Racial and Ethnic Disparities in European Breast Cancer Clinical Trials
Abstract
Simple Summary
Abstract
1. Introduction
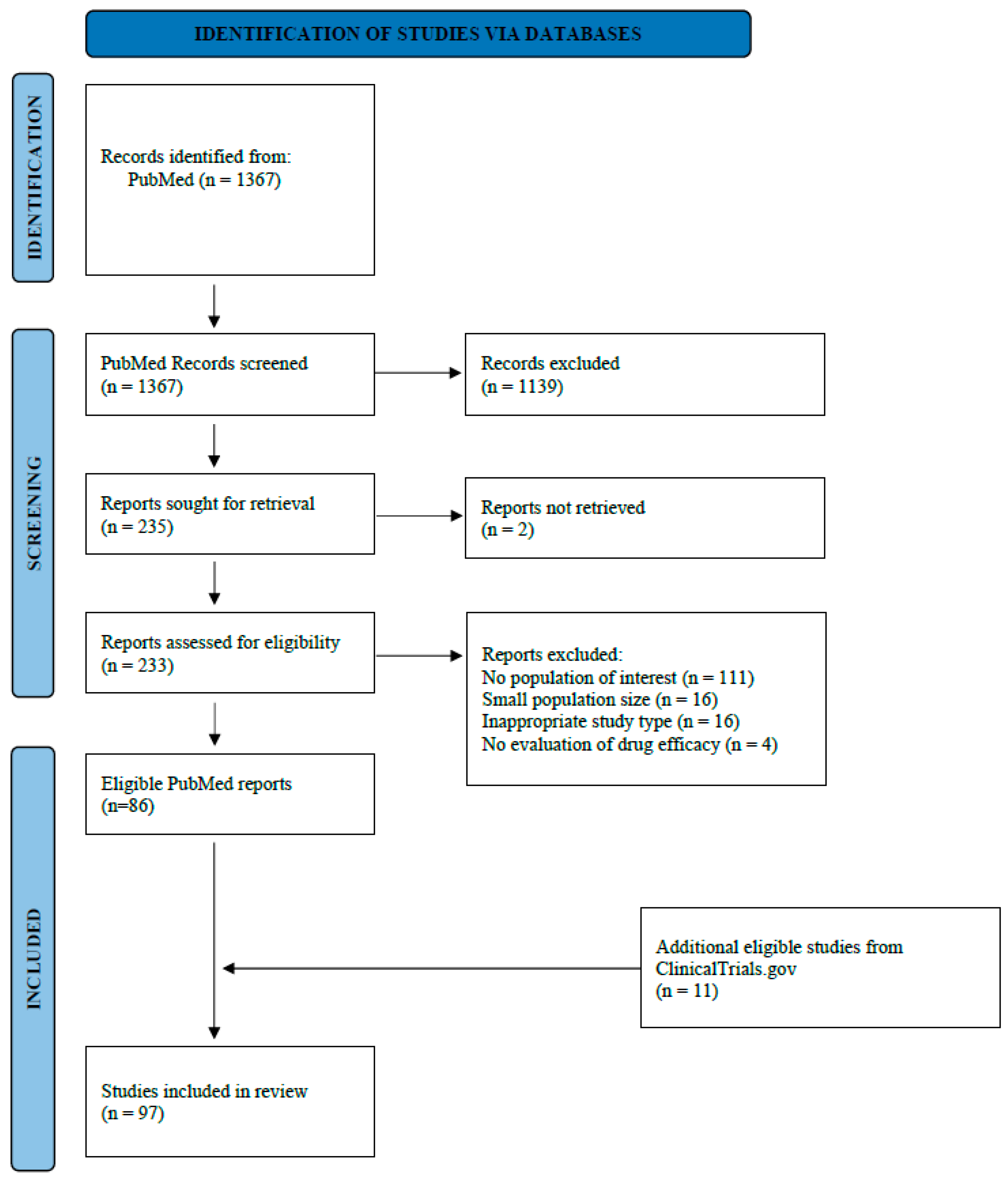
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Extraction
2.3. Statistical Analysis
3. Results
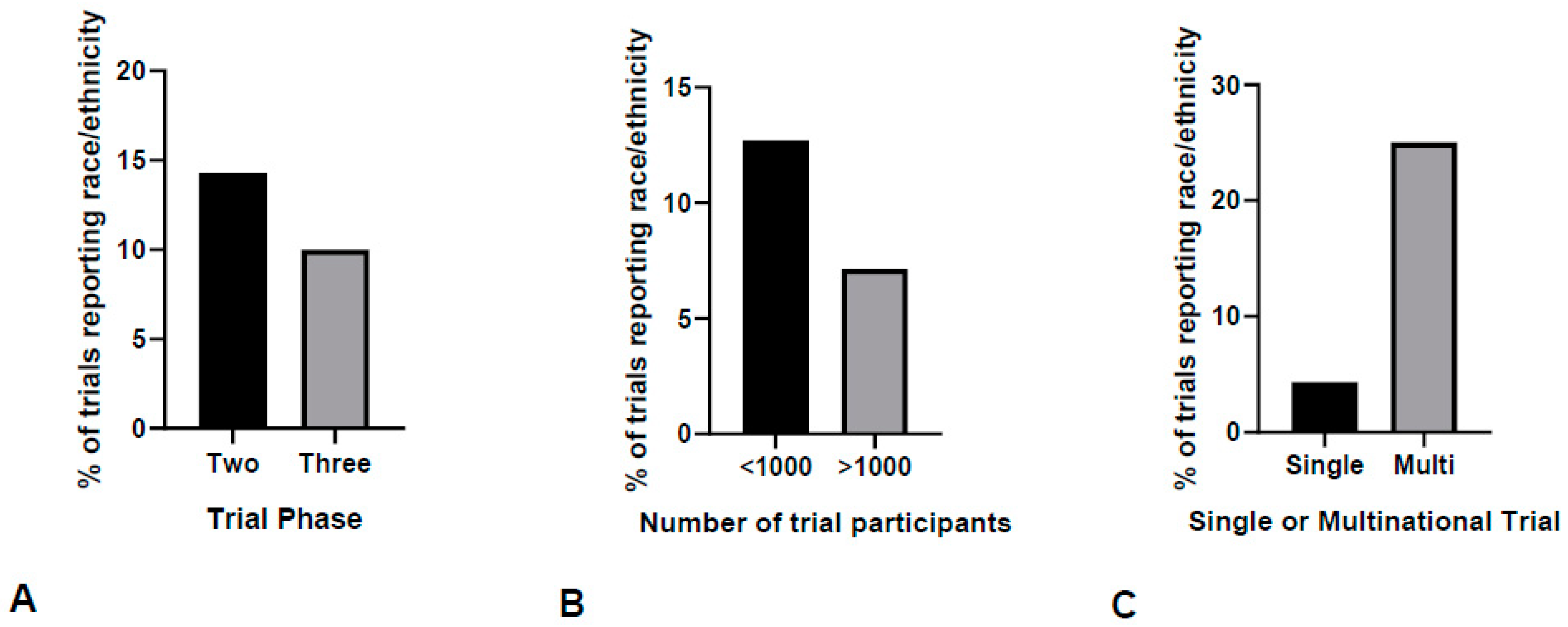
3.1. Race and Ethnicity Reporting in European Breast Cancer Clinical Trials
3.2. Inclusion of Racial and Ethnic Minorities in European Breast Cancer Clinical Trials
3.3. Representation of Racial Minorities in Breast Cancer Clinical Trials
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022; American Association for Cancer Research: Philadelphia, PA, USA, 2022. [Google Scholar]
- Sung, H.; DeSantis, C.; Jemal, A. Subtype-Specific Breast Cancer Incidence Rates in Black versus White Men in the United States. JNCI Cancer Spectr. 2019, 4, pkz091. [Google Scholar] [CrossRef] [PubMed]
- Fejerman, L.; John, E.M.; Huntsman, S.; Beckman, K.; Choudhry, S.; Perez-Stable, E.; Burchard, E.G.; Ziv, E. Genetic Ancestry and Risk of Breast Cancer among U.S. Latinas. Cancer Res. 2008, 68, 9723–9728. [Google Scholar] [CrossRef]
- Fejerman, L.; Romieu, I.; John, E.M.; Lazcano-Ponce, E.; Huntsman, S.; Beckman, K.B.; Pérez-Stable, E.J.; González Burchard, E.; Ziv, E.; Torres-Mejía, G. European Ancestry Is Positively Associated with Breast Cancer Risk in Mexican Women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1074–1082. [Google Scholar] [CrossRef]
- Aldrighetti, C.M.; Niemierko, A.; Van Allen, E.; Willers, H.; Kamran, S.C. Racial and Ethnic Disparities Among Participants in Precision Oncology Clinical Studies. JAMA Netw. Open 2021, 4, e2133205. [Google Scholar] [CrossRef]
- Duma, N.; Vera Aguilera, J.; Paludo, J.; Haddox, C.L.; Gonzalez Velez, M.; Wang, Y.; Leventakos, K.; Hubbard, J.M.; Mansfield, A.S.; Go, R.S.; et al. Representation of Minorities and Women in Oncology Clinical Trials: Review of the Past 14 Years. J. Oncol. Pract. 2018, 14, e1–e10. [Google Scholar] [CrossRef]
- Al Hadidi, S.; Mims, M.; Miller-Chism, C.N.; Kamble, R. Participation of African American Persons in Clinical Trials Supporting U.S. Food and Drug Administration Approval of Cancer Drugs. Ann. Intern. Med. 2020, 173, 320–322. [Google Scholar] [CrossRef]
