Real-World Experience among Elderly Metastatic Breast Cancer Patients Treated with CDK4/6 Inhibitor-Based Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Tissue Selection and Subtyping
2.3. Statistical Analysis
3. Results
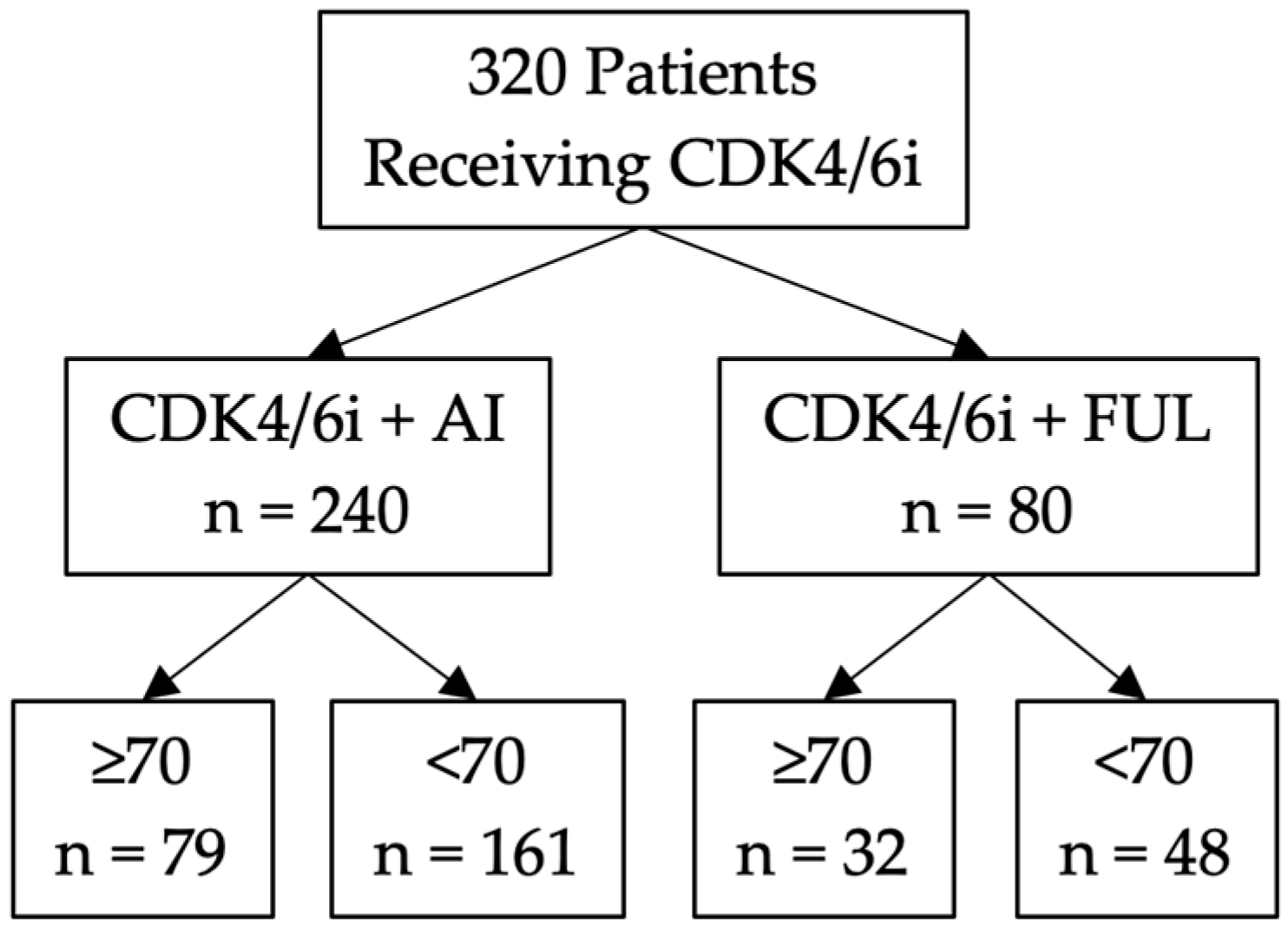
3.1. Study Design
3.2. Clinicopathological Variates
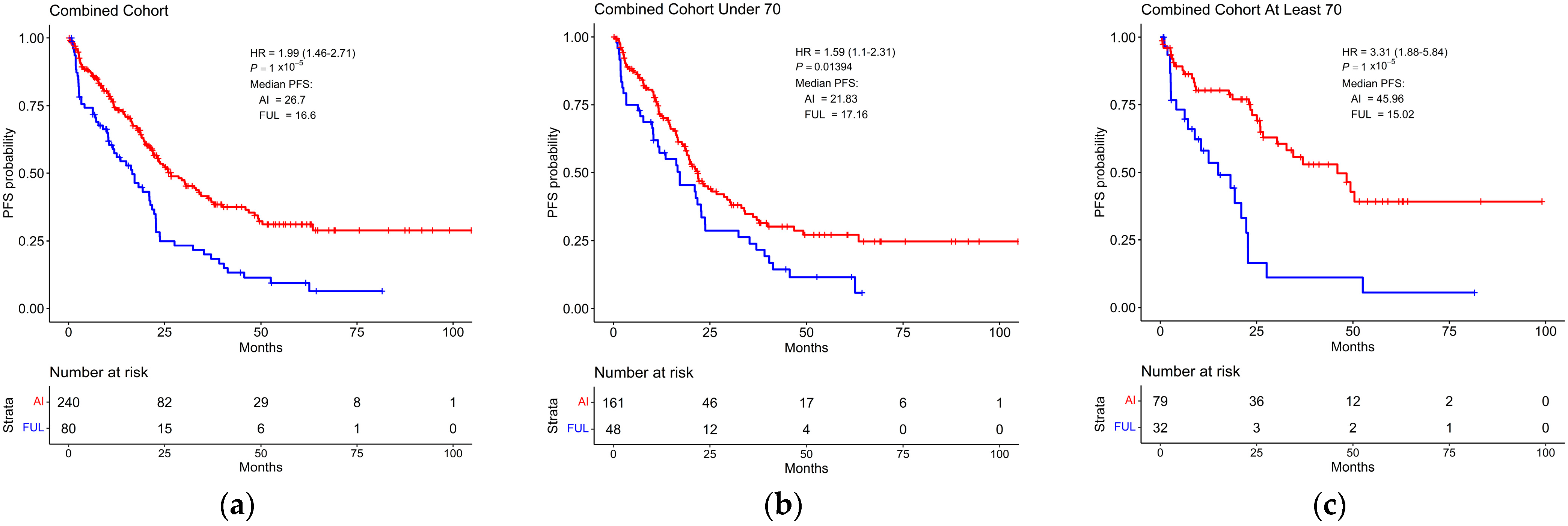
3.3. Survival Based on Endocrine Therapy
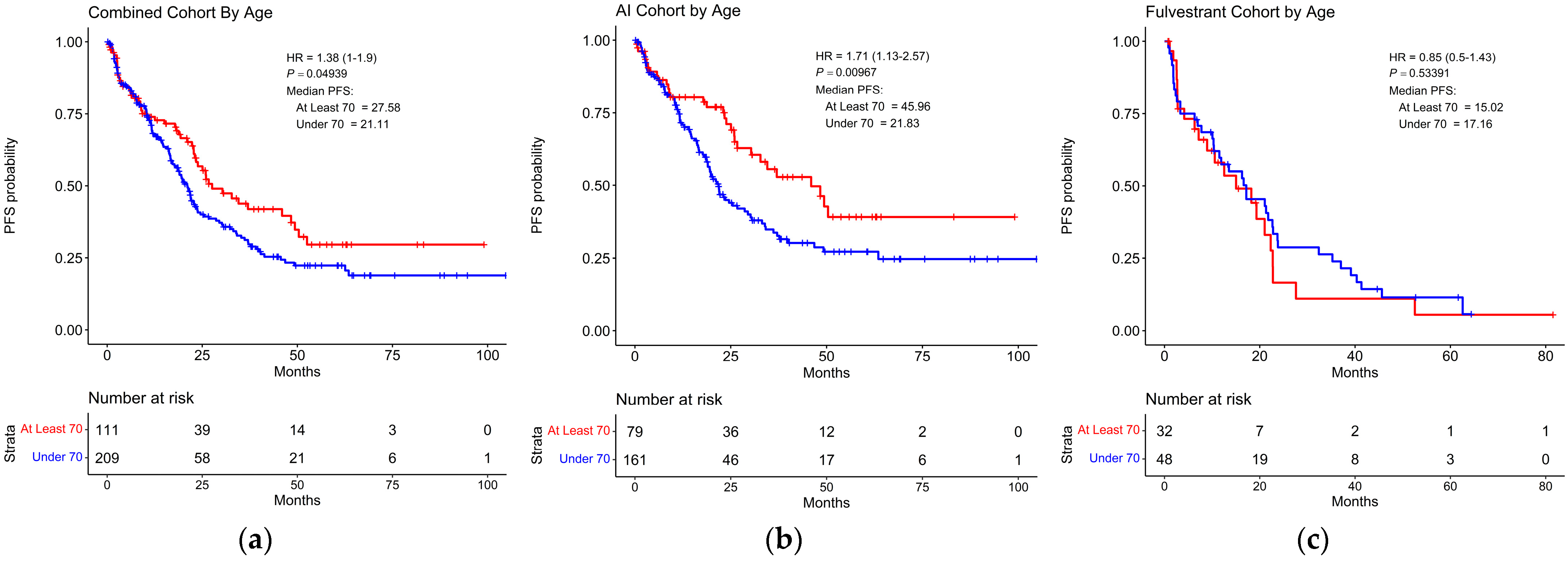
3.4. Survival Based on Age
3.5. Adverse Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, G.; Ma, C.X. Clinical Challenges in the Management of Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: A Literature Review. Adv. Ther. 2021, 38, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef]
- Turner, N.C.; Ro, J.; André, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Kumarasamy, V.; Nambiar, R.; Pearson, J.D.; Vail, P.; Rosenheck, H.; Wang, J.; Eng, K.; Bremner, R.; Schramek, D.; et al. CDK/cyclin dependencies define extreme cancer cell-cycle heterogeneity and collateral vulnerabilities. Cell Rep. 2022, 38, 110448. [Google Scholar] [CrossRef]
- André, F.; Su, F.; Solovieff, N.; Hortobagyi, G.; Chia, S.; Neven, P.; Bardia, A.; Tripathy, D.; Lu, Y.S.; Lteif, A.; et al. Pooled ctDNA analysis of MONALEESA phase III advanced breast cancer trials. Ann. Oncol. 2023, 34, 1003–1014. [Google Scholar] [CrossRef]
- Asghar, U.S.; Kanani, R.; Roylance, R.; Mittnacht, S. Systematic Review of Molecular Biomarkers Predictive of Resistance to CDK4/6 Inhibition in Metastatic Breast Cancer. JCO Precis. Oncol. 2022, 6, e2100002. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Pruitt, S.C.; Hershberger, P.A.; Witkiewicz, A.K.; Goodrich, D.W. Cell Cycle and Beyond: Exploiting New RB1 Controlled Mechanisms for Cancer Therapy. Trends Cancer 2019, 5, 308–324. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Witkiewicz, A.K.; Rubin, S.M. Cancer takes many paths through G1/S. Trends Cell Biol. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, V.; Nambiar, R.; Wang, J.; Rosenheck, H.; Witkiewicz, A.K.; Knudsen, E.S. RB loss determines selective resistance and novel vulnerabilities in ER-positive breast cancer models. Oncogene 2022, 41, 3524–3538. [Google Scholar] [CrossRef]
