Anticancer Activity of Encapsulated Pearl Millet Polyphenol-Rich Extract against Proliferating and Non-Proliferating Breast Cancer Cells In Vitro
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Pearl Millet Extract
2.2. Lipid Extraction
2.3. HPLC Analysis
2.4. Preparation of Microcapsules
2.5. Dynamic Light Scattering (DLS)
2.6. Raman Spectroscopy
2.7. Analysis of Total Polyphenol Content Using Folin–Ciocalteu Assay
2.8. Protection against Fluorescein Bleaching Test
2.9. Cell Lines and Culture Conditions
2.10. MTT Assay
2.11. Encapsulated Polyphenolic Extract-Mediated Apoptotic Cell Death
2.12. The Analysis of Microcapsule Uptake and Encapsulated Polyphenolic Extract-Mediated Antiproliferative, Immunomodulatory, and Autophagy-Inducing Effects in Senescent Breast Cancer Cells
2.13. Statistical Analysis
3. Results and Discussion
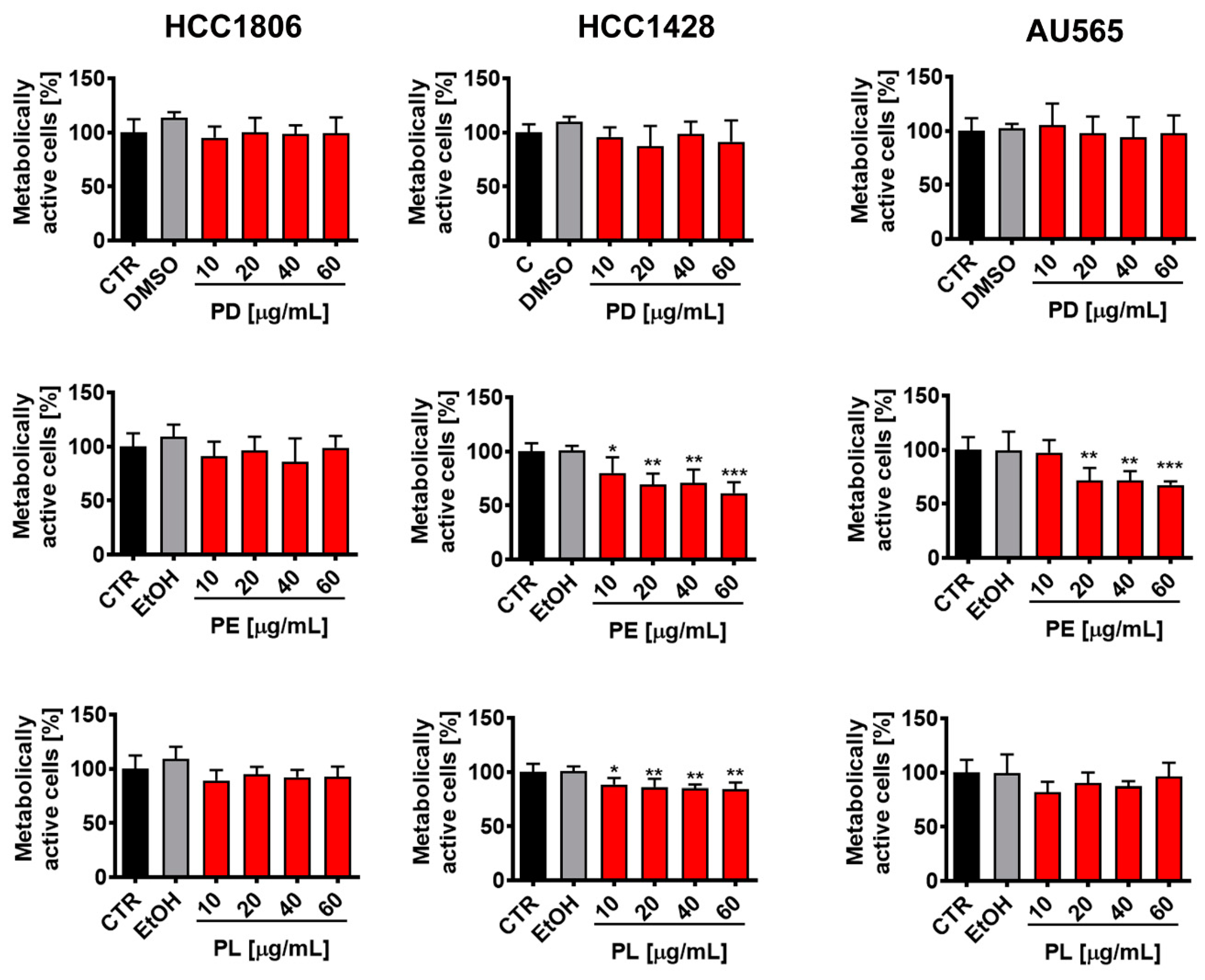
3.1. Polyphenol Content and Initial Analysis of Anti-Breast Cancer Effects of Pearl Millet Grain Extracts
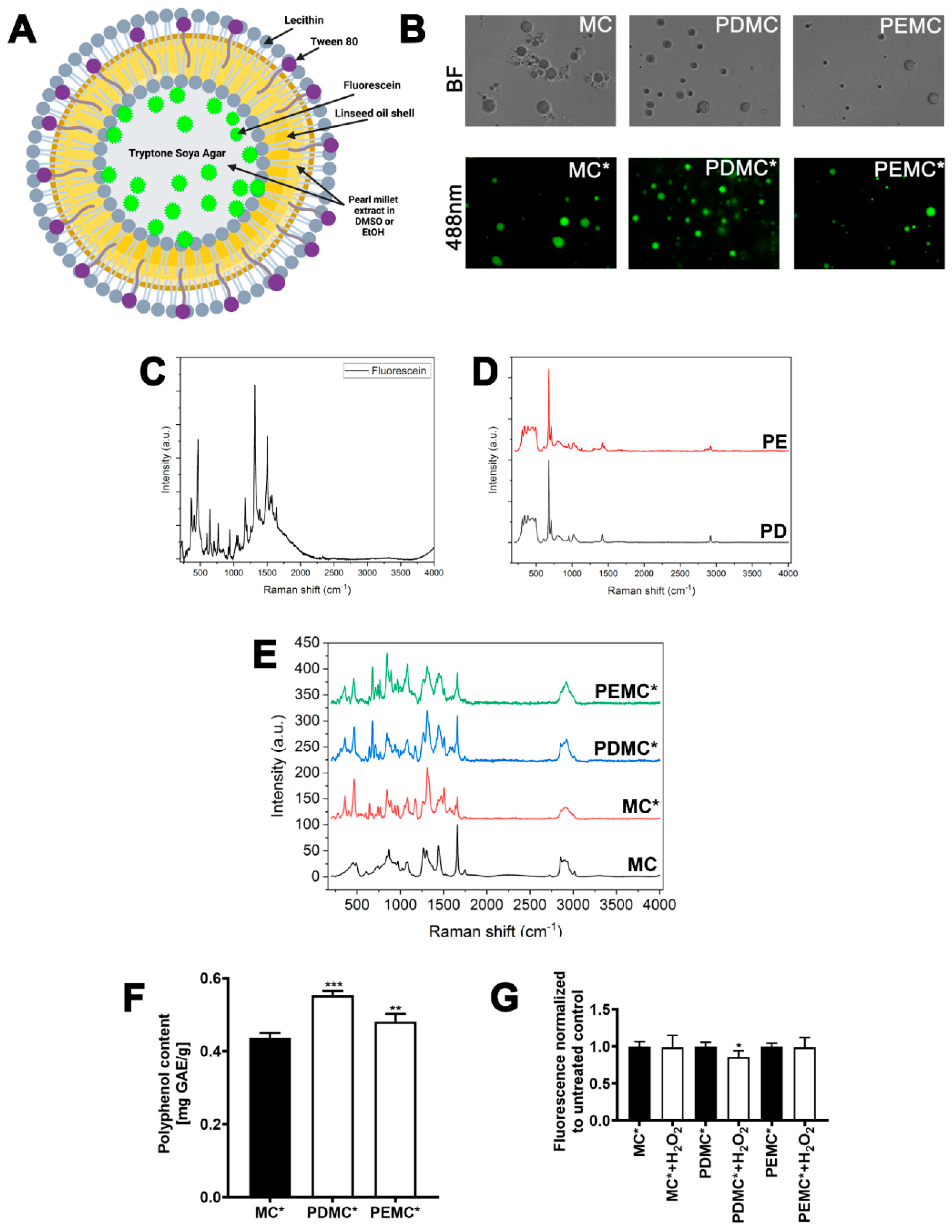
3.2. Physico-Chemical Characterization of Microcapsules
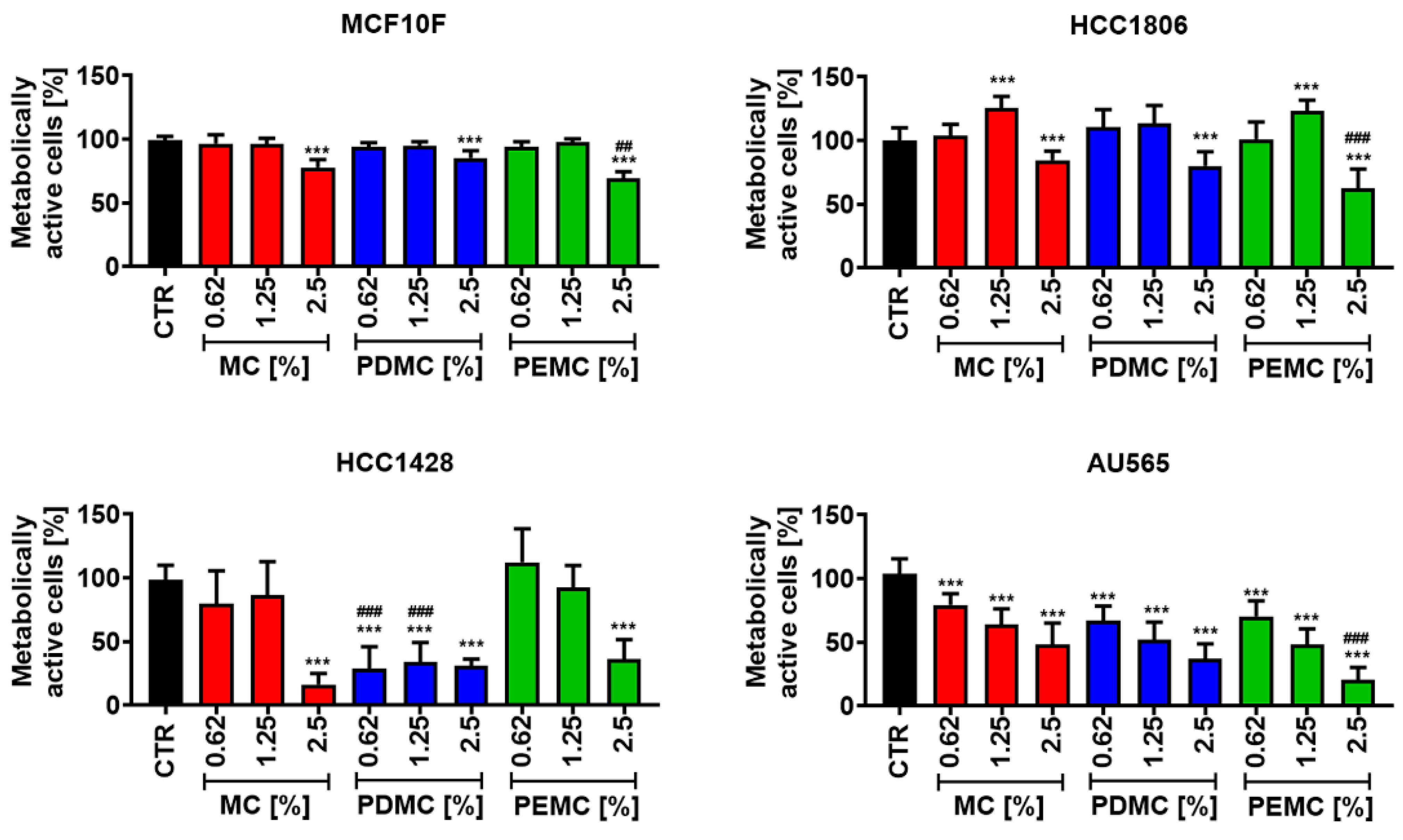
3.3. The Anticancer Activity of Encapsulated Polyphenolic Extracts against Breast Cancer Cells
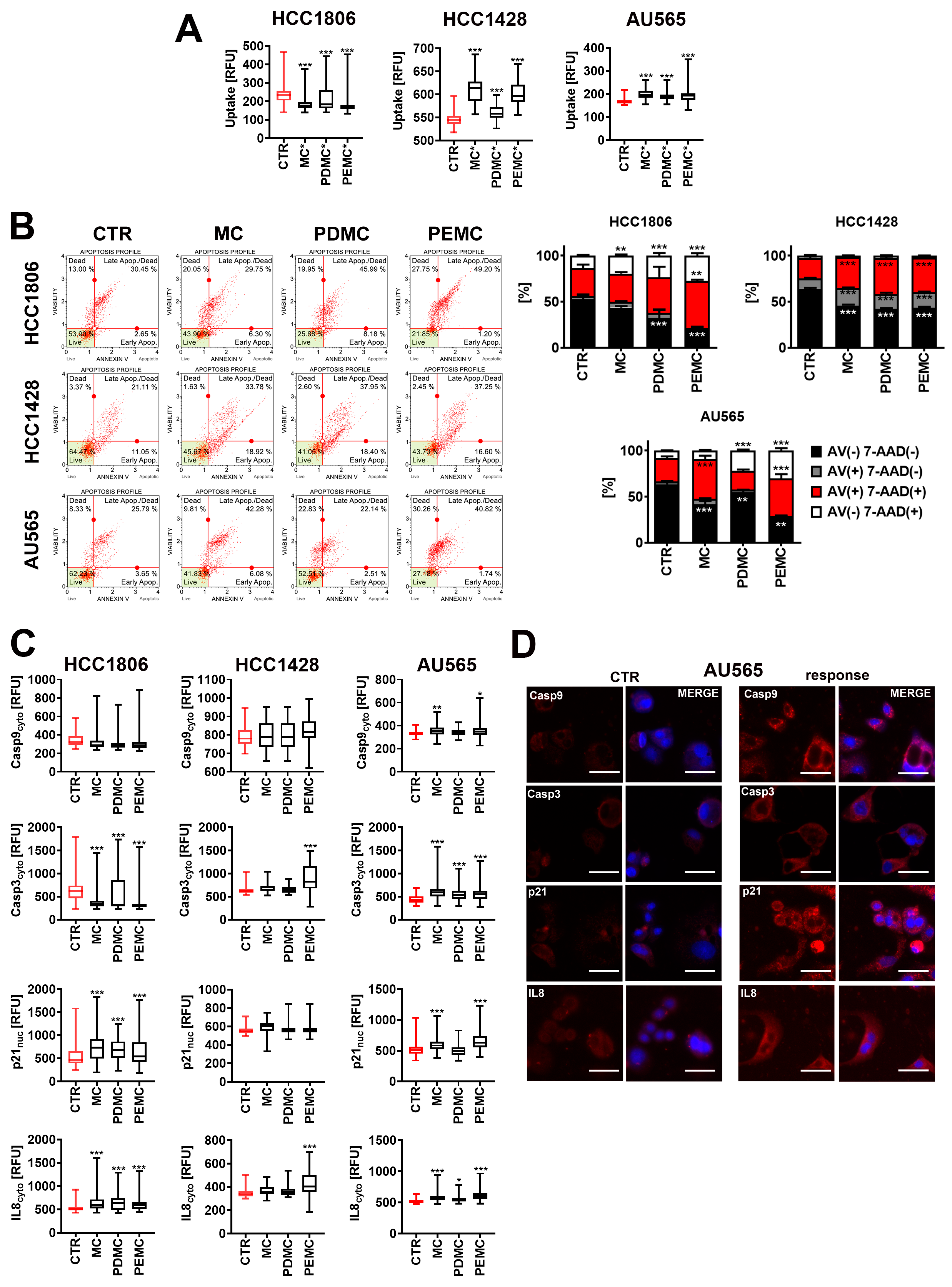
3.4. The Anticancer Activity of Encapsulated Polyphenolic Extracts against Drug-Induced Senescent Breast Cancer Cells
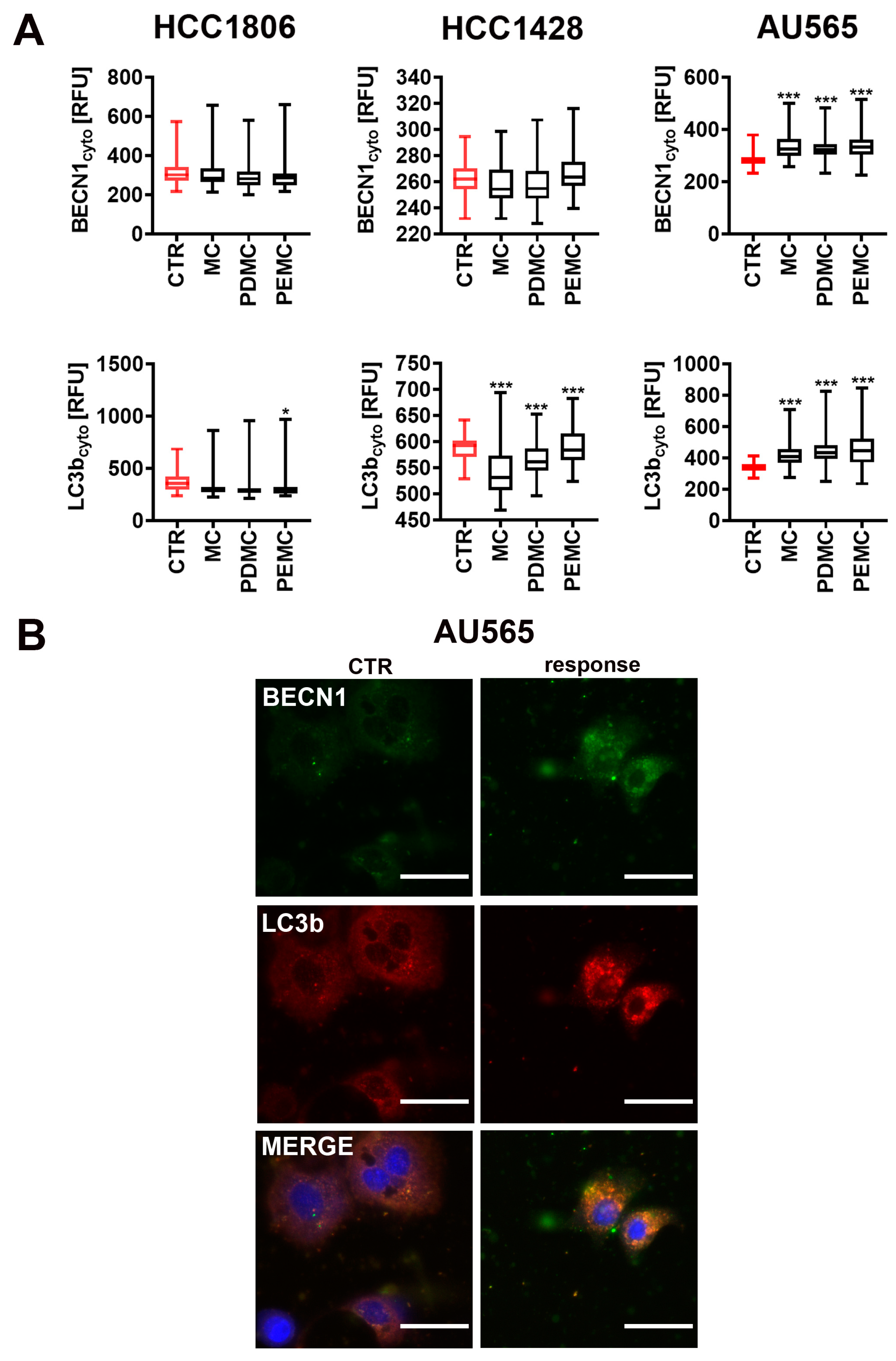