- Grette, K.V.; White, A.L.; Awad, E.K.; Scalici, J.M.; Young-Pierce, J.; Rocconi, R.P.; Jones, N.L. Not Immune to Inequity: Minority under-Representation in Immunotherapy Trials for Breast and Gynecologic Cancers. Int. J. Gynecol. Cancer 2021, 31, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, A.; Knepper, T.C.; Merenda, C.; Mendoza, M.; McLeod, H.L.; Bull, J.; Zhang, L.; Pacanowski, M. Demographic Composition of Select Oncologic New Molecular Entities Approved by the FDA Between 2008 and 2017. Clin. Pharmacol. Ther. 2018, 104, 940–948. [Google Scholar] [CrossRef]
- Ajewole, V.B.; Akindele, O.; Abajue, U.; Ndulue, O.; Marshall, J.J.; Mossi, Y.T. Cancer Disparities and Black American Representation in Clinical Trials Leading to the Approval of Oral Chemotherapy Drugs in the United States between 2009 and 2019. JCO Oncol. Pract. 2021, 17, e623–e628. [Google Scholar] [CrossRef]
- Katz, R.V.; Green, B.L.; Kressin, N.R.; Claudio, C.; Wang, M.Q.; Russell, S.L. Willingness of Minorities to Participate in Biomedical Studies: Confirmatory Findings from a Follow-up Study Using the Tuskegee Legacy Project Questionnaire. J. Natl. Med. Assoc. 2007, 99, 1052–1060. [Google Scholar] [PubMed]
- Andersson, M.; Lidbrink, E.; Bjerre, K.; Wist, E.; Enevoldsen, K.; Jensen, A.B.; Karlsson, P.; Tange, U.B.; Sørensen, P.G.; Møller, S.; et al. Phase III Randomized Study Comparing Docetaxel Plus Trastuzumab with Vinorelbine Plus Trastuzumab As First-Line Therapy of Metastatic or Locally Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: The HERNATA Study. J. Clin. Oncol. 2011, 29, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Kellokumpu-Lehtinen, P.-L.; Huovinen, R.; Jukkola-Vuorinen, A.; Tanner, M.; Kokko, R.; Ahlgren, J.; Auvinen, P.; Saarni, O.; Helle, L.; et al. Outcome of Patients with HER2−Positive Breast Cancer Treated with or without Adjuvant Trastuzumab in the Finland Capecitabine Trial (FinXX). Acta Oncol. 2014, 53, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Perrone, F.; Nuzzo, F.; Di Rella, F.; Gravina, A.; Iodice, G.; Labonia, V.; Landi, G.; Pacilio, C.; Rossi, E.; De Laurentiis, M.; et al. Weekly Docetaxel versus CMF as Adjuvant Chemotherapy for Older Women with Early Breast Cancer: Final Results of the Randomized Phase III ELDA Trial. Ann. Oncol. 