299. [Google Scholar] [CrossRef]
- Madeddu, C.; Sanna, E.; Gramignano, G.; Tanca, L.; Cherchi, M.C.; Mola, B.; Petrillo, M.; Macciò, A. Correlation of Leptin, Proinflammatory Cytokines and Oxidative Stress with Tumor Size and Disease Stage of Endometrioid (Type I) Endometrial Cancer and Review of the Underlying Mechanisms. Cancers 2022, 14, 268. [Google Scholar] [CrossRef]
- Bosse, T.; Peters, E.E.; Creutzberg, C.L.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Mens, J.W.M.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Smit, V.T.; Nout, R.A. Substantial Lymph-Vascular Space Invasion (LVSI) is A Significant Risk Factor for Recurrence in Endometrial Cancer—A Pooled Analysis of Portec 1 and 2 Trials. Eur. J. Cancer 2015, 51, 1742–1750. [Google Scholar] [CrossRef]
- Song, J.; Li, H.; Guo, H.; Cai, Y. Microcystic, Elongated and Fragmented (MELF) Pattern in Endometrial Carcinoma: Clinicopathologic Analysis and Prognostic Implications. Medicine 2022, 101, e31369. [Google Scholar] [CrossRef] [PubMed]
- Kanopiene, D.; Vidugiriene, J.; Valuckas, K.P.; Smailyte, G.; Uleckiene, S.; Bacher, J. Endometrial Cancer and Microsatellite Instability Status. Open Med. 2015, 10, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Lukanova, A.; Rinaldi, S.; Allen, N.; Cust, A.E.; Becker, S.; Tjonneland, A.; Hansen, L.; Overvad, K.; Chabbert-Buffet, N.; et al. Hormonal, Metabolic, and Inflammatory Profiles and Endometrial Cancer Risk within the EPIC Cohort—A Factor Analysis. Am. J. Epidemiol. 2013, 177, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kadowaki, T. Adiponectin Receptor as a Key Player in Healthy Longevity and Obesity-Related Diseases. Cell Metab. 2013, 17, 185–196. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and Related Cytokines as the Critical Lynchpins between Inflammation and Cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Brahmkhatri, V.P.; Prasanna, C.; Atreya, H.S. Insulin-like Growth Factor System in Cancer: Novel Targeted Therapies. BioMed Res. Int. 2015, 2015, 538019. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Woo, Y.; Wang, Y.; Yeung, C.; Xu, A.; Lam, K. Obesity, Adipokines and Cancer: An Update. Clin. Endocrinol. 2014, 83, 147–156. [Google Scholar] [CrossRef]
- Tanabe, K.; Matsushima-Nishiwaki, R.; Yamaguchi, S.; Iida, H.; Dohi, S.; Kozawa, O. Mechanisms of Tumor Necrosis Factor-α-Induced Interleukin-6 Synthesis in Glioma Cells. J. Neuroinflammation 2010, 7, 16. [Google Scholar] [CrossRef]
- Orrù, S.; Nigro, E.; Mandola, A.; Alfieri, A.; Buono, P.; Daniele, A.; Mancini, A.; Imperlini, E. A Functional Interplay between IGF-1 and Adiponectin. Int. J. Mol. Sci. 2017, 18, 2145. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wen, K.; Han, X.; Liu, R.; Qu, Q. Adiponectin Mediates Antiproliferative and Apoptotic Responses in Endometrial Carcinoma by the AdipoRs/AMPK Pathway. Gynecol. Oncol. 2015, 137, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Daley-Brown, D.; Harbuzariu, A.; Kurian, A.A.; Oprea-Ilies, G.; Gonzalez-Perez, R.R. Leptin-Induced Notch and IL-1 Signaling Crosstalk in Endometrial Adenocarcinoma is Associated with Invasiveness and Chemoresistance. World J. Clin. Oncol. 2019, 10, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhu, Y.; Wang, Y.; Teng, F.; Zhang, H.; Liu, G.; Ma, X.; Sun, D.; Rohan, T.; Xue, F. Visfatin, a Potential Biomarker and Prognostic Factor for Endometrial Cancer. Gynecol. Oncol. 2013, 129, 505–512. [Google Scholar] [CrossRef]
- Moon, H.-S.; Chamberland, J.P.; Aronis, K.; Tseleni-Balafouta, S.; Mantzoros, C.S. Direct Role of Adiponectin and Adiponectin Receptors in Endometrial Cancer: In Vitro and Ex Vivo Studies in Humans. Mol. Cancer Ther. 2011, 10, 2234–2243. [Google Scholar] [CrossRef]
- Physical Status: The Use and Interpretation of Anthropometry. Report of A WHO Expert Committee; World Health Organization Technical Report Series; Europe PMC: Oxford, UK, 1995; Volume 854.