3.5. Encapsulated Polyphenolic Extract-Induced Cytotoxic Autophagy in Senescent AU565 Breast Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA. Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Yardim-Akaydin, S.; Karahalil, B.; Baytas, S.N. New Therapy Strategies in the Management of Breast Cancer. Drug Discov. Today 2022, 27, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; De Angelis, C.; Licata, L.; Gianni, L. Treatment Landscape of Triple-Negative Breast Cancer—Expanded Options, Evolving Needs. Nat. Rev. Clin. Oncol. 2022, 19, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The Role of Polyphenols in Overcoming Cancer Drug Resistance: A Comprehensive Review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. The Anticancer Mechanism of Action of Selected Polyphenols in Triple-Negative Breast Cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Wu, X.; Li, M.; Xiao, Z.; Daglia, M.; Dragan, S.; Delmas, D.; Vong, C.T.; Wang, Y.; Zhao, Y.; Shen, J.; et al. Dietary Polyphenols for Managing Cancers: What Have We Ignored? Trends Food Sci. Technol. 2020, 101, 150–164. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Jafari, S.M. Biopolymer Nano-Particles and Natural Nano-Carriers for Nano-Encapsulation of Phenolic Compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of Hydrophobic and Low-Soluble Food Bioactive Compounds within Different Nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids Nanoparticles in Cancer: Treatment, Prevention