2015, 26, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Janni, W.; Harbeck, N.; Rack, B.; Augustin, D.; Jueckstock, J.; Wischnik, A.; Annecke, K.; Scholz, C.; Huober, J.; Zwingers, T.; et al. Randomised Phase III Trial of FEC120 vs. EC-Docetaxel in Patients with High-Risk Node-Positive Primary Breast Cancer: Final Survival Analysis of the ADEBAR Study. Br. J. Cancer 2016, 114, 863–871. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gnant, M.; Fitzal, F.; Rinnerthaler, G.; Steger, G.G.; Greil-Ressler, S.; Balic, M.; Heck, D.; Jakesz, R.; Thaler, J.; Egle, D.; et al. Duration of Adjuvant Aromatase-Inhibitor Therapy in Postmenopausal Breast Cancer. N. Engl. J. Med. 2021, 385, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.-Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espié, M.; Fumoleau, P.; Serin, D.; et al. 6 Months versus 12 Months of Adjuvant Trastuzumab for Patients with HER2−Positive Early Breast Cancer (PHARE): A Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 741–748. [Google Scholar] [CrossRef]
- Martín, M.; Ruiz, A.; Borrego, M.R.; Barnadas, A.; González, S.; Calvo, L.; Vila, M.M.; Antón, A.; Rodríguez-Lescure, A.; Seguí-Palmer, M.A.; et al. Fluorouracil, Doxorubicin, and Cyclophosphamide (FAC) Versus FAC Followed by Weekly Paclitaxel As Adjuvant Therapy for High-Risk, Node-Negative Breast Cancer: Results From the GEICAM/2003-02 Study. J. Clin. Oncol. 2013, 31, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.; Robertson, J.; Kilburn, L.; Wilcox, M.; Evans, A.; Holcombe, C.; Horgan, K.; Kirwan, C.; Mallon, E.; Sibbering, M.; et al. Long-Term Outcome and Prognostic Value of Ki67 after Perioperative Endocrine Therapy in Postmenopausal Women with Hormone-Sensitive Early Breast Cancer (POETIC): An Open-Label, Multicentre, Parallel-Group, Randomised, Phase 3 Trial. Lancet Oncol. 2020, 21, 1443–1454. [Google Scholar] [CrossRef]