- McShane, L.M.; for the Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Reporting Recommendations for Tumour MARKer Prognostic Studies (REMARK). Br. J. Cancer 2005, 93, 387–391. [Google Scholar] [CrossRef]
- van Vugt, J.L.A.; van Putten, Y.; van der Kall, I.M.; Buettner, S.; D’ancona, F.C.H.; Dekker, H.M.; Kimenai, H.J.A.N.; de Bruin, R.W.F.; Warlé, M.C.; Ijzermans, J.N.M. Estimated skeletal muscle mass and density values measured on computed tomography examinations in over 1000 living kidney donors. Eur. J. Clin. Nutr. 2019, 73, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Kuznia, P.; Heshka, S.; Albu, J.; Heymsfield, S.B.; Goodpaster, B.; Visser, M.; Harris, T.B. Adipose Tissue in Muscle: A Novel Depot Similar in Size to Visceral Adipose Tissue. Am. J. Clin. Nutr. 2005, 81, 903–910. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO Staging for Carcinoma of the Vulva, Cervix, and Endometrium. Int. J. Gynecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Bokhman, J.V. Two Pathogenetic Types of Endometrial Carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Voss, M.A.; Ganesan, R.; Ludeman, L.; McCarthy, K.; Gornall, R.; Schaller, G.; Wei, W.; Sundar, S. Should Grade 3 Endometrioid Endometrial Carcinoma be Considered a Type 2 cancer—A Clinical and Pathological Evaluation. Gynecol. Oncol. 2012, 124, 15–20. [Google Scholar] [CrossRef]
- Pelikan, H.; Trum, J.; Bakers, F.; Beets-Tan, R.; Smits, L.; Kruitwagen, R. Diagnostic Accuracy of Preoperative Tests for Lymph Node Status in Endometrial Cancer: A Systematic Review. Cancer Imaging 2013, 13, 314–322. [Google Scholar] [CrossRef]
- Bellone, S.; Watts, K.; Cane’, S.; Palmieri, M.; Cannon, M.J.; Burnett, A.; Roman, J.J.; Pecorelli, S.; Santin, A.D. High Serum Levels of Interleukin-6 in Endometrial Carcinoma are Associated with Uterine Serous PAPILLARY HISTOlogy, A Highly Aggressive and Chemotherapy-resistant Variant of endometrial Cancer. Gynecol. Oncol. 2005, 98, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and Cancer Risk: New Mechanistic Insights from Epidemiology. Nat. Rev. Cancer 2015, 15, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Ashizawa, N.; Yahata, T.; Quan, J.; Adachi, S.; Yoshihara, K.; Tanaka, K. Serum Leptin–Adiponectin Ratio and Endometrial Cancer Risk in Postmenopausal Female Subjects. Gynecol. Oncol. 2010, 119, 65–69. [Google Scholar] [CrossRef]
- Zeng, F.; Shi, J.; Long, Y.; Tian, H.; Li, X.; Zhao, A.Z.; Li, R.F.; Chen, T. Adiponectin and Endometrial Cancer: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2015, 36, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.E.; Barron, G.A.; Bermano, G. Adipocytokines and Their Relationship to Endometrial Cancer Risk: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2020, 158, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Dossus, L.; Rinaldi, S.; Becker, S.; Lukanova, A.; Tjonneland, A.; Olsen, A.; Stegger, J.; Overvad, K.; Chabbert-Buffet, N.; Jimenez-Corona, A.; et al. Obesity, Inflammatory Markers, and Endometrial Cancer risk: A Prospective Case–Control Study. Endocr. Relat. Cancer 2010, 17, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.; Sezer, S.; Baser, E.; Gun-Eryilmaz, O.; Gungor, T.; Uysal, S.; Yilmaz, F.M. Evaluating Vaspin and Adiponectin in Postmenopausal Women with Endometrial Cancer. Endocr. Relat. Cancer 2013, 20, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Petridou, E.; Mantzoros, C.; Dessypris, N.; Koukoulomatis, P.; Addy, C.; Voulgaris, Z.; Chrousos, G.; Trichopoulos, D. Plasma Adiponectin Concentrations in Relation to Endometrial Cancer: A Case-Control Study in Greece. J. Clin. Endocrinol. Metab. 2003, 88, 993–997. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yabushita, H.; Iwasaki, K.; Wakatsuki, A. Role of Adiponectin and Leptin in Non-Diabetic, Non-Obese Patients with Endometrial Cancer. Eur. J. Gynaecol. Oncol. 2018, 39, 199–204. [Google Scholar] [CrossRef]
- Terlikowska, K.M.; Dobrzycka, B.; Terlikowski, R.; Sienkiewicz, A.; Kinalski, M.; Terlikowski, S.J. Clinical Value of Selected Markers of Angiogenesis, Inflammation, Insulin Resistance and Obesity in Type 1 Endometrial Cancer. BMC Cancer 2020, 20, 921. [Google Scholar] [CrossRef] [PubMed]
- Cymbaluk, A.; Chudecka-Głaz, A.; Rzepka-Górska, I. Leptin Levels in Serum Depending on Body Mass Index in Patients with Endometrial Hyperplasia and cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 136, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Lecoultre, V.; Ravussin, E. Novel Strategy for the Use of Leptin for Obesity Therapy. Expert Opin. Biol. Ther. 2011, 11, 1677–1685. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, S.-H.; Nagalingam, A.; Saxena, N.K.; Singh, S.V.; Sharma, D. Benzyl Isothioacyanate Inhiabits Oncogenic Actions of Leptin in Human Breast Cancer Cells by Suppressing Activation of Signal Transducer and Activator of Transcription 3. Carcinogenesis 2010, 32, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.R.; Leavis, P.C. A Peptide Derived from the Human Leptin Molecule Is a Potent Inhibitor of the Leptin Receptor Function in Rabbit Endometrial Cells. Endocrine 2003, 21, 185–196. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Salvador, J.; Colina, I.; Gómez-Ambrosi, J. Adiponectin-leptin Ratio is a Functional Biomarker of Adipose Tissue Inflammation. Nutrients 2019, 11, 454. [Google Scholar] [CrossRef]
- Gong, T.; Wu, Q.; Wang, Y.; Ma, X. Circulating Adiponectin, Leptin and Adiponectin–Leptin Ratio and Endometrial Cancer Risk: Evidence from a Meta-Analysis of Epidemiologic Studies. Int. J. Cancer 2015, 137, 1967–1978. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Langley, A.R.; Speidel, T.P.; Lau, D.C.; Courneya, K.S.; Csizmadi, I.; Magliocco, A.M.; Yasui, Y.; Cook, L.S. Case–Control Study of Inflammatory Markers and the Risk of Endometrial Cancer. Eur. J. Cancer Prev. 2013, 22, 374–379. [Google Scholar] [CrossRef]
- Wang, T.; Rohan, T.E.; Gunter, M.J.; Xue, X.; Wactawski-Wende, J.; Rajpathak, S.N.; Cushman, M.; Strickler, H.D.; Kaplan, R.C.; Wassertheil-Smoller, S.; et al. A Prospective Study of Inflammation Markers and Endometrial Cancer Risk in Postmenopausal Hormone Nonusers. Cancer Epidemiol. Biomark. Prev. 2011, 20, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Ohannesian, J.P.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Magosin, S.; Marco, C.; Caro, J.F. Nocturnal Rise of Leptin in Lean, Obese, and Non-Insulin-Depeandent Diabetes Mellitus Subjects. J. Clin. Investig. 1996, 97, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Posaci, C.; Altunyurt, S.; Islekel, H.; Onvural, A. Effects of HRT on Serum Levels of IGF-I in Postmenopausal Women. Maturitas 2001, 40, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Nasu, M.; Sugimoto, T.; Chihara, M.; Hiraumi, M.; Kurimoto, F.; Chihara, K. Effect of Natural Menopause on Serum Levels of IGF-I and IGF-binding Proteins: Relationship with Bone Mineral Density and Lipid Metabolism in Perimenopausal Women. Eur. J. Endocrinol. 1997, 136, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Sawada, S.; Satoh, M.; Asai, Y.; Kodama, S.; Sato, T.; Tomiyama, S.; Seike, J.; Takahashi, K.; Kaneko, K.; et al. Insulin-like Growth Factor-1 Levels are Associated with High Comorbidity of Metabolic Disorders in obaese Subjects; A Japanese single-Center, Retrospective-STUDY. Sci. Rep. 2022, 12, 20130. [Google Scholar] [CrossRef]
- Florescu, A.; Branisteanu, D.; Bilha, S.; Scripcariu, D.; Florescu, I.; Scripcariu, V.; Dimofte, G.; Grigoras, I. Leptin and Adiponectin Dynamics at Patients with Rectal Neoplasm—Gender Differences. PLoS ONE 2019, 14, e0212471. [Google Scholar] [CrossRef]
- Shi, W.; Lou, J.; Zhang, X.; Ji, Y.; Weng, X.; Du, J. Adipose Tissue Alleviates the Stress Response by Releasing Adiponectin during Laparoscopic Surgery in Patients with Colorectal Cancer. Lipids Health Dis. 2021, 20, 166. [Google Scholar] [CrossRef]
- Coskun, T.; Kosova, F.; Ari, Z.; Sakarya, A.; Kaya, Y. Effect of Oncological Treatment on Serum Adipocytokine Levels in Patients with Stage II–III Breast Cancer. Mol. Clin. Oncol. 2016, 4, 893–897. [Google Scholar] [CrossRef][Green Version]
- Holdaway, I.M.; Lethaby, A.E.; Mason, B.H.; Singh, V.; Harman, J.E.; MacCormick, M.; Civil, I.D. Effect of Breast Surgery on Serum Levels of Insulin-like Growth Factors (IGF-I, IGF-II, and IGF Binding Protein-3) in Women with Benign and Malignant Breast Lesions. Ann. Surg. Oncol. 2001, 8, 25–31. [Google Scholar] [CrossRef]
- Wu, Q.J.; Li, Y.Y.; Tu, C.; Zhu, J.; Qian, K.Q.; Feng, T.B.; Li, C.; Wu, L.; Ma, X.X. Parity and endometrial cancer risk: A meta-analysis of epidemiological studies OPEN. Nat. Publ. Group 2015, 5, 14243. [Google Scholar] [CrossRef]
- Dossus, L.; Allen, N.; Kaaks, R.; Bakken, K.; Lund, E.; Tjonneland, A.; Olsen, A.; Overvad, K.; Clavel-Chapelon, F.; Fournier, A.; et al. Reproductive risk factors and endometrial cancer: The European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2010, 127, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yang, L.; Sun, Q.; Cong, R.; Gu, H.; Tang, N.; Zhu, H.; Wang, B. Cigarette smoking and the risk of endometrial cancer: A meta-analysis. Am. J. Med. 2008, 121, 501–508. [Google Scholar] [CrossRef] [PubMed]
- American Society of Clinical Oncology. Uterine Cancer: Risk Factors and Prevention. Available online: https://www.cancer.net/cancer-types/uterine-cancer/risk-factors-and-prevention (accessed on 18 September 2022).
- The American Cancer Society Medical and Editorial Content Team. Endometrial Cancer Risk Factors; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Brinton, L.A.; Felix, A.S. Menopausal hormone therapy and risk of endometrial cancer. J. Steroid Biochem. Mol. Biol. 2014, 142, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Su, P.Y.; Hao, J.H.; Sun, Y.H. The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: A meta-analysis of prospective cohort studies. Int. J. Gynecol. Cancer 2013, 23, 294–303. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Vatten, L.J. Hypertension and the risk of endometrial cancer: A systematic review and meta-analysis of case-control and cohort studies. Sci. Rep. 2017, 7, 44808. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef]
| Parameters | Study Population (n = 50) | Control Population (n = 50) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Number | Percentage | Number | Percentage | ||||
| 30–39 | 3 | 6% | 3 | 6% | 1 | ||
| Age range (years) | 40–49 | 3 | 6% | 3 | 6% | ||
| 50–59 | 11 | 22% | 11 | 22% | |||
| Mean age (years) | 60–69 | 10 | 20% | 10 | 20% | ||
| Study | Control | 70–79 | 16 | 32% | 16 | 32% | |
| 65.7 | 66.1 | 80–89 | 6 | 12% | 6 | 12% | |
| 90–99 | 1 | 2% | 1 | 2% | |||
| BMI | 18.5–24.9 | 9 | 18% | 20 | 40% | 0.022 * | |
| Average BMI | 25.0–29.9 | 15 | 30% | 19 | 38% | ||
| Study | Control | ≥30.0 | 26 | 52% | 11 | 22% | |
| 32.1 | 26.6 | ||||||
| Parity | P0 (nulliparous) | 11 | 22% | 3 | 6% | 0.041 * | |
| ≥P0 | 39 | 78% | 47 | 94% | |||
| Ethnicity | Caucasian | 43 | 86% | 48 | 96% | 0.112 | |
| Asian | 6 | 12% | 1 | 2% | |||
| Afro-Caribbean | 1 | 2% | 1 | 2% | |||
| Smoking status | Yes | 3 | 6% | 1 | 2% | 0.47 | |
| No | 35 | 70% | 33 | 66% | |||
| Ex-smoker | 12 | 24% | 16 | 32% | |||
| Menopausal status | Yes | 39 | 78% | 42 | 84% | 0.611 | |
| No | 11 | 22% | 8 | 16% | |||
| Hormonal Contraception | Yes | 30 | 60% | 32 | 64% | 0.837 | |
| No | 20 | 40% | 18 | 36% | |||
| HRT (menopausal women only) | Yes | 10 (n = 39) | 25.60% | 20 (n = 42) | 47.60% | 0.05 | |
| No | 29 | 74.40% | 22 | 52.40% | |||
| Diabetes | Yes | 13 | 26% | 3 | 6% | 0.012 * | |
| No | 37 | 74% | 47 | 94% | |||
| Hypertension | Yes | 22 | 44% | 20 | 40% | 0.84 | |
| No | 28 | 56% | 30 | 60% | |||
| Past history of | Yes | 6 | 12% | 14 | 28% | 0.078 | |
| cancer | No | 44 | 88% | 36 | 72% | ||
| Family history of | Yes | 22 | 44% | 28 | 56% | 0.317 | |
| cancer | No | 28 | 56% | 22 | 44% | ||
| Parameter | Sub-Groups | n (%) | Parameter | Sub-Groups | n (%) | |
|---|---|---|---|---|---|---|
| Grade | 1 | 23 (46) | LVSI | Yes | 15 (30) | |
| 2 | 13 (26) | No | 35 (70) | |||
| 3 | 14 (28) | |||||
| Stage | IA | 32 (64) | MELF | Yes | 6 (17) | |
| IB | 9 (18) | No | 29 (83) | |||
| II | 2 (4) | |||||
| III | 7 (14) | |||||
| Histology | Type 1 | Endometrioid | 36 (72) | MSI | Yes | 10 (24) |
| Type 2 | 14 (28) | No | 32 (76) | |||
| Serous | 6 (12) | |||||
| Mucinous | 1 (2) | |||||
| Endometrioid (G3) | 5 (10) | |||||
| Carcinosarcoma | 2 (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ray, I.; Möller-Levet, C.S.; Michael, A.; Butler-Manuel, S.; Chatterjee, J.; Tailor, A.; Ellis, P.E.; Meira, L.B. Circulating Adipocytokines and Insulin Like-Growth Factors and Their Modulation in Obesity-Associated Endometrial Cancer. Cancers 2024, 16, 531. https://doi.org/10.3390/cancers16030531
Ray I, Möller-Levet CS, Michael A, Butler-Manuel S, Chatterjee J, Tailor A, Ellis PE, Meira LB. Circulating Adipocytokines and Insulin Like-Growth Factors and Their Modulation in Obesity-Associated Endometrial Cancer. Cancers. 2024; 16(3):531. https://doi.org/10.3390/cancers16030531
Chicago/Turabian StyleRay, Irene, Carla S. Möller-Levet, Agnieszka Michael, Simon Butler-Manuel, Jayanta Chatterjee, Anil Tailor, Patricia E. Ellis, and Lisiane B. Meira. 2024. "Circulating Adipocytokines and Insulin Like-Growth Factors and Their Modulation in Obesity-Associated Endometrial Cancer" Cancers 16, no. 3: 531. https://doi.org/10.3390/cancers16030531
APA StyleRay, I., Möller-Levet, C. S., Michael, A., Butler-Manuel, S., Chatterjee, J., Tailor, A., Ellis, P. E., & Meira, L. B. (2024). Circulating Adipocytokines and Insulin Like-Growth Factors and Their Modulation in Obesity-Associated Endometrial Cancer. Cancers, 16(3), 531. https://doi.org/10.3390/cancers16030531