- Pandey, K.; An, H.J.; Kim, S.K.; Lee, S.A.; Kim, S.; Lim, S.M.; Kim, G.M.; Sohn, J.; Moon, Y.W. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int. J. Cancer 2019, 145, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Im, S.A.; Park, K.; Wen, J.; Lee, K.H.; Choi, Y.L.; Lee, W.C.; Min, A.; Bonato, V.; Park, S.; et al. Longitudinal multi-omics study of palbociclib resistance in HR-positive/HER2-negative metastatic breast cancer. Genome Med. 2023, 15, 55. [Google Scholar] [CrossRef]
- Freedman, R.A.; Foster, J.C.; Seisler, D.K.; Lafky, J.M.; Muss, H.B.; Cohen, H.J.; Mandelblatt, J.; Winer, E.P.; Hudis, C.A.; Partridge, A.H.; et al. Accrual of Older Patients with Breast Cancer to Alliance Systemic Therapy Trials Over Time: Protocol A151527. J. Clin. Oncol. 2017, 35, 421–431. [Google Scholar] [CrossRef]
- Mariotto, A.B.; Etzioni, R.; Hurlbert, M.; Penberthy, L.; Mayer, M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Pacilio, C.; Rosati, G.; Crispo, A.; Bimonte, S.; di Rella, F.; Nuzzo, F.; de Laurentiis, M. An Overview of the Roles of CDK4/6 Inhibitors in Metastatic Breast Cancer Elderly Patients. In Vivo 2023, 37, 1445–1449. [Google Scholar] [CrossRef]
- Singh, H.; Kanapuru, B.; Smith, C.; Fashoyin-Aje, L.A.; Myers, A.; Kim, G.; Pazdur, R. FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: A 10-year experience by the U.S. Food and Drug Administration. J. Clin. Oncol. 2017, 35, 10009. [Google Scholar] [CrossRef]
- Howie, L.J.; Singh, H.; Bloomquist, E.; Wedam, S.; Amiri-Kordestani, L.; Tang, S.; Sridhara, R.; Sanchez, J.; Prowell, T.M.; Kluetz, P.G.; et al. Outcomes of Older Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor-Negative Metastatic Breast Cancer Treated With a CDK4/6 Inhibitor and an Aromatase Inhibitor: An FDA Pooled Analysis. J. Clin. Oncol. 2019, 37, 3475–3483. [Google Scholar] [CrossRef]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef]
- Chadha, M.; White, J.; Swain, S.M.; Rakovitch, E.; Jagsi, R.; Whelan, T.; Sparano, J.A. Optimal adjuvant therapy in older (≥70 years of age) women with low-risk early-stage breast cancer. NPJ Breast Cancer 2023, 9, 99. [Google Scholar] [CrossRef]