and Clinical Prospects. Semin. Cancer Biol. 2021, 69, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid Nanocarriers for the Loading of Polyphenols—A Comprehensive Review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological Approaches to Enhance the Bioavailability and Therapeutic Efficacy of Green Tea Polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]
- Shahidi, F.; Chandrasekara, A. Millet Grain Phenolics and Their Role in Disease Risk Reduction and Health Promotion: A Review. J. Funct. Foods 2013, 5, 570–581. [Google Scholar] [CrossRef]
- Nithiyanantham, S.; Kalaiselvi, P.; Mahomoodally, M.F.; Zengin, G.; Abirami, A.; Srinivasan, G. Nutritional and Functional Roles of Millets—A Review. J. Food Biochem. 2019, 43, e12859. [Google Scholar] [CrossRef] [PubMed]
- Majid, A.; Priyadarshini, C.G.P. Millet Derived Bioactive Peptides: A Review on Their Functional Properties and Health Benefits. Crit. Rev. Food Sci. Nutr. 2020, 60, 3342–3351. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhaka, N.; Dhewa, T.; Puniya, A.K. Nutritional and Health-Promoting Attributes of Millet: Current and Future Perspectives. Nutr. Rev. 2023, 81, 684–704. [Google Scholar] [CrossRef]
- Nani, A.; Belarbi, M.; Ksouri-Megdiche, W.; Abdoul-Azize, S.; Benammar, C.; Ghiringhelli, F.; Hichami, A.; Khan, N.A. Effects of Polyphenols and Lipids from Pennisetum Glaucum Grains on T-Cell Activation: Modulation of Ca2+ and ERK1/ERK2 Signaling. BMC Complement. Altern. Med. 2015, 15, 426. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R. Kiwi Fruit Residues from Industry Processing: Study for a Maximum Phenolic Recovery Yield. J. Food Sci. Technol. 2020, 57, 4265–4276. [Google Scholar] [CrossRef] [PubMed]
- Hudecki, A.; Rzeszutek, I.; Lewińska, A.; Warski, T.; Baranowska-Korczyc, A.; Wojnarowska-Nowak, R.; Betlej, G.; Deręgowska, A.; Hudecki, J.; Łyko-Morawska, D.; et al. Electrospun Fiber-Based Micro- and Nano-System for Delivery of High Concentrated Quercetin to Cancer Cells. Biomater. Adv. 2023, 153, 213582. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, P.; Lewińska, A.; Rzeszutek, I.; Błoniarz, D.; Moskal, A.; Betlej, G.; Deręgowska, A.; Cybularczyk-Cecotka, M.; Szmatoła, T.; Litwinienko, G.; et al. Mutation Status and Glucose Availability Affect the Response to Mitochondria-Targeted Quercetin Derivative in Breast Cancer Cells. Cancers 2023, 15, 5614. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Tchinda, C.F.; Mambe, F.T.; Beng, V.P.; Efferth, T. Cytotoxicity of Methanol Extracts of 10 Cameroonian Medicinal Plants towards Multi-Factorial Drug-Resistant Cancer Cell Lines. BMC Complement. Altern. Med. 2016, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Shan, S.; Li, Z.; Li, H.; Li, X.; Li, Z. Bound Polyphenol from Foxtail Millet Bran Induces Apoptosis in HCT-116 Cell through ROS Generation. J. Funct. Foods 2015, 17, 958–968. [Google Scholar] [CrossRef]
- Espina, A.; Sanchez-Cortes, S.; Jurašeková, Z. Vibrational Study (Raman, SERS, and IR) of Plant Gallnut Polyphenols Related to the Fabrication of Iron Gall Inks. Molecules 2022, 27, 279. [Google Scholar] [CrossRef]
- Corredor, C.; Teslova, T.; Cañamares, M.V.; Chen, Z.; Zhang, J.; Lombardi, J.R.; Leona, M. Raman and Surface-Enhanced Raman Spectra of Chrysin, Apigenin and Luteolin. Vib. Spectrosc. 2009, 49, 190–195. [Google Scholar] [CrossRef]
- de Siqueira e Oliveira, F.S.; Giana, H.E.; Silveira, L. Discrimination of Selected Species of Pathogenic Bacteria Using Near-Infrared Raman Spectroscopy and Principal Components Analysis. J. Biomed. Opt. 2012, 17, 107004. [Google Scholar] [CrossRef]
- Schönemann, A.; Edwards, H.G.M. Raman and FTIR Microspectroscopic Study of the Alteration of Chinese Tung Oil and Related Drying Oils during Ageing. Anal. Bioanal. Chem. 2011, 400, 1173–1180. [Google Scholar] [CrossRef]
- Siwak, J.; Lewinska, A.; Wnuk, M.; Bartosz, G. Protection of Flavonoids against Hypochlorite-Induced Protein Modifications. Food Chem. 2013, 141, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Parama, D.; Daimari, E.; Girisa, S.; Banik, K.; Harsha, C.; Dutta, U.; Kunnumakkara, A.B. Rationalizing the Therapeutic Potential of Apigenin against Cancer. Life Sci. 2021, 267, 118814. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Dia, V.P.; Baek, S.J.; Zhong, Q. Nanoencapsulation of Apigenin with Whey Protein Isolate: Physicochemical Properties, in Vitro Activity against Colorectal Cancer Cells, and Bioavailability. LWT 2022, 154, 112751. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Zahmatkeshan, M.; Akbarzadeh, A.; Rabiee, N.; Ahmadi, S.; Keyhanvar, P.; Rezayat, S.M.; Seifalian, A.M. Chemotherapeutic Effects of Apigenin in Breast Cancer: Preclinical Evidence and Molecular Mechanisms; Enhanced Bioavailability by Nanoparticles. Biotechnol. Rep. 2022, 34, e00730. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lankhorst, L.; Bernards, R. Exploiting Senescence for the Treatment of Cancer. Nat. Rev. Cancer 2022, 22, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ Heel of Senescent Cells: From Transcriptome to Senolytic Drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Recent Advances in the Discovery of Senolytics. Mech. Ageing Dev. 2021, 200, 111587. [Google Scholar] [CrossRef]
- Lewińska, A.; Przybylski, P.; Adamczyk-Grochala, J.; Błoniarz, D.; Litwinienko, G.; Wnuk, M. Senolysis-Based Elimination of Chemotherapy-Induced Senescent Breast Cancer Cells by Quercetin Derivative with Blocked Hydroxy Groups. Cancers 2022, 14, 605. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chang, C.-Y.; Lee, K.-R.; Lin, H.-J.; Chen, T.-H.; Wan, L. Flavones Inhibit Breast Cancer Proliferation through the Akt/FOXO3a Signaling Pathway. BMC Cancer 2015, 15, 958. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.-Y.; Campisi, J. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the P53 Tumor Suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Perrott, K.M.; Wiley, C.D.; Desprez, P.-Y.; Campisi, J. Apigenin Suppresses the Senescence-Associated Secretory Phenotype and Paracrine Effects on Breast Cancer Cells. GeroScience 2017, 39, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. The Four Faces of Autophagy: Implications for Cancer Therapy. Cancer Res. 2014, 74, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, J.M.; Galluzzi, L.; Tajeddine, N.; Ortiz, C.; Criollo, A.; Tasdemir, E.; Morselli, E.; Ben Younes, A.; Maiuri, M.C.; Lavandero, S.; et al. Senescence, Apoptosis or Autophagy? Gerontology 2008, 54, 92–99. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; La, X.; Li, S.; Wen, L.; Liu, T.; Li, H.; Li, A.; Wu, H.; Wu, C.; et al. The Bound Polyphenols of Foxtail Millet (Setaria Italica) Inner Shell Inhibit Breast Cancer by Promoting Lipid Accumulation-Induced Autophagic Death. Food Chem. Toxicol. 2023, 177, 113855. [Google Scholar] [CrossRef]
- Huang, H.-C.; Syu, K.-Y.; Lin, J.-K. Chemical Composition of Solanum Nigrum Linn Extract and Induction of Autophagy by Leaf Water Extract and Its Major Flavonoids in AU565 Breast Cancer Cells. J. Agric. Food Chem. 2010, 58, 8699–8708. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajri, L.; Lewińska, A.; Rzeszutek, I.; Oklejewicz, B.; Wojnarowska-Nowak, R.; Krogul-Sobczak, A.; Szpyrka, E.; Aires, A.; Ghodbane, S.; Ammari, M.; et al. Anticancer Activity of Encapsulated Pearl Millet Polyphenol-Rich Extract against Proliferating and Non-Proliferating Breast Cancer Cells In Vitro. Cancers 2024, 16, 1750. https://doi.org/10.3390/cancers16091750
Hajri L, Lewińska A, Rzeszutek I, Oklejewicz B, Wojnarowska-Nowak R, Krogul-Sobczak A, Szpyrka E, Aires A, Ghodbane S, Ammari M, et al. Anticancer Activity of Encapsulated Pearl Millet Polyphenol-Rich Extract against Proliferating and Non-Proliferating Breast Cancer Cells In Vitro. Cancers. 2024; 16(9):1750. https://doi.org/10.3390/cancers16091750
Chicago/Turabian StyleHajri, Latifa, Anna Lewińska, Iwona Rzeszutek, Bernadetta Oklejewicz, Renata Wojnarowska-Nowak, Agnieszka Krogul-Sobczak, Ewa Szpyrka, Alfredo Aires, Soumaya Ghodbane, Mohamed Ammari, and et al. 2024. "Anticancer Activity of Encapsulated Pearl Millet Polyphenol-Rich Extract against Proliferating and Non-Proliferating Breast Cancer Cells In Vitro" Cancers 16, no. 9: 1750. https://doi.org/10.3390/cancers16091750
APA StyleHajri, L., Lewińska, A., Rzeszutek, I., Oklejewicz, B., Wojnarowska-Nowak, R., Krogul-Sobczak, A., Szpyrka, E., Aires, A., Ghodbane, S., Ammari, M., & Wnuk, M. (2024). Anticancer Activity of Encapsulated Pearl Millet Polyphenol-Rich Extract against Proliferating and Non-Proliferating Breast Cancer Cells In Vitro. Cancers, 16(9), 1750. https://doi.org/10.3390/cancers16091750









