Simple Summary
Meningiomas are the most common intracranial tumors, and significant advances have been made in our understanding of the biology that leads to meningioma growth and aggressiveness. This review summarizes molecular advances and the historical contexts that led to them.
Abstract
Meningioma classification and treatment have evolved over the past eight decades. Since Bailey, Cushing, and Eisenhart’s description of meningiomas in the 1920s and 1930s, there have been continual advances in clinical stratification by histopathology, radiography and, most recently, molecular profiling, to improve prognostication and predict response to therapy. Precise and accurate classification is essential to optimizing management for patients with meningioma, which involves surveillance imaging, surgery, primary or adjuvant radiotherapy, and consideration for clinical trials. Currently, the World Health Organization (WHO) grade, extent of resection (EOR), and patient characteristics are used to guide management. While these have demonstrated reliability, a substantial number of seemingly benign lesions recur, suggesting opportunities for improvement of risk stratification. Furthermore, the role of adjuvant radiotherapy for grade 1 and 2 meningioma remains controversial. Over the last decade, numerous studies investigating the molecular drivers of clinical aggressiveness have been reported, with the identification of molecular markers that carry clinical implications as well as biomarkers of radiotherapy response. Here, we review the historical context of current practices, highlight recent molecular discoveries, and discuss the challenges of translating these findings into clinical practice.
Keywords:
meningioma; molecular; methylation; WHO grade; copy number variant; gene expression panel; history 1. Introduction
Meningioma is the most common primary central nervous system tumor, with over 37,000 cases per year in the United States, greater than the 16,000 gliomas of all grades per year [1]. While most meningiomas are sporadic, they can occur in genetic syndromes, including neurofibromatosis type 2, BAP1 tumor predisposition syndrome, and Gorlin syndrome [2]. Meningioma classification and treatment have evolved over the past eight decades. Since Bailey, Cushing, and Eisenhart’s description of meningiomas in the 1920s and 1930s [3,4,5], there have been continual advances in clinical stratification by histopathology, radiography and, most recently, molecular profiling, to improve prognostication and predict response to therapy. Precise and accurate classification is essential to optimizing management for patients with meningioma, which involves surveillance imaging, surgery, primary or adjuvant radiotherapy, and consideration for clinical trials. This process is challenging, given outcomes that range from an indolent tumor that never grows to aggressive meningiomas that cause mortality. Currently, the World Health Organization (WHO) grade, extent of resection (EOR), and patient characteristics are used to guide management. While these have demonstrated reliability, a substantial number of seemingly benign lesions recur, suggesting opportunities for improvement of risk stratification. Furthermore, the role of adjuvant radiotherapy for grade 1 and 2 meningioma remains controversial, with debate about whether to radiate subtotally resected WHO grade 1 tumors or gross totally resected WHO grade 2 tumors [6,7]. Over the last decade, numerous studies investigating the molecular drivers of clinical aggressiveness have been reported, with the identification of key markers that may carry clinical utility (Figure 1). We review the historical context of current practices (Figure 2), highlight recent molecular discoveries, and discuss the challenges of translating these findings into clinical practice.
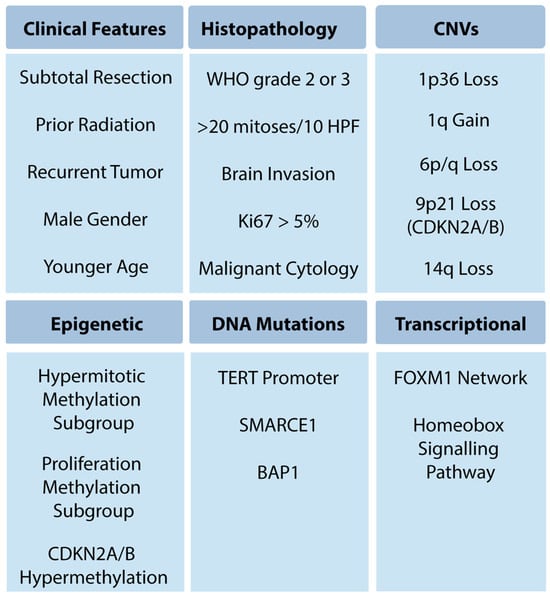
Figure 1.
High-risk meningioma features.
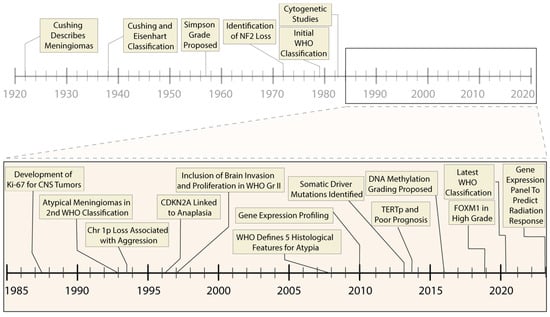
Figure 2.
Timeline of prognostic advances in meningiomas. Progressive molecular and histological characterization has led to refinement in the clinical treatment of meningiomas, including improvement in prognostic accuracy and selection of appropriate therapeutic interventions. The past thirty-five years have been marked by rapid molecular advances (inset panel), including high-throughput sequencing and genome-wide epigenetic studies.
2. Current Clinical and Pathological Classification with Historical Context
2.1. Clinical Features
Several preoperative patient factors predict WHO grade and recurrence. Multiple studies, including one with 1113 patients, found that WHO grade 2 tumors were more likely to be associated with tumor size >3.2 cm, a non-skull base location, and male gender [8,9,10,11,12,13]. Young age is associated with shorter PFS [14]. Finally, malignant meningiomas are more common in African Americans [15]. Machine learning algorithms have shown that patient demographics, radiographic features, and radiomic features such as sphericity have similar accuracy in predicting PFS and overall survival as models based on meningioma grade and EOR [16]. As standardization, generalizability, and reproducibility improve, radiomics may play a role in the evaluation and management of meningioma [17].
2.2. Simpson Grade
The importance of EOR in decreasing recurrence has been foundational in meningioma care since the Simpson grade was described in 1957 [18]. The scale is graded I–V, with a grade I resection being complete removal of the tumor, the involved dura, and the involved bone; grade II being complete removal of the tumor and coagulation of the dura; grade III being complete removal of the tumor without coagulating the dura; grade IV being subtotal removal; and grade V being a biopsy or simple decompression. The scale is significantly associated with five-year recurrence [19,20]. A Simpson grade 0, defined as resection of a 2 cm margin of dura around the tumor, which may reduce the rate of recurrence, is often impractical anatomically [21]. Likewise, the ability to achieve a Simpson 1 resection is often limited by anatomical considerations, such as involvement of a patent’s superior sagittal sinus.
The ability to perform safe and complete resections has improved greatly since the 1950s, based on improvements in imaging, instrumentation, intraoperative navigation, and the operative microscope. Thus, the relevance of the Simpson grade is debated [22]. Several studies failed to show a significant difference in patient recurrence between Simpson grade I, II, and III resections [23,24,25,26,27]. However, Simpson grade IV and V resections are still associated with shorter PFS [28]. These findings are likely related to improvements in microsurgical technique, resulting in small tumor volume with subtotal resection [22]. Many surgeons continue to use Simpson grade to communicate EOR, as complete safe resection remains the operative goal.
2.3. WHO Grade
The first WHO classification (1979) categorized meningiomas based on histopathological architecture into meningothelial, fibrous, transitional, psammomatous, angiomatous, hemangioblastic, hemangiopericytotic, and papillary subtypes, or as anaplastic, which behaved aggressively [29]. Meningiomas were defined as extra-parenchymal tumors that could be cured surgically [30]. In 1993, the WHO classification added atypical meningiomas, defined as tumors with brisk mitotic activity (4+ mitotic figures per 10 HPF) and hypercellularity without frank anaplasia [31]. Notably, counting mitosis per 10 HPF has more than a 20% discordance between observers [32]. The 2000 WHO classification added clear cell and chordoid histology and brain invasion as a criteria for the atypical grade [33,34,35]. In 2007, additional histopathological criteria (three or more of spontaneous necrosis, sheeting, high cellularity, high nuclear-to-cytoplasmic ratio, and prominent nucleoli) were added to classify a meningioma as grade 2 [36]. Brain invasion alone became sufficient for the diagnosis of atypical meningioma in the 2016 WHO classification [36,37].
The current 2021 WHO classification categorizes meningiomas into three grades with 15 histopathologic subtypes [38]. Nine variants are grade 1, while clear cell and chordoid histology are grade 2. Rhabdoid and papillary histology no longer are criteria for grade 3. The majority of meningiomas are classified as WHO grade 1 and have an 86% 5-year progression-free survival (PFS) regardless of EOR and 96% 5-year PFS after gross total resection (GTR) [37,39,40,41]. Nevertheless, long-term studies show up to 38% of gross totally resected grade 1 meningiomas eventually recur, suggesting that WHO grade alone is not sufficient to predict clinical outcomes [19,39,42,43]. WHO grade 2 (atypical) and 3 (anaplastic) meningiomas have a more aggressive clinical course, with recurrence rates of 20–70% at 5 years, despite surgical resection and adjuvant radiotherapy [37,44]. Survival is shorter for high-grade meningiomas, with a median of 80% of WHO grade 2 and 40% of WHO grade 3 patients surviving 5 years after diagnosis [45]. Finally, molecular changes have been added to the WHO diagnostic criteria, with TERT promoter mutations and homozygous CDKN2A/B loss being sufficient for grade 3 classification. The WHO classification remains the foundation of pathological diagnosis; however, limitations remain due to interobserver variability in histologic analysis, potential under-sampling due to intratumor heterogeneity, and questionable reliability of brain invasion to predict recurrence [32,46,47,48].
2.4. Proliferation Index
The proliferation index (MIB-1 index or Ki-67 antigen) is a continuous variable that reports the percentage of cells dividing within a tumor. Despite multiple studies, there is no clear-cut point to define the threshold that independently predicts meningioma recurrence [49,50,51]. Interobserver and institutional differences in evaluating and reporting the MIB-1 index and intratumoral heterogeneity, with hotspots of high Ki-67 activity and areas of low activity, limit generalizability. In particular, there can be differences in the staining methodology; in particular, a lack of accurate quantification and relying on an estimate of the percent staining creates significant interobserver variability. Various studies have suggested MIB-1 cut-off points of 3%, 5%, or 10% as clinically meaningful based on an association with increased risk of progression or recurrence [26,40,46,52]. Notably, the MIB-1 index carries greater prognostic utility when considered in conjunction with histopathology and EOR. One study found that for WHO grade 1 meningiomas undergoing Simpson grade II or III resection, an MIB-1 value of 3% or higher was associated with greater recurrence [26]. Another study of 239 WHO grade I meningiomas found that after GTR, when the MIB-1 index was >4.5%, the recurrence rate was the same (18%) as after subtotal resection (STR) [53]. Among atypical meningiomas after GTR, adjuvant radiation therapy (RT) was associated with a longer PFS when the MIB-1 index was >7% [44]. Thus, the MIB-1 index may be useful in refining predictions of post-operative recurrence when considered with additional factors, but its role as a sole prognostic variable remains unclear. Technical advances, including automated quantification, may improve reproducibility and allow integration of MIB-1 into generalizable prognostic criteria for meningioma [54].
3. Recent Advances and Current Evidence for Molecular-Based Classification
Contemporary management of meningiomas depends entirely on histologic, radiographic, and surgical (EOR) data. Despite progressive improvements in these modalities, many patients have recurrences that are unexpected based on benign histopathology, while others do not recur despite having aggressive histopathology. The challenges for clinicians to reliably classify patients into risk groups suggest room for improvement in our current methods. Over the last two decades, scientific advances in high-throughput sequencing have led to an exponential rise in molecular research on meningiomas. Though the clinical significance of these results has not been validated in prospective studies due to the long natural history of meningioma, they are increasingly informing prognostication [55,56]. Analogous discoveries in glioma and other forms of central nervous system (CNS) neoplasia have led to dramatic re-classifications of these lesions, with recognition that the underlying biology is the principal determinate of clinical course. Next, we review and summarize published molecular findings, with a focus on well-validated studies with translational potential.
3.1. Chromosomal Copy Number Variants
Meningiomas have been extensively studied with karyotyping and cytogenetics, beginning in the 1970s [57,58,59,60,61]. Most sporadic meningiomas exhibit inactivation of the NF2 tumor suppressor gene, which is lost via copy number variants and/or somatic damaging mutations in 60–80% of cases [62,63,64,65]. NF2 inactivation is associated with atypia and enriched in higher-grade meningiomas [66]. TIMP3 is another gene located near NF2 on chromosome 22 that has tumor suppressor-like properties in addition to inhibiting matrix metalloproteinases, and can be hypermethylated in meningiomas. This hypermethylation is associated with more aggressive high-risk meningiomas and may play a role in malignant conversion of meningiomas [67,68]. High-grade meningiomas often have frequent chromosomal aberrations, such as losses of 1p, 9p (CDKN2A/B), 10q, and 14q, and/or gains of 1q [69,70,71,72,73]. The number of chromosomal aberrations typically increases with histopathologic grade [43,70,74,75,76,77,78,79,80]. Loss of chromosomes 1p and 14q are the second and third most common chromosomal changes, respectively, and are associated with higher-grade meningiomas and reduced PFS [81,82,83,84,85]. Other events have been documented at a lower frequency, including loss of chromosomes 10, 6q, 18q, and sex chromosomes and gains of chromosomes 9q, 12q, 15q, 17q, and 20q [81,86]. Deletions of chr9p21, which includes the tumor suppressor genes CDKN2A (p16) and CDKN2B, are associated with anaplastic meningiomas, poor prognosis, and aggressive clinical behavior [86,87,88,89,90,91].
There have been several proposed methods for meningioma risk stratification and prediction of recurrence using karyotyping and cytogenetics [14,43,74,89,92,93,94]; however, the ability of copy number variation (CNV) to predict clinical behavior is still debated. Complex karyotypes are associated with worsened outcomes [14,43,74,83], though the sensitivity for predicting aggressiveness is often limited. The inclusion of additional clinical features, such as gender, age, tumor size, and location may improve prognostic value. Muti-factorial assessments, including mitotic count, CDKN2A/B status, and CNVs, can differentiate between aggressive WHO grade 1 and well-behaved WHO grade 2 meningiomas, which is useful for risk stratification [86]. Meningioma cytogenetics are not yet included in the WHO guidelines, though loss of chromosome 1p, 6, 9p, and 14, or gain of 1q, should trigger clinical concern for aggressive course and consideration of adjuvant radiotherapy [86,95].
3.2. Somatic Mutations
Multiple large-cohort sequencing studies have established the landscape of somatic mutations in meningiomas, identifying mutually exclusive genomic subgroups that account for over 80% of all cases [9,66,96,97]. Bi-allelic loss of the tumor suppressor NF2 is associated with approximately one-half of sporadic meningiomas and most syndromic cases. These alterations can co-occur with mutations in SMARCB1, which also resides on chromosome 22 near the NF2 gene, with meningiomas typically found near the midline falx [98]. Among non-NF2 cases, activating mutations in the oncogenic PI3K (AKT1, PIK3CA) and Hedgehog (SMO) pathways are found primarily in the skull base [66,96]. Other variants in genes previously unreported in cancer have also described, such as TNF receptor-associated factor 7 (TRAF7), Krupple-like factor 4 (KLF4), and RNA-polymerase II (POLR2A) [66]. Meningiomas with KLF4 or PI3K pathway variants nearly always harbor a concomitant mutation in TRAF7, which occurs in the WD40-repeat region of TRAF7. KLF4 is one of four Yamanaka factors that can induce pluripotency in somatic cells [99], while POLR2A encodes the enzyme responsible for transcription of eukaryotic messenger RNA [9]. The oncogenic mechanisms associated with these variants are under investigation.
The reported mutations stratify into mutually exclusive genomic groups that exhibit correlations with molecular, pathological, and clinical features, including location, histology, and WHO grade [9,100,101]. Non-NF2 meningiomas are found along the skull base [9,101,102], with those harboring Hedgehog activating events located in the ventral midline [66,96,103]. By contrast, NF2-mutated meningiomas are located along the convexity and falx and are often the transitional/fibroblastic subtype [104,105]. Secretory meningiomas, a rare subtype associated with peritumoral edema, uniformly harbor co-mutation of TRAF7/KLF4 [66,106,107]. The association of mutations with clinical features suggests different underlying oncogenic mechanisms or cells of origin.
Somatic variants are currently not evaluated in the routine management of meningiomas, but they may predict clinical course [103,108,109]. Mutations in the promotor region of Telomerase Reverse Transcriptase (TERTp) are the one exception and are associated with malignant progression and an independent criterion for WHO grade 3 diagnosis [110,111,112,113]. Activating mutations in the PI3K pathway are predictive of shorter time to recurrence [108,109], while meningiomas in the hedgehog subgroup exhibit an overall increased rate of recurrence [103,108]. Variants in SMARCB1 and SMARCE1, components of the SWI/SNF chromatin remodeling complex, are enriched in higher-grade meningiomas [56,98,101], and BAP1 alterations are associated with rhabdoid morphology [114], though the predictive ability of these events is not established. Prospective studies that stratify patients according to TERTp and other somatic mutations will be essential before widespread clinical adoption.
3.3. Epigenetic and Transcriptional Classification
The role of epigenetic profiling in clinical practice remains an emerging area, which includes assays measuring DNA methylation, H3K27ac ChIP-seq, and other non-coding alterations. In meningiomas, several studies have reported associations between epigenetic alterations and outcome [95,100,115,116], defining associated molecular pathways and transcriptional changes that could underlie tumor aggressiveness. DNA methylation profiling is increasingly being used to define high-risk molecular groups, though availability of testing remains a barrier at many hospitals and institutions [117,118].
Several methylation classification schemes have been proposed using genome-wide clustering approaches [28,95,100,116,119,120]. Sahm et al. described unsupervised clustering of 497 meningiomas into three major epigenetic groups, with subdivision into six subgroups that correlated with clinical outcome [28]. Maas et al. built on this system and combined the Sahm methylation class with WHO grade and CNVs in 1p, 6q and 14q to develop an integrated score to predict outcome, but this still requires methylation profiling [118]. Furthermore, they proposed a schema to guide molecular workup, which is useful when working with limited resources. By clustering multiple molecular profiling approaches, including methylation, Nassiri et al. proposed four methylation subgroups with distinct clinical outcomes [116]. Choudhury et al. profiled 565 meningiomas with long-term clinical follow-up and identified three methylation classes—Merlin-intact, Immune-enriched, and Hypermitotic—and identified biological mechanisms distinct to each subgroup [95]. The two high-risk groups proposed by Nassiri et al. are subgroups within the Hypermitotic methylation group [121]. The Choudhury methylation groups predicted PFS with greater accuracy than the WHO grade and proposed inhibition of CDK4/6 as a rational treatment for Hypermitotic meningiomas. The results and post hoc analysis of Alliance A071401, which has an arm testing Abemaciclib that has completed accrual, are pending. Challenges to the clinical adoption of DNA methylation profiling include availability and cost, as well as the labor and variability in data analysis and post-processing pipelines.
Transcriptional studies have also identified key pathways associated with meningioma development and progression, as well as insights into features associated with poor clinical outcomes. Several studies have identified gene expression signatures that are predictive of clinical course, including WHO grade, recurrence, and mortality [122,123,124]. Recently, Chen et al. reported a 34-gene panel that independently predicted recurrence with a higher area under the curve (AUC) than the WHO grade or any of the proposed CNV-based grading schemes [125]. Furthermore, this gene panel was the first biomarker to predict radiotherapy responses and was validated across 12 sites and more than 1800 meningiomas. In the RTOG-0539 meningioma samples, the gene expression panel would have refined management, either to give radiation, or to withhold it, in nearly 30% of the patients. As this panel becomes prospectively validated and commercially available (currently a research tool), it should help refine care for patients.
The role of specific genes or gene expression pathways has also been investigated, with the hypothesis that select mediators may drive progression or therapeutic resistance. FOXM1, one of the earliest transcription factors found to be overexpressed in meningiomas [126], has emerged as a candidate driver of aggressive phenotypes. Expression of this gene is elevated in high-grade meningiomas and is associated with decreased PFS and overall survival [115]. Interestingly, the FOXM1 and E2F transcriptional pathways have been found to underlie de novo formation of atypical meningiomas, while progressing meningiomas harbor TERT promotor mutations [100]. The downstream mechanisms associated with FOXM1 activation are yet to be fully elucidated; however, a FOXM1/WNT signaling axis that drives proliferative activity has recently been proposed [115]. Consistent with this hypothesis, previous studies have identified increased WNT pathway expression among higher-grade meningiomas [127].
3.4. PET Imaging and Radiomics
Somatostatin receptor 2A (SSTR2A)-based PET imaging, such as DOTATATE-PET, is emerging as a powerful tool to guide meningioma care and is useful in defining radiotherapy targets as well as differentiating recurrence from scar tissue [128,129,130]. It can highlight intraosseous extension of tumors and can improve radiotherapy control when used to define radiation target volumes [131,132]. There are case reports and on-going trials of DOTATATE coupled to Lutitium-77 as a theranostic treatment strategy [133].
Analyses of MRI imaging data have been extensively reported in a search for prognostic radiographic features that predict grade, invasion, recurrence, and survival [134,135,136,137,138,139]. These studies use various methods of machine learning, including random forests, support vector machines, and convolutional neural networks, to extract non-discrete imaging features related to lesion shape and texture. The most significant features are then used to predict clinical parameters and prognostic features. Clinically significant radiomic features have been reported in the literature to specifically include gray-level co-occurrence and gray-level non-uniformity (cluster prominence) across T1, T2, and fluid-attenuated inversion recovery (FLAIR) sequences and have been predictive in tumor grading [134,138,139,140]. These features have been combined with radiologic parameters, such as cystic components, to have a greater prediction of high-risk atypical meningiomas [139,141]. Additional radiologic features that correspond to higher grade, local recurrence, and poor overall survival include apparent diffusion coefficient (ADC) hypointensity, peritumoral edema, irregular shape, and heterogeneous enhancement [136]. The utility of radiomic approaches, however, is markedly limited by the challenges in generalizing this technology into a clinical workflow.
4. Conclusions
Molecular characterization of meningioma has progressed rapidly, and insights revealed by these studies are being translated into clinical practice. Emerging molecular data are increasingly being incorporated into clinical practice, including high-risk copy number variants, especially the loss of 1p in combination with 22q loss, as well as CDKN2A/B loss or hypermethylation and TERT promoter mutation (Figure 1 and Figure 3). Emerging data support evaluation of these variants to assess recurrence risk and trigger consideration of adjuvant radiotherapy. How DNA methylation profiling and gene expression-based profiling will be incorporated into clinical practice will be determined in the coming years, something that is currently under discussion by the c-IMPACT NOW working group, and offers the potential to refine care for a significant number of patients. Emerging molecular markers are already being used clinically to inform care (Figure 4) but will require prospective validation. By understanding the molecular pathology driving meningioma growth, we anticipate novel targeted treatments can be developed. At present, molecularly stratified clinical trials are beginning to bring precision medicine to meningioma (Alliance A071401). In the future, we anticipate a consensus molecular classification of meningioma, as well as radiomic and blood-based biomarkers to predict tumor grade and inform clinical management non-invasively. The future is exciting, as the bench-to-bedside cycle continues for meningioma, increasing our understanding of the biology of the disease and using those insights to inform management and develop novel therapeutics.
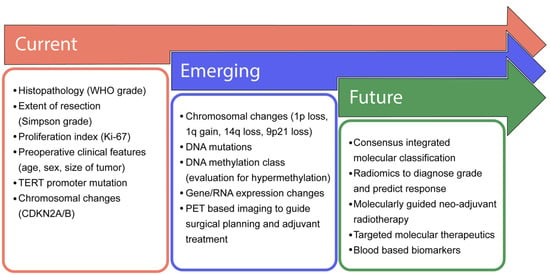
Figure 3.
Progressive advancement in clinical management of meningiomas. In most clinical settings, the current management of meningiomas is determined by histopathological markers and the extent of surgical resection. Emerging diagnostics include the consideration of copy number events and advanced molecular profiling. As our understanding of meningioma biology matures, the emergence of consensus-integrated paradigms will guide the selection of adjuvant therapies and the need for more frequent follow-up and imaging.
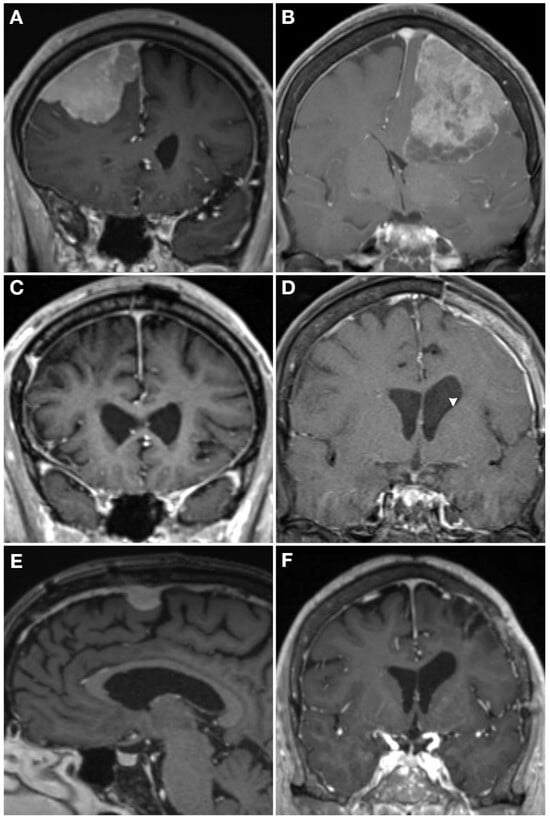
Figure 4.
Case examples demonstrating how molecular profiling can inform care. T1 post-contrast MRI from two WHO grade 2 meningiomas (A,B) who both presented with a seizure and underwent gross total resection (C,D). The patient on the left had a hypermitotic methylation profile and chromosome losses at 1p, 6, 14, haplo-insufficiency of CDKN2A, and 22q, making it a Driver et al. integrated grade 3 tumor, with a high Chen et al. gene expression risk score. He underwent 59.4 Gy adjuvant radiotherapy and had an in-field recurrence (E) at 15 months post-operative. The patient on the right also had a WHO grade 2 meningioma that had an immune-enriched methylation profile and chromosome 8 loss with haplo-insufficiency of chromosome 22, making it a Driver et al. integrated grade 1 tumor [86], and had a low Chen et al. gene expression risk score [126]. She was observed given the favorable molecular profile without adjuvant radiotherapy and had no recurrence at 2 years post-operative (F).
Author Contributions
Conceptualization, S.J.T. and S.T.M.; methodology, S.J.T., M.W.Y. and S.T.M.; writing—original draft preparation, S.J.T., M.W.Y., C.L.K. and N.K.M.; writing—review and editing, all authors; visualization, M.W.Y. and S.T.M.; supervision, S.T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This was an unfunded study. S.T.M. and C.M.H. are supported by the Northwestern Medicine Malnati Brain Tumor Institute of the Lurie Cancer Center.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro-Oncology 2023, 25 (Suppl. S4), iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, Y.S. Molecular characteristics of meningiomas. J. Pathol. Transl. Med. 2020, 54, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Cushing, H.; Eisenhardt, L. Meningiomas, Their Classification, Regional Behaviour, Life History, and Surgical End Results; Charles C Thomas: Springfield, IL, USA, 1938. [Google Scholar]
- Cushing, H. The meningiomas (dural endotheliomas): Their source, and favoured seats of origin1. Brain 1922, 45, 282–316. [Google Scholar] [CrossRef]
- Bailey, P. The origin and nature of meningeal tumors. Am. J. Cancer 1931, 15, 15–54. [Google Scholar]
- Soyuer, S.; Chang, E.L.; Selek, U.; Shi, W.; Maor, M.; Franco, D.M. Radiotherapy after surgery for benign cerebral meningioma. Radiother. Oncol. 2004, 71, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, S.M.; Ghadjar, P.; Grün, A.; Badakhshi, H.; Zschaeck, S.; Senger, C.; Acker, G.; Misch, M.; Budach, V.; David, K. Adjuvant radiotherapy improves progression-free survival in intracranial atypical meningioma. Radiat. Oncol. 2019, 14, 160. [Google Scholar] [CrossRef] [PubMed]
- Boskos, C.; Feuvret, L.; Noel, G.; Habrand, J.-L.; Pommier, P.; Alapetite, C.; Mammar, H.; Ferrand, R.; Boisserie, G.; Jean-Jacques, M. Combined proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Harmanci, A.S.; Bai, H.; Youngblood, M.W.; Lee, T.I.; Baranoski, J.F.; Ercan-Sencicek, A.G.; Abraham, B.J.; Weintraub, A.S.; Hnisz, D.; et al. Recurrent somatic mutations in POLR2A define a distinct subset of meningiomas. Nat. Genet. 2016, 48, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.-F.; Xiu, Y.-J.; Wang, X.; Li, M.; Yang, Y.; Mao, Q.; Liu, Y.-H. The potential risk factors for atypical and anaplastic meningiomas: Clinical series of 1,239 cases. Int. J. Clin. Exp. Med. 2014, 7, 5696–5700. [Google Scholar]
- Kano, H.; Takahashi, J.A.; Katsuki, T.; Araki, N.; Oya, N.; Hiraoka, M.; Nobuo, H. Stereotactic radiosurgery for atypical and anaplastic meningiomas. J. Neurooncol. 2007, 84, 41–47. [Google Scholar] [CrossRef]
- Rohringer, M.; Sutherland, G.R.; Louw, D.F.; Anders, A.F.S. Incidence and clinicopathological features of meningioma. J. Neurosurg. 1989, 71, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.T.; Young, J.S.; Chae, R.; Aghi, M.K.; Theodosopoulos, P.V.; McDermott, M.W. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg. Focus 2018, 44, E4. [Google Scholar] [CrossRef]
- Domingues, P.H.; Sousa, P.; Otero, Á.; Gonçalves, J.M.; Ruiz, L.; de Oliveira, C.; Lopes, M.C.; Orfao, A.; Maria, D.T. Proposal for a new risk stratification classification for meningioma based on patient age, WHO tumor grade, size, localization, and karyotype. Neuro-Oncology 2014, 16, 735–747. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Jill, S.B.-S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21 (Suppl. S5), v1–v100. [Google Scholar] [CrossRef]
- Gennatas, E.D.; Wu, A.; Braunstein, S.E.; Morin, O.; Chen, W.C.; Magill, S.T.; Gopinath, C.; Villaneueva-Meyer, J.E.; Perry, A.; McDermott, M.W.; et al. Preoperative and postoperative prediction of long-term meningioma outcomes. PLoS ONE 2018, 13, e0204161. [Google Scholar] [CrossRef]
- Ugga, L.; Perillo, T.; Cuocolo, R.; Stanzione, A.; Romeo, V.; Green, R.; Cantoni, V.; Arturo, B. Meningioma MRI radiomics and machine learning: Systematic review, quality score assessment, and meta-analysis. Neuroradiology 2021, 63, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef]
- Jääskeläinen, J. Seemingly complete removal of histologically benign intracranial meningioma: Late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg. Neurol. 1986, 26, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Stafford, S.L.; Perry, A.; Suman, V.J.; Meyer, F.B.; Scheithauer, B.W.; Lohse, C.M.; Edward, G.S. Primarily resected meningiomas: Outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin. Proc. 1998, 73, 936–942. [Google Scholar] [CrossRef]
- Kinjo, T.; Al-Mefty, O.; Kanaan, I. Grade zero removal of supratentorial convexity meningiomas. Neurosurgery 1993, 33, 394–399; discussion 399. [Google Scholar]
- Schwartz, T.H.; McDermott, M.W. The Simpson grade: Abandon the scale but preserve the message. J. Neurosurg. 2020, 135, 488–495. [Google Scholar] [CrossRef]
- Mathiesen, T.; Lindquist, C.; Kihlström, L.; Bengt, K. Recurrence of cranial base meningiomas. Neurosurgery 1996, 39, 2–9. [Google Scholar] [CrossRef]
- Nakasu, S.; Fukami, T.; Jito, J.; Kazuhiko, N. Recurrence and regrowth of benign meningiomas. Brain Tumor Pathol. 2009, 26, 69–72. [Google Scholar] [CrossRef]
- Naumann, M.; Meixensberger, J. Factors influencing meningioma recurrence rate. Acta Neurochir. 1990, 107, 108–111. [Google Scholar] [CrossRef]
- Oya, S.; Kawai, K.; Nakatomi, H.; Nobuhito, S. Significance of Simpson grading system in modern meningioma surgery: Integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. J. Neurosurg. 2012, 117, 121–128. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Kane, A.J.; Shangari, G.; Rutkowski, M.J.; McDermott, M.W.; Berger, M.S.; Andrew, T.P. The relevance of Simpson Grade I and II resection in modern neurosurgical treatment of World Health Organization Grade I meningiomas. J. Neurosurg. 2010, 113, 1029–1035. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017, 18, 682–694. [Google Scholar] [CrossRef]
- Zulch, K.J. Histological Typing of Tumours of the Central Nervous System; World Health Organization. Geneva, Switzerland; 1979.
- Zulch, K. Brain Tumours. Their Biology and Pathology, 2nd ed.; Heinemann Medical: London, UK, 1965. [Google Scholar]
- Kleihues, P.; Burger, P.C.; Scheithauer, B.W. The new WHO classification of brain tumours. Brain Pathol. 1993, 3, 255–268. [Google Scholar] [CrossRef]
- Rogers, C.L.; Perry, A.; Pugh, S.; Vogelbaum, M.A.; Brachman, D.; McMillan, W.; Jenrette, J.; Barani, I.; Shrieve, D.; Sloan, A.; et al. Pathology concordance levels for meningioma classification and grading in NRG Oncology RTOG Trial 0539. Neuro-Oncology 2016, 18, 565–574. [Google Scholar] [CrossRef]
- Kleihues, P.; Louis, D.N.; Scheithauer, B.W.; Rorke, L.B.; Reifenberger, G.; Burger, P.C.; Webster, K.C. The WHO Classification of Tumors of the Nervous System. J. Neuropathol. Exp. Neurol. 2002, 61, 215–225. [Google Scholar] [CrossRef]
- Perry, A.; Stafford, S.L.; Scheithauer, B.W.; Suman, V.J.; Lohse, C.M. Meningioma grading: An analysis of histologic parameters. Am. J. Surg. Pathol. 1997, 21, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Scheithauer, B.W.; Stafford, S.L.; Lohse, C.M.; Wollan, P.C. “Malignancy” in meningiomas: A clinicopathologic study of 116 patients, with grading implications. Cancer 1999, 85, 2046–2056. [Google Scholar] [PubMed]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Pettersson-Segerlind, J.; Orrego, A.; Lönn, S.; Mathiesen, T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurg. 2011, 76, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Gousias, K.; Schramm, J.; Simon, M. The Simpson grading revisited: Aggressive surgery and its place in modern meningioma management. J. Neurosurg. 2016, 125, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Zhang, P.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Brachman, D.; Jenrette, J.M.; et al. Intermediate-risk meningioma: Initial outcomes from NRG Oncology RTOG 0539. J. Neurosurg. 2018, 129, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.J.; Jenkinson, M.D.; Brodbelt, A.R.; Mills, S.J.; Chavredakis, E. WHO grade 1 meningioma recurrence: Are location and Simpson grade still relevant? Clin. Neurol. Neurosurg. 2016, 141, 117–121. [Google Scholar] [CrossRef]
- Maíllo, A.; Orfao, A.; Sayagués, J.M.; Díaz, P.; Gómez-Moreta, J.A.; Caballero, M.; Santamarta, D.; Santos-Briz, A.; Morales, F.; Tabernero, M.D. New classification scheme for the prognostic stratification of meningioma on the basis of chromosome 14 abnormalities, patient age, and tumor histopathology. J. Clin. Oncol. 2003, 21, 3285–3295. [Google Scholar] [CrossRef]
- Chen, W.C.; Magill, S.T.; Wu, A.; Vasudevan, H.N.; Morin, O.; Aghi, M.K.; Theodosopoulos, P.V.; Perry, A.; McDermott, M.W.; Sneed, P.K.; et al. Histopathological features predictive of local control of atypical meningioma after surgery and adjuvant radiotherapy. J. Neurosurg. JNS 2019, 130, 443–450. [Google Scholar] [CrossRef]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef]
- Baumgarten, P.; Gessler, F.; Schittenhelm, J.; Skardelly, M.; Tews, D.S.; Senft, C.; Dunst, M.; Imoehl, L.; Plate, K.H.; Wagner, M.; et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol. 2016, 132, 479–481. [Google Scholar] [CrossRef]
- Pizem, J.; Velnar, T.; Prestor, B.; Mlakar, J.; Popovic, M. Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin. Neuropathol. 2014, 33, 354–363. [Google Scholar]
- Spille, D.C.; Heß, K.; Sauerland, C.; Sanai, N.; Stummer, W.; Paulus, W.; Brokinkel, B. Brain Invasion in Meningiomas: Incidence and Correlations with Clinical Variables and Prognosis. World Neurosurg. 2016, 93, 346–354. [Google Scholar] [CrossRef]
- Roser, F.; Samii, M.; Ostertag, H.; Bellinzona, M. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir. 2004, 146, 37–44; discussion 44. [Google Scholar] [CrossRef]
- Abramovich, C.M.; Prayson, R.A. Histopathologic features and MIB-1 labeling indices in recurrent and nonrecurrent meningiomas. Arch. Pathol. Lab Med. 1999, 123, 793–800. [Google Scholar] [CrossRef]
- Schiffer, D.; Ghimenti, C.; Fiano, V. Absence of histological signs of tumor progression in recurrences of completely resected meningiomas. J. Neurooncol. 2005, 73, 125–130. [Google Scholar] [CrossRef]
- Marciscano, A.E.; Stemmer-Rachamimov, A.O.; Niemierko, A.; Larvie, M.; Curry, W.T.; Barker, F.G.; Martuza, R.L.; McGuone, D.; Oh, K.S.; Loeffler, J.S.; et al. Benign meningiomas (WHO Grade I) with atypical histological features: Correlation of histopathological features with clinical outcomes. J. Neurosurg. 2016, 124, 106–114. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Kanungo, I.; Sudhir, S.; Chen, J.-S.; Raleigh, D.R.; Magill, S.T.; McDermott, M.W.; Aghi, M.K. WHO Grade I Meningioma Recurrence: Identifying High Risk Patients Using Histopathological Features and the MIB-1 Index. Front. Oncol. 2020, 10, 1522. [Google Scholar] [CrossRef]
- Behling, F.; Fodi, C.; Wang, S.; Hempel, J.-M.; Hoffmann, E.; Tabatabai, G.; Honegger, J.; Tatagiba, M.; Schittenhelm, J.; Skardelly, M. Increased proliferation is associated with CNS invasion in meningiomas. J. Neurooncol. 2021, 155, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.M.; Vetsa, S.; Nadar, A.; Vasandani, S.; Youngblood, M.W.; Gorelick, E.; Jin, L.; Marianayagam, N.; Erson-Omay, E.Z.; Günel, M.; et al. The integrated multiomic diagnosis of sporadic meningiomas: A review of its clinical implications. J. Neurooncol. 2022, 156, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Galani, V.; Lampri, E.; Varouktsi, A.; Alexiou, G.; Mitselou, A.; Kyritsis, A.P. Genetic and epigenetic alterations in meningiomas. Clin. Neurol. Neurosurg. 2017, 158, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Al Saadi, A.; Latimer, F.; Madercic, M.; Robbins, T. Cytogenetic studies of human brain tumors and their clinical significance. II. Meningioma. Cancer Genet. Cytogenet. 1987, 26, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Zang, K.D. Cytological and cytogenetical studies on human meningioma. Cancer Genet. Cytogenet. 1982, 6, 249–274. [Google Scholar] [CrossRef] [PubMed]
- Zankl, H.; Zang, K.D. Cytological and cytogenetical studies on brain tumors. 4. Identification of the missing G chromosome in human meningiomas as no. 22 by fluorescence technique. Humangenetik 1972, 14, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.; Levan, G.; Mitelman, F. Identification by fluorescence of the G chromosome lost in human meningomas. Hereditas 1972, 71, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ketter, R.; Kim, Y.-J.; Storck, S.; Rahnenführer, J.; Romeike, B.F.M.; Steudel, W.-I.; Zang, K.D.; Henn, W. Hyperdiploidy defines a distinct cytogenetic entity of meningiomas. J. Neurooncol. 2007, 83, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ruttledge, M.H.; Sarrazin, J.; Rangaratnam, S.; Phelan, C.M.; Twist, E.; Merel, P.; Delattre, O.; Thomas, G.; Nordenskjöld, M.; Collins, V.P.; et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat. Genet. 1994, 6, 180–184. [Google Scholar] [CrossRef]
- Papi, L.; De Vitis, L.R.; Vitelli, F.; Montali, E.; Bigozzi, U.; Ammannati, F.; Mennonna, P. Somatic mutations in the neurofibromatosis type 2 gene in sporadic meningiomas. Hum. Genet. 1995, 95, 347–351. [Google Scholar] [CrossRef]
- Meese, E.; Blin, N.; Zang, K.D. Loss of heterozygosity and the origin of meningioma. Hum. Genet. 1987, 77, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, R.; Kraus, J.A.; Lenartz, D.; Menon, A.G.; Schramm, J.; Louis, D.N.; Ramesh, V.; Gusella, J.F.; Wiestler, O.D.; Von Deimling, A. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am. J. Pathol. 1995, 146, 827–832. [Google Scholar] [PubMed]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Ozduman, K.; Avsar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Barski, D.; Wolter, M.; Reifenberger, G.; Riemenschneider, M.J. Hypermethylation and transcriptional downregulation of the TIMP3 gene is associated with allelic loss on 22q12.3 and malignancy in meningiomas. Brain Pathol. 2010, 20, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Linsler, S.; Kraemer, D.; Driess, C.; Oertel, J.; Kammers, K.; Rahnenführer, J.; Ketter, R.; Urbschat, S. Molecular biological determinations of meningioma progression and recurrence. PLoS ONE 2014, 9, e94987. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Von Deimling, A.; Larson, J.J.; Wellenreuther, R.; Kaskel, P.; Waha, A.; Warnick, R.E.; Tew, J.M.; Menon, A.G. Allelic losses on chromosomes 14, 10, and 1 in atypical and malignant meningiomas: A genetic model of meningioma progression. Cancer Res. 1995, 55, 4696–4701. [Google Scholar]
- Ketter, R.; Henn, W.; Niedermayer, I.; Steilen-Gimbel, H.; Konig, J.; Zang, K.D.; Steudel, W.I. Predictive value of progression-associated chromosomal aberrations for the prognosis of meningiomas: A retrospective study of 198 cases. J. Neurosurg. 2001, 95, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.M.; Bruder, C.; Nordenskjold, M.; Dumanski, J.P. 1p and 3p deletions in meningiomas without detectable aberrations of chromosome 22 identified by comparative genomic hybridization. Genes Chromosomes Cancer 1997, 20, 419–424. [Google Scholar] [CrossRef]
- Gabeau-Lacet, D.; Engler, D.; Gupta, S.; Scangas, G.A.; Betensky, R.A.; Barker, F.G.; Loeffler, J.S.; Louis, D.N.; Mohapatra, G. Genomic profiling of atypical meningiomas associates gain of 1q with poor clinical outcome. J. Neuropathol. Exp. Neurol. 2009, 68, 1155–1165. [Google Scholar] [CrossRef]
- Rempel, S.A.; Schwechheimer, K.; Davis, R.L.; Cavenee, W.K.; Rosenblum, M.L. Loss of heterozygosity for loci on chromosome 10 is associated with morphologically malignant meningioma progression. Cancer Res. 1993, 53, 2386–2392. [Google Scholar]
- Aizer, A.A.; Abedalthagafi, M.; Bi, W.L.; Horvath, M.C.; Arvold, N.D.; Al-Mefty, O.; Lee, E.Q.; Nayak, L.; Rinne, M.L.; Norden, A.D.; et al. A prognostic cytogenetic scoring system to guide the adjuvant management of patients with atypical meningioma. Neuro-Oncology 2016, 18, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Yew, A.; Trang, A.; Nagasawa, D.T.; Spasic, M.; Choy, W.; Garcia, H.M.; Yang, I. Chromosomal alterations, prognostic factors, and targeted molecular therapies for malignant meningiomas. J. Clin. Neurosci. 2013, 20, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Al-Mefty, O.; Kadri, P.A.S.; Pravdenkova, S.; Sawyer, J.R.; Stangeby, C.; Husain, M. Malignant progression in meningioma: Documentation of a series and analysis of cytogenetic findings. J. Neurosurg. 2004, 101, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, W.K.; Coons, S.W.; Aboul-Enein, F.; Hendricks, W.P.; Scheck, A.C.; Preul, M.C. Implicating chromosomal aberrations with meningioma growth and recurrence: Results from FISH and MIB-I analysis of grades I and II meningioma tissue. J. Neurooncol. 2008, 87, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Maillo, A.; Orfao, A.; Espinosa, A.B.; Sayagues, J.M.; Merino, M.; Sousa, P.; Lara, M.; Tabernero, M.D. Early recurrences in histologically benign/grade I meningiomas are associated with large tumors and coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone. Neuro-Oncology 2007, 9, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Barbera, S.; Miguel, T.S.; Gil-Benso, R.; Muñoz-Hidalgo, L.; Roldan, P.; Gonzalez-Darder, J.; Cerda-Nicolas, M.; Lopez-Gines, C. Genetic changes with prognostic value in histologically benign meningiomas. Clin. Neuropathol. 2013, 32, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.X.; Banerjee, R.; Scheithauer, B.W.; Lohse, C.M.; Kleinschmidt-Demasters, B.K.; Perry, A. Chromosome 1p and 14q FISH analysis in clinicopathologic subsets of meningioma: Diagnostic and prognostic implications. J. Neuropathol. Exp. Neurol. 2001, 60, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Domingues, P.; González-Tablas, M.; Otero, Á.; Pascual, D.; Ruiz, L.; Miranda, D.; Sousa, P.; Gonçalves, J.M.; Lopes, M.C.; Orfao, A.; et al. Genetic/molecular alterations of meningiomas and the signaling pathways targeted. Oncotarget 2015, 6, 10671–10688. [Google Scholar] [CrossRef] [PubMed]
- Mawrin, C.; Perry, A. Pathological classification and molecular genetics of meningiomas. J. Neurooncol. 2010, 99, 379–391. [Google Scholar] [CrossRef]
- Tabernero, M.D.; Maíllo, A.; Nieto, A.B.; Diez-Tascón, C.; Lara, M.; Sousa, P.; Otero, A.; Castrillo, A.; Patino-Alonso, M.d.C.; Espinosa, A.; et al. Delineation of commonly deleted chromosomal regions in meningiomas by high-density single nucleotide polymorphism genotyping arrays. Genes Chromosomes Cancer 2012, 51, 606–617. [Google Scholar] [CrossRef]
- Arslantas, A.; Artan, S.; Oner, U.; Durmaz, R.; Müslümanoğlu, H.; Atasoy, M.A.; Başaran, N.; Tel, E. Comparative genomic hybridization analysis of genomic alterations in benign, atypical and anaplastic meningiomas. Acta Neurol. Belg. 2002, 102, 53–62. [Google Scholar] [PubMed]
- Buckley, P.G.; Jarbo, C.; Menzel, U.; Mathiesen, T.; Scott, C.; Gregory, S.G.; Langford, C.F.; Dumanski, J.P. Comprehensive DNA copy number profiling of meningioma using a chromosome 1 tiling path microarray identifies novel candidate tumor suppressor loci. Cancer Res. 2005, 65, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.; Hoffman, S.E.; Tavakol, S.; Woodward, E.; Maury, E.A.; Bhave, V.; Greenwald, N.F.; Nassiri, F.; Aldape, K.; Zadeh, G.; et al. A molecularly integrated grade for meningioma. Neuro-Oncology 2022, 24, 796–808. [Google Scholar] [CrossRef]
- Choy, W.; Kim, W.; Nagasawa, D.; Stramotas, S.; Yew, A.; Gopen, Q.; Parsa, A.T.; Yang, I. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg. Focus 2011, 30, E6. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Banerjee, R.; Lohse, C.M.; Kleinschmidt-DeMasters, B.K.; Scheithauer, B.W. A role for chromosome 9p21 deletions in the malignant progression of meningiomas and the prognosis of anaplastic meningiomas. Brain Pathol. 2002, 12, 183–190. [Google Scholar] [PubMed]
- Weber, R.G.; Boström, J.; Wolter, M.; Baudis, M.; Collins, V.P.; Reifenberger, G.; Lichter, P. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: Toward a genetic model of meningioma progression. Proc. Natl. Acad. Sci. USA 1997, 94, 14719–14724. [Google Scholar] [CrossRef] [PubMed]
- Boström, J.; Meyer-Puttlitz, B.; Wolter, M.; Blaschke, B.; Weber, R.G.; Lichter, P.; Ichimura, K.; Collins, V.P.; Reifenberger, G. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am. J. Pathol. 2001, 159, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.B.; English, C.W.; Chen, W.C.; Athukuri, P.; Bayley, J.C.; Brandt, V.L.; Shetty, A.; Hadley, C.C.; Choudhury, A.; Lu, H.-C.; et al. Even heterozygous loss of CDKN2A/B greatly accelerates recurrence in aggressive meningioma. Acta Neuropathol. 2023, 145, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Ketter, R.; Urbschat, S.; Henn, W.; Feiden, W.; Beerenwinkel, N.; Lengauer, T.; Steudel, W.; Zang, K.D.; Rahnenfuhrer, J. Application of oncogenetic trees mixtures as a biostatistical model of the clonal cytogenetic evolution of meningiomas. Int. J. Cancer 2007, 121, 1473–1480. [Google Scholar] [CrossRef]
- Urbschat, S.; Rahnenfuhrer, J.; Henn, W.; Feiden, W.; Wemmert, S.; Linsler, S.; Zang, K.D.; Oertel, J.; Ketter, R. Clonal cytogenetic progression within intratumorally heterogeneous meningiomas predicts tumor recurrence. Int. J. Oncol. 2011, 39, 1601–1608. [Google Scholar]
- da Silva, C.E.; de Freitas, P.E.P. Classification of Meningiomas Based on Their Surgical Removal, World Health Organization Grade, and Cytogenetic Profile: A Treatment Algorithm. World Neurosurg. 2017, 105, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Magill, S.T.; Eaton, C.D.; Prager, B.C.; Chen, W.C.; Cady, M.A.; Seo, K.; Lucas, C.-H.G.; Casey-Clyde, T.J.; Vasudevan, H.N.; et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat. Genet. 2022, 54, 649–659. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescandolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic Sequencing of Meningiomas Identifies Oncogenic SMO and AKT1 Mutations. Nat. Genet. 2013, 45, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Nassiri, F.; Mawrin, C.; Zadeh, G. Genomic Landscape of Meningiomas. Adv. Exp. Med. Biol. 2023, 1416, 137–158. [Google Scholar] [PubMed]
- Munckhof, P.v.D.; Christiaans, I.; Kenter, S.B.; Baas, F.; Hulsebos, T.J.M. Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri. Neurogenetics 2012, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Harmancı, A.S.; Youngblood, M.W.; Clark, V.E.; Coşkun, S.; Henegariu, O.; Duran, D.; Erson-Omay, E.Z.; Kaulen, L.D.; Lee, T.I.; Abraham, B.J.; et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat. Commun. 2018, 9, 16215. [Google Scholar] [CrossRef]
- Youngblood, M.W.; Duran, D.; Montejo, J.D.; Li, C.; Omay, S.B.; Ozduman, K.; Sheth, A.H.; Zhao, A.Y.; Tyrtova, E.; Miyahishima, D.F.; et al. Correlations between genomic subgroup and clinical features in a cohort of more than 3000 meningiomas. J. Neurosurg. 2019, 133, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Abedalthagafi, M.; Bi, W.L.; Aizer, A.A.; Merrill, P.H.; Brewster, R.; Agarwalla, P.K.; Listewnik, M.L.; Dias-Santagata, D.; Thorner, A.R.; Hummelen, P.V.; et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro-Oncology 2016, 18, 649–655. [Google Scholar] [CrossRef]
- Boetto, J.; Bielle, F.; Sanson, M.; Peyre, M.; Kalamarides, M. SMO mutation status defines a distinct and frequent molecular subgroup in olfactory groove meningiomas. Neuro-Oncology 2017, 19, 345–351. [Google Scholar]
- Kros, J.; de Greve, K.; van Tilborg, A.; Hop, W.; Pieterman, H.; Avezaat, C.; Deprez, R.L.D.; Zwarthoff, E. NF2 status of meningiomas is associated with tumour localization and histology. J. Pathol. 2001, 194, 367–372. [Google Scholar] [CrossRef]
- Tabor, J.K.; O’Brien, J.; Vasandani, S.; Vetsa, S.; Lei, H.; Jalal, M.I.; Marianayagam, N.J.; Jin, L.; Chavez, M.M.; Haynes, J.; et al. Clinical and genomic differences in supratentorial versus infratentorial NF2 mutant meningiomas. J. Neurosurg. 2023, 139, 1648–1656. [Google Scholar] [CrossRef]
- Yuzawa, S.; Nishihara, H.; Tanaka, S. Genetic landscape of meningioma. Brain Tumor Pathol. 2016, 33, 237–247. [Google Scholar] [CrossRef]
- Reuss, D.E.; Piro, R.M.; Jones, D.T.; Simon, M.; Ketter, R.; Kool, M.; Becker, A.; Sahm, F.; Pusch, S.; Meyer, J.; et al. Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations. Acta Neuropathol. 2013, 125, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, M.W.; Miyagishima, D.F.; Jin, L.; Gupte, T.; Li, C.; Duran, D.; Montejo, J.D.; Zhao, A.; Sheth, A.; Tyrtova, E.; et al. Associations of Meningioma Molecular Subgroup and Tumor Recurrence. Neuro-Oncology 2021, 23, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Yesilöz, Ü.; Kirches, E.; Hartmann, C.; Scholz, J.; Kropf, S.; Sahm, F.; Nakamura, M.; Mawrin, C. Frequent AKT1 E17K mutations in skull base meningiomas are associated with mTOR and ERK1/2 activation and reduced time to tumor recurrence. Neuro-Oncology 2017, 19, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. J. Natl. Cancer Inst. 2016, 108, djv377. [Google Scholar] [CrossRef]
- Abedalthagafi, M.S.; Bi, W.L.; Merrill, P.H.; Gibson, W.J.; Rose, M.F.; Du, Z.; Francis, J.M.; Du, R.; Dunn, I.F.; Ligon, A.H.; et al. ARID1A and TERT promoter mutations in dedifferentiated meningioma. Cancer Genet. 2015, 208, 345–350. [Google Scholar] [CrossRef]
- Goutagny, S.; Nault, J.C.; Mallet, M.; Henin, D.; Rossi, J.Z.; Kalamarides, M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014, 24, 184–189. [Google Scholar] [CrossRef]
- Lu, V.M.; Goyal, A.; Lee, A.; Jentoft, M.; Quinones-Hinojosa, A.; Chaichana, K.L. The prognostic significance of TERT promoter mutations in meningioma: A systematic review and meta-analysis. J. Neurooncol. 2019, 142, 1–10. [Google Scholar] [CrossRef]
- Shankar, G.M.; Abedalthagafi, M.; Vaubel, R.A.; Merrill, P.H.; Nayyar, N.; Gill, C.M.; Brewster, R.; Bi, W.L.; Agarwalla, P.K.; Thorner, A.R.; et al. Germline and somatic BAP1 mutations in high-grade rhabdoid meningiomas. Neuro-Oncology 2017, 19, 535–545. [Google Scholar] [CrossRef]
- Vasudevan, H.N.; Braunstein, S.E.; Phillips, J.J.; Pekmezci, M.; Tomlin, B.A.; Wu, A.; Reis, G.F.; Magill, S.T.; Zhang, J.; Feng, F.Y.; et al. Comprehensive Molecular Profiling Identifies FOXM1 as a Key Transcription Factor for Meningioma Proliferation. Cell Rep. 2018, 22, 3672–3683. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.C.; Hadley, C.C.; Harmanci, A.O.; Harmanci, A.S.; Klisch, T.J.; Patel, A.J. Multiple approaches converge on three biological subtypes of meningioma and extract new insights from published studies. Sci. Adv. 2022, 8, eabm6247. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Stichel, D.; Hielscher, T.; Sievers, P.; Berghoff, A.S.; Schrimpf, D.; Sill, M.; Euskirchen, P.; Blume, C.; Patel, A.; et al. Integrated Molecular-Morphologic Meningioma Classification: A Multicenter Retrospective Analysis, Retrospectively and Prospectively Validated. J. Clin. Oncol. 2021, 39, 3839–3852. [Google Scholar] [CrossRef] [PubMed]
- Olar, A.; Wani, K.M.; Wilson, C.D.; Zadeh, G.; DeMonte, F.; Jones, D.T.W.; Pfister, S.M.; Sulman, E.P.; Aldape, K.D. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017, 133, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Nassiri, F.; Mamatjan, Y.; Suppiah, S.; Badhiwala, J.H.; Mansouri, S.; Karimi, S.; Saarela, O.; Gepfner-Tuma, I.; Schittenhelm, J.; Ng, H.-K.; et al. DNA methylation profiling to predict recurrence risk in meningioma: Development and validation of a nomogram to optimize clinical management. Neuro-Oncology 2019, 21, 901–910. [Google Scholar] [CrossRef]
- Choudhury, A.; Chen, W.; Lucas, C.-H.; Magill, S.; Raleigh, D. Hypermitotic meningiomas harbor DNA methylation subgroups with distinct biological and clinical features. Neuro-Oncology 2023, 25, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Olar, A.; Goodman, L.D.; Wani, K.M.; Boehling, N.S.; Sharma, D.S.; Mody, R.R.; Gumin, J.; Claus, E.B.; Lang, F.F.; Cloughesy, T.F.; et al. A gene expression signature predicts recurrence-free survival in meningioma. Oncotarget 2018, 9, 16087–16098. [Google Scholar] [CrossRef]
- Stuart, J.E.; Lusis, E.A.; Scheck, A.C.; Coons, S.W.; Lal, A.; Perry, A.; Gutmann, D.H. Identification of gene markers associated with aggressive meningioma by filtering across multiple sets of gene expression arrays. J. Neuropathol. Exp. Neurol. 2011, 70, 1–12. [Google Scholar] [CrossRef]
- Chen, W.C.; Vasudevan, H.N.; Choudhury, A.; Pekmezci, M.; Lucas, C.-H.G.; Phillips, J.; Magill, S.T.; Susko, M.S.; Braunstein, S.E.; Bush, N.A.O.; et al. A Prognostic Gene-Expression Signature and Risk Score for Meningioma Recurrence After Resection. Neurosurgery 2020, 88, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Choudhury, A.; Youngblood, M.W.; Polley, M.-Y.C.; Lucas, C.-H.G.; Mirchia, K.; Maas, S.L.N.; Suwala, A.K.; Won, M.; Bayley, J.C.; et al. Targeted gene expression profiling predicts meningioma outcomes and radiotherapy responses. Nat. Med. 2023, 29, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Laurendeau, I.; Ferrer, M.; Garrido, D.; D’haene, N.; Ciavarelli, P.; Basso, A.; Vidaud, M.; Bieche, I.; Salmon, I.; Szijan, I. Gene expression profiling of the hedgehog signaling pathway in human meningiomas. Mol. Med. 2010, 16, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, G.; Roerig, P.; Kokocinski, F.; Neben, K.; Hahn, M.; Reifenberger, G.; Lichter, P. Microarray-based gene expression profiling of benign, atypical and anaplastic meningiomas identifies novel genes associated with meningioma progression. Int. J. Cancer 2005, 114, 249–256. [Google Scholar] [CrossRef]
- Rachinger, W.; Stoecklein, V.M.; Terpolilli, N.A.; Haug, A.R.; Ertl, L.; Pöschl, J.; Schüller, U.; Schichor, C.; Thon, N.; Tonn, J.-C. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J. Nucl. Med. 2015, 56, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.N.; Perlow, H.K.; Bovi, J.; Braunstein, S.E.; Ivanidze, J.; Kalapurakal, J.A.; Kleefisch, C.; Knisely, J.P.; Mehta, M.P.; Prevedello, D.M.; et al. (68)Ga-DOTATATE PET: The Future of Meningioma Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Ivanidze, J.; Roytman, M.; Lin, E.; Magge, R.S.; Pisapia, D.J.; Liechty, B.; Karakatsanis, N.; Ramakrishna, R.; Knisely, J.; Schwartz, T.H.; et al. Gallium-68 DOTATATE PET in the Evaluation of Intracranial Meningiomas. J. Neuroimaging 2019, 29, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.G.; Jungblut, L.M.; Kazmierczak, P.M.; Vettermann, F.J.; Bollenbacher, A.; Tonn, J.C.; Schichor, C.; Rominger, A.; Albert, N.L. Improved Detection of Transosseous Meningiomas Using (68)Ga-DOTATATE PET/CT Compared with Contrast-Enhanced MRI. J. Nucl. Med. 2017, 58, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Hadi, I.; Biczok, A.; Terpolilli, N.; Thorsteinsdottir, J.; Forbrig, R.; Albert, N.L.; Yanchovski, P.; Zollner, B.; Bodensohn, R.; Corradini, S.; et al. Multimodal therapy of cavernous sinus meningioma: Impact of surgery and (68)Ga-DOTATATE PET-guided radiation therapy on tumor control and functional outcome. Neurooncol. Adv. 2021, 3, vdab114. [Google Scholar] [CrossRef]
- Zahid, A.; Johnson, D.R.; Kizilbash, S.H. Efficacy of (177)Lu-Dotatate Therapy in the Treatment of Recurrent Meningioma. Mayo Clin. Proc. Innov. Qual. Outcomes 2021, 5, 236–240. [Google Scholar] [CrossRef]
- Ko, C.-C.; Zhang, Y.; Chen, J.-H.; Chang, K.-T.; Chen, T.-Y.; Lim, S.-W.; Wu, T.-C.; Su, M.-Y. Pre-operative MRI Radiomics for the Prediction of Progression and Recurrence in Meningiomas. Front. Neurol. 2021, 12, 636235. [Google Scholar] [CrossRef] [PubMed]
- Joo, L.; Park, J.E.; Park, S.Y.; Nam, S.J.; Kim, Y.-H.; Kim, J.H.; Kim, H.S. Extensive peritumoral edema and brain-to-tumor interface MRI features enable prediction of brain invasion in meningioma: Development and validation. Neuro-Oncology 2021, 23, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Morin, O.; Chen, W.C.; Nassiri, F.; Susko, M.; Magill, S.T.; Vasudevan, H.N.; Wu, A.; Vallières, M.; Gennatas, E.D.; Valdes, G.; et al. Integrated models incorporating radiologic and radiomic features predict meningioma grade, local failure, and overall survival. Neurooncol. Adv. 2019, 1, vdz011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, J.; Han, T.; Zhao, Z.; Cao, Y.; Zhang, G.; Zhou, J. Radiomic features of magnetic resonance images as novel preoperative predictive factors of bone invasion in meningiomas. Eur. J. Radiol. 2020, 132, 109287. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhou, X.; Duan, C.; Zhao, J.; Sui, Q.; Liu, X.; Zhang, X. Differentiation Researches on the Meningioma Subtypes by Radiomics from Contrast-Enhanced Magnetic Resonance Imaging: A Preliminary Study. World Neurosurg. 2019, 126, e646–e652. [Google Scholar] [CrossRef] [PubMed]
- Kalasauskas, D.; Kronfeld, A.; Renovanz, M.; Kurz, E.; Leukel, P.; Krenzlin, H.; Brockmann, M.A.; Sommer, C.J.; Ringel, F.; Keric, N. Identification of High-Risk Atypical Meningiomas According to Semantic and Radiomic Features. Cancers 2020, 12, 2942. [Google Scholar] [CrossRef] [PubMed]
- Laukamp, K.R.; Shakirin, G.; Baeßler, B.; Thiele, F.; Zopfs, D.; Hokamp, N.G.; Timmer, M.; Kabbasch, C.; Perkuhn, M.; Borggrefe, J. Accuracy of Radiomics-Based Feature Analysis on Multiparametric Magnetic Resonance Images for Noninvasive Meningioma Grading. World Neurosurg. 2019, 132, e366–e390. [Google Scholar] [CrossRef]
- Khanna, O.; Kazerooni, A.F.; Farrell, C.J.; Baldassari, M.P.; Alexander, T.D.; Karsy, M.; Greenberger, B.A.; Garcia, J.A.; Sako, C.; Evans, J.J.; et al. Machine Learning Using Multiparametric Magnetic Resonance Imaging Radiomic Feature Analysis to Predict Ki-67 in World Health Organization Grade I Meningiomas. Neurosurgery 2021, 89, 928–936. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).