- Clifton, K.; Min, Y.; Kimmel, J.; Litton, J.; Tripathy, D.; Karuturi, M. Progression-free survival (PFS) and toxicities of palbociclib in a geriatric population. Breast Cancer Res. Treat. 2019, 175, 667–674. [Google Scholar] [CrossRef]
- de Boer, A.Z.; Derks, M.G.M.; de Glas, N.A.; Bastiaannet, E.; Liefers, G.J.; Stiggelbout, A.M.; van Dijk, M.A.; Kroep, J.R.; Ropela, A.; van den Bos, F.; et al. Metastatic breast cancer in older patients: A longitudinal assessment of geriatric outcomes. J. Geriatr. Oncol. 2020, 11, 969–975. [Google Scholar] [CrossRef]
- Pla, H.; Felip, E.; Obadia, V.; Pernas, S.; Viñas, G.; Margelí, M.; Fort-Culillas, R.; Del Barco, S.; Sabaté, N.; Fort, E.; et al. Elderly patients with hormone receptor-positive HER2-negative metastatic breast cancer treated with CDK4/6 inhibitors in a multicentre cohort. Clin. Transl. Oncol. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Saura, C.; Pascual, T.; Hernando, C.; Muñoz, M.; Paré, L.; González Farré, B.; Fernández, P.L.; Galván, P.; Chic, N.; et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Sobrini-Morillo, P.; Ravot, C.; Herlédan, C.; Sánchez-Castellano, C.; Cruz-Jentoft, A.J.; Falandry, C. Real-world experience with CDK4-6 inhibition in the old and oldest old with a diagnosis of breast cancer. Semin. Oncol. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Tang, H.; Yeo, D.; De Souza, K.; Ahmad, O.; Shafiq, T.; Ofor, O.; Anand, A.; Karim, S.; Khan, S.; Madhusudan, S. Clinical Impact of CDK4/6 Inhibitors in De Novo or PR- or Very Elderly Post-Menopausal ER+/HER2- Advanced Breast Cancers. Cancers 2023, 15, 5164. [Google Scholar] [CrossRef]
- Biganzoli, L.; Battisti, N.M.L.; Wildiers, H.; McCartney, A.; Colloca, G.; Kunkler, I.H.; Cardoso, M.J.; Cheung, K.L.; de Glas, N.A.; Trimboli, R.M.; et al. Updated recommendations regarding the management of older patients with breast cancer: A joint paper from the European Society of Breast Cancer Specialists (EUSOMA) and the International Society of Geriatric Oncology (SIOG). Lancet Oncol. 2021, 22, e327–e340. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Im, S.A.; Joy, A.A.; Shparyk, Y.; Walshe, J.M.; Sleckman, B.; Loi, S.; Theall, K.P.; Kim, S.; Huang, X.; et al. Effect of palbociclib plus endocrine therapy on time to chemotherapy across subgroups of patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Post hoc analyses from PALOMA-2 and PALOMA-3. Breast 2022, 66, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Turner, N.C.; Finn, R.S.; Joy, A.A.; Verma, S.; Harbeck, N.; Masuda, N.; Im, S.A.; Huang, X.; Kim, S.; et al. Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: A pooled analysis of randomised PALOMA clinical studies. Eur. J. Cancer 2018, 101, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; André, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Im, S.A.; Lu, Y.S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef]
- Tripathy, D.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Harbeck, N.; Hurvitz, S.A.; Chow, L.; Sohn, J.; Lee, K.S.; et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018, 19, 904–915. [Google Scholar] [CrossRef]
- Goetz, M.P.; Okera, M.; Wildiers, H.; Campone, M.; Grischke, E.M.; Manso, L.; André, V.A.M.; Chouaki, N.; San Antonio, B.; Toi, M.; et al. Safety and efficacy of abemaciclib plus endocrine therapy in older patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: An age-specific subgroup analysis of MONARCH 2 and 3 trials. Breast Cancer Res. Treat. 2021, 186, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Trédan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.J.; Cheng, J.; Bloomquist, E.; Sanchez, J.; Wedam, S.B.; Singh, H.; Amiri-Kordestani, L.; Ibrahim, A.; Sridhara, R.; Goldberg, K.B.; et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2020, 21, 250–260. [Google Scholar] [CrossRef]
- Sotaniemi, E.A.; Arranto, A.J.; Pelkonen, O.; Pasanen, M. Age and cytochrome P450-linked drug metabolism in humans: An analysis of 226 subjects with equal histopathologic conditions. Clin. Pharmacol. Ther. 1997, 61, 331–339. [Google Scholar] [CrossRef]
- Witkiewicz, A.K.; Schultz, E.; Wang, J.; Hamilton, D.; Levine, E.; O’Connor, T.; Knudsen, E.S. Determinants of response to CDK4/6 inhibitors in the real-world setting. NPJ Precis. Oncol. 2023, 7, 90. [Google Scholar] [CrossRef]
- Paquet, E.R.; Hallett, M.T. Absolute assignment of breast cancer intrinsic molecular subtype. J. Natl. Cancer Inst. 2015, 107, 357. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Schultz, E.; Hamilton, D.; Attwood, K.; Edge, S.; O’Connor, T.; Levine, E.; Witkiewicz, A.K. Real-World Experience with CDK4/6 Inhibitors for Metastatic HR+/HER2- Breast Cancer at a Single Cancer Center. Oncologist 2022, 27, 646–654. [Google Scholar] [CrossRef]
- Rakha, E.A.; Reis-Filho, J.S.; Baehner, F.; Dabbs, D.J.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Breast cancer prognostic classification in the molecular era: The role of histological grade. Breast Cancer Res. 2010, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Ismail, R.K.; van Breeschoten, J.; Wouters, M.; van Dartel, M.; van der Flier, S.; Reyners, A.K.L.; de Graeff, P.; Pasmooij, A.M.G.; de Boer, A.; Broekman, K.E.; et al. Palbociclib dose reductions and the effect on clinical outcomes in patients with advanced breast cancer. Breast 2021, 60, 263–271. [Google Scholar] [CrossRef]
- McAndrew, N.P.; Finn, R.S. Clinical Review on the Management of Hormone Receptor-Positive Metastatic Breast Cancer. JCO Oncol. Pract. 2022, 18, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Mouabbi, J.A.; Osborne, C.K.; Schiff, R.; Rimawi, M.F. Management of hormone receptor-positive, human epidermal growth factor 2-negative metastatic breast cancer. Breast Cancer Res. Treat. 2021, 190, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Karuturi, M.S.; Cappelleri, J.C.; Blum, J.L.; Patel, K.; Telivala, B.; McCune, S.; Cuevas, J.D.; Lakhanpal, S.; Montelongo, M.Z.; Wang, Y.; et al. Measures of functional status in older patients treated with palbociclib for advanced breast cancer. J. Geriatr. Oncol. 2024, 15, 101670. [Google Scholar] [CrossRef]
- Wekking, D.; Lambertini, M.; Dessì, M.; Denaro, N.; Bardanzellu, F.; Garrone, O.; Scartozzi, M.; Solinas, C. CDK4/6 inhibitors in the treatment of metastatic breast cancer: Focus on toxicity and safety. Semin. Oncol. 2023, 50, 131–139. [Google Scholar] [CrossRef]
- Damodaran, S.; Zhao, F.; Deming, D.A.; Mitchell, E.P.; Wright, J.J.; Gray, R.J.; Wang, V.; McShane, L.M.; Rubinstein, L.V.; Patton, D.R.; et al. Phase II Study of Copanlisib in Patients With Tumors With PIK3CA Mutations: Results From the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol Z1F. J. Clin. Oncol. 2022, 40, 1552–1561. [Google Scholar] [CrossRef]
- Layman, R.M.; Han, H.S.; Rugo, H.S.; Stringer-Reasor, E.M.; Specht, J.M.; Dees, E.C.; Kabos, P.; Suzuki, S.; Mutka, S.C.; Sullivan, B.F.; et al. Gedatolisib in combination with palbociclib and endocrine therapy in women with hormone receptor-positive, HER2-negative advanced breast cancer: Results from the dose expansion groups of an open-label, phase 1b study. Lancet Oncol. 2024, 25, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Lim, J.S.J.; Macpherson, I.R.; Armstrong, A.C.; Ring, A.; Okines, A.F.C.; Cutts, R.J.; Herrera-Abreu, M.T.; Garcia-Murillas, I.; Pearson, A.; et al. Triplet Therapy with Palbociclib, Taselisib, and Fulvestrant in PIK3CA-Mutant Breast Cancer and Doublet Palbociclib and Taselisib in Pathway-Mutant Solid Cancers. Cancer Discov. 2021, 11, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; Kumarasamy, V.; Sanidas, I.; Knudsen, E.S. Cancer cell cycle dystopia: Heterogeneity, plasticity, and therapy. Trends Cancer 2022, 8, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Talarico, L.; Chen, G.; Pazdur, R. Enrollment of elderly patients in clinical trials for cancer drug registration: A 7-year experience by the US Food and Drug Administration. J. Clin. Oncol. 2004, 22, 4626–4631. [Google Scholar] [CrossRef]
- Battisti, N.M.L.; De Glas, N.; Sedrak, M.S.; Loh, K.P.; Liposits, G.; Soto-Perez-de-Celis, E.; Krok-Schoen, J.L.; Menjak, I.B.; Ring, A. Use of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in older patients with ER-positive HER2-negative breast cancer: Young International Society of Geriatric Oncology review paper. Ther. Adv. Med. Oncol. 2018, 10, 1758835918809610. [Google Scholar] [CrossRef]
- Scotté, F.; Bossi, P.; Carola, E.; Cudennec, T.; Dielenseger, P.; Gomes, F.; Knox, S.; Strasser, F. Addressing the quality of life needs of older patients with cancer: A SIOG consensus paper and practical guide. Ann. Oncol. 2018, 29, 1718–1726. [Google Scholar] [CrossRef]
| CDK4/6i + AI | CDK4/6i + FUL | |||
|---|---|---|---|---|
| <70 (n = 70) | ≥70 (n = 45) | <70 (n = 24) | ≥70 (n = 12) | |
| Basal | 1 (1.4) | 1 (2.2) | 0 (0.0) | 0 (0.0) |
| HER2 | 1 (1.4) | 2 (4.4) | 4 (16.7) | 2 (16.7) |
| LumA | 32 (45.7) | 29 (64.4) | 13 (54.2) | 3 (25.0) |
| LumB | 34 (48.6) | 13 (28.9) | 6 (25.0) | 6 (50.0) |
| Normal | 2 (2.9) | 0 (0.0) | 1 (4.2) | 1 (8.3) |
| All Patients (n = 320) (<70 = 209; ≥70 = 111) | CDK4/6i + AI Patients (n = 240) (<70 = 161; ≥70 = 79) | CDK4/6i + FUL Patients (n = 80) (<70 = 48; ≥70 = 32) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥70 vs. <70 | <70 | ≥70 | HR | p val. | <70 | ≥70 | HR | p val. | <70 | ≥70 | HR | p val. |
| HER2 0+ | 37 (17.7) | 15 (13.5) | 1.34 | 0.6 | 33 (20.5) | 12 (15.2) | 1.17 | 0.8 | 4 (8.3) | 3 (9.4) | 2.40 | 0.4 |
| HER2 1+ | 73 (34.9) | 40 (36.0) | 1.55 | 0.1 | 54 (33.5) | 30 (38.0) | 2.20 | 0.04 | 19 (39.6) | 10 (31.3) | 0.63 | 0.3 |
| HER2 2+ | 74 (35.4) | 41 (36.9) | 1.36 | 0.2 | 56 (34.8) | 32 (40.5) | 1.57 | 0.1 | 18 (37.5) | 9 (28.1) | 0.70 | 0.4 |
| PR− | 74 (35.4) | 42 (37.8) | 1.36 | 0.2 | 50 (31.1) | 28 (35.4) | 1.64 | 0.1 | 24 (50) | 14 (43.8) | 0.92 | 0.8 |
| PR+ | 115 (55.0) | 56 (50.5) | 1.50 | 0.1 | 98 (60.9) | 48 (60.8) | 1.78 | 0.05 | 17 (35.4) | 8 (25.0) | 0.69 | 0.4 |
| SBR1 | 20 (9.6) | 15 (13.5) | 0.97 | 1 | 12 (7.5) | 11 (13.9) | 0.56 | 0.5 | 8 (16.7) | 4 (12.5) | 2.13 | 0.3 |
| SBR2 | 112 (53.6) | 66 (59.5) | 1.29 | 0.2 | 85 (52.8) | 45 (57.0) | 1.77 | 0.04 | 27 (56.3) | 21 (65.6) | 0.73 | 0.4 |
| SBR3 | 59 (28.2) | 18 (16.2) | 1.14 | 0.7 | 48 (29.8) | 13 (16.5) | 1.26 | 0.6 | 11 (22.9) | 5 (15.6) | 0.68 | 0.7 |
| Non-Visceral | 119 (56.9) | 49 (44.1) | 1.52 | 0.1 | 97 (60.2) | 38 (48.1) | 1.80 | 0.06 | 22 (45.8) | 11 (34.4) | 0.94 | 0.9 |
| Visceral | 90 (43.1) | 62 (55.9) | 1.38 | 0.1 | 64 (39.8) | 41 (51.9) | 1.74 | 0.05 | 26 (54.2) | 21 (65.6) | 0.83 | 0.6 |
| Distant Mets | 195 (93.3) | 110 (99.1) | 1.45 | 0.02 | 152 (94.4) | 78 (98.7) | 1.79 | 0.005 | 43 (89.6) | 32 (100.0) | 0.93 | 0.8 |
| Local Mets | 14 (6.7) | 1 (0.9) | - | - | 9 (5.6) | 1 (1.3) | - | - | 5 (10.4) | 0 (0.0) | - | - |
| De novo Mets | 74 (35.4) | 29 (26.1) | 1.31 | 0.41 | 66 (41.0) | 27 (34.2) | 1.34 | 0.43 | 8 (16.7) | 2 (6.3) | 0.39 | 0.31 |
| Recurrent Mets | 135 (64.6) | 82 (73.9) | 1.45 | 0.05 | 95 (59.0) | 52 (65.8) | 2.00 | 0.007 | 40 (83.3) | 30 (93.8) | 0.87 | 0.63 |
| Prior ET | 133 (63.6) | 76 (68.5) | 1.20 | 0.3 | 89 (55.3) | 45 (57.0) | 1.50 | 0.1 | 44 (91.7) | 31 (96.9) | 0.96 | 0.9 |
| No Prior ET | 76 (36.4) | 35 (31.5) | 1.86 | 0.06 | 72 (44.7) | 34 (43.0) | 1.95 | 0.05 | 4 (8.3) | 1 (3.1) | - | - |
| CDK4/6i + AI | CDK4/6i + FUL | |||
|---|---|---|---|---|
| <70 (n = 161) | ≥70 (n = 79) | <70 (n = 48) | ≥70 (n = 32) | |
| AE leading to dose reduction and/or hold | 79 (49.1) | 48 (60.8) | 23 (47.9) | 18 (56.3) |
| AE leading to discontinuation | 9 (5.6) | 6 (7.6) | 0 (0.0) | 5 (15.6) |
| Fatigue | 66 (41.0) | 43 (54.4) | 16 (33.3) | 17 (53.1) |
| Diarrhea | 28 (17.4) | 20 (25.3) | 9 (18.8) | 8 (25.0) |
| Leukopenia/Neutropenia | 84 (52.2) | 48 (60.8) | 25 (52.1) | 15 (46.9) |
| Thrombocytopenia | 16 (9.9) | 17 (21.5) | 2 (4.2) | 7 (21.9) |
| Anemia | 38 (23.6) | 28 (35.4) | 8 (16.7) | 9 (18.8) |
| Abnormal ALT/AST | 11 (6.8) | 3 (3.8) | 1 (2.1) | 1 (3.1) |
| Nausea/Vomiting | 53 (32.9) | 19 (24.1) | 19 (39.6) | 9 (18.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, T.N.; Schultz, E.; Wang, J.; O’Connor, T.; Levine, E.; Knudsen, E.S.; Witkiewicz, A.K. Real-World Experience among Elderly Metastatic Breast Cancer Patients Treated with CDK4/6 Inhibitor-Based Therapy. Cancers 2024, 16, 1749. https://doi.org/10.3390/cancers16091749
O’Connor TN, Schultz E, Wang J, O’Connor T, Levine E, Knudsen ES, Witkiewicz AK. Real-World Experience among Elderly Metastatic Breast Cancer Patients Treated with CDK4/6 Inhibitor-Based Therapy. Cancers. 2024; 16(9):1749. https://doi.org/10.3390/cancers16091749
Chicago/Turabian StyleO’Connor, Thomas N., Emily Schultz, Jianxin Wang, Tracey O’Connor, Ellis Levine, Erik S. Knudsen, and Agnieszka K. Witkiewicz. 2024. "Real-World Experience among Elderly Metastatic Breast Cancer Patients Treated with CDK4/6 Inhibitor-Based Therapy" Cancers 16, no. 9: 1749. https://doi.org/10.3390/cancers16091749
APA StyleO’Connor, T. N., Schultz, E., Wang, J., O’Connor, T., Levine, E., Knudsen, E. S., & Witkiewicz, A. K. (2024). Real-World Experience among Elderly Metastatic Breast Cancer Patients Treated with CDK4/6 Inhibitor-Based Therapy. Cancers, 16(9), 1749. https://doi.org/10.3390/cancers16091749