- Del Mastro, L.; Levaggi, A.; Michelotti, A.; Cavazzini, G.; Adami, F.; Scotto, T.; Piras, M.; Danese, S.; Garrone, O.; Durando, A.; et al. 5-Fluorouracil, Epirubicin and Cyclophosphamide versus Epirubicin and Paclitaxel in Node-Positive Early Breast Cancer: A Phase-III Randomized GONO-MIG5 Trial. Breast Cancer Res. Treat. 2016, 155, 117–126. [Google Scholar] [CrossRef]
- Del Mastro, L.; Mansutti, M.; Bisagni, G.; Ponzone, R.; Durando, A.; Amaducci, L.; Campadelli, E.; Cognetti, F.; Frassoldati, A.; Michelotti, A.; et al. Extended Therapy with Letrozole as Adjuvant Treatment of Postmenopausal Patients with Early-Stage Breast Cancer: A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Del Mastro, L.; Poggio, F.; Blondeaux, E.; De Placido, S.; Giuliano, M.; Forestieri, V.; De Laurentiis, M.; Gravina, A.; Bisagni, G.; Rimanti, A.; et al. Fluorouracil and Dose-Dense Adjuvant Chemotherapy in Patients with Early-Stage Breast Cancer (GIM2): End-of-Study Results from a Randomised, Phase 3 Trial. Lancet Oncol. 2022, 23, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Ejlertsen, B.; Tuxen, M.K.; Jakobsen, E.H.; Jensen, M.-B.; Knoop, A.S.; Højris, I.; Ewertz, M.; Balslev, E.; Danø, H.; Vestlev, P.M.; et al. Adjuvant Cyclophosphamide and Docetaxel with or without Epirubicin for Early TOP2A-Normal Breast Cancer: DBCG 07-READ, an Open-Label, Phase III, Randomized Trial. J. Clin. Oncol. 2017, 35, 2639–2646. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Heck, D.; Menzel, C.; Jakesz, R.; Seifert, M.; Hubalek, M.; Pristauz, G.; et al. Adjuvant Endocrine Therapy plus Zoledronic Acid in Premenopausal Women with Early-Stage Breast Cancer: 62-Month Follow-up from the ABCSG-12 Randomised Trial. Lancet Oncol. 2011, 12, 631–641. [Google Scholar] [CrossRef]
- Martín, M.; Ruiz Simón, A.; Ruiz Borrego, M.; Ribelles, N.; Rodríguez-Lescure, Á.; Muñoz-Mateu, M.; González, S.; Margelí Vila, M.; Barnadas, A.; Ramos, M.; et al. Epirubicin Plus Cyclophosphamide Followed by Docetaxel Versus Epirubicin Plus Docetaxel Followed by Capecitabine As Adjuvant Therapy for Node-Positive Early Breast Cancer: Results From the GEICAM/2003-10 Study. J. Clin. Oncol. 2015, 33, 3788–3795. [Google Scholar] [CrossRef]
- Barrett-Lee, P.; Casbard, A.; Abraham, J.; Hood, K.; Coleman, R.; Simmonds, P.; Timmins, H.; Wheatley, D.; Grieve, R.; Griffiths, G.; et al. Oral Ibandronic Acid versus Intravenous Zoledronic Acid in Treatment of Bone Metastases from Breast Cancer: A Randomised, Open Label, Non-Inferiority Phase 3 Trial. Lancet Oncol. 2014, 15, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Moebus, V.; Jackisch, C.; Lueck, H.-J.; du Bois, A.; Thomssen, C.; Kurbacher, C.; Kuhn, W.; Nitz, U.; Schneeweiss, A.; Huober, J.; et al. Intense Dose-Dense Sequential Chemotherapy with Epirubicin, Paclitaxel, and Cyclophosphamide Compared with Conventionally Scheduled Chemotherapy in High-Risk Primary Breast Cancer: Mature Results of an AGO Phase III Study. J. Clin. Oncol. 2010, 28, 2874–2880. [Google Scholar] [CrossRef]
- Gogas, H.; Dafni, U.; Karina, M.; Papadimitriou, C.; Batistatou, A.; Bobos, M.; Kalofonos, H.P.; Eleftheraki, A.G.; Timotheadou, E.; Bafaloukos, D.; et al. Postoperative Dose-Dense Sequential versus Concomitant Administration of Epirubicin and Paclitaxel in Patients with Node-Positive Breast Cancer: 5-Year Results of the Hellenic Cooperative Oncology Group HE 10/00 Phase III Trial. Breast Cancer Res. Treat. 2012, 132, 609–619. [Google Scholar] [CrossRef]
- Martín, M.; Seguí, M.A.; Antón, A.; Ruiz, A.; Ramos, M.; Adrover, E.; Aranda, I.; Rodríguez-Lescure, A.; Große, R.; Calvo, L.; et al. Adjuvant Docetaxel for High-Risk, Node-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 2200–2210. [Google Scholar] [CrossRef]
- Steenbruggen, T.G.; Steggink, L.C.; Seynaeve, C.M.; van der Hoeven, J.J.M.; Hooning, M.J.; Jager, A.; Konings, I.R.; Kroep, J.R.; Smit, W.M.; Tjan-Heijnen, V.C.G.; et al. High-Dose Chemotherapy with Hematopoietic Stem Cell Transplant in Patients with High-Risk Breast Cancer and 4 or More Involved Axillary Lymph Nodes: 20-Year Follow-up of a Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 528–534. [Google Scholar] [CrossRef]
- Ruíz-Borrego, M.; Guerrero-Zotano, A.; Bermejo, B.; Ramos, M.; Cruz, J.; Baena-Cañada, J.M.; Cirauqui, B.; Rodríguez-Lescure, Á.; Alba, E.; Martínez-Jáñez, N.; et al. Phase III Evaluating the Addition of Fulvestrant (F) to Anastrozole (A) as Adjuvant Therapy in Postmenopausal Women with Hormone Receptor-Positive HER2−Negative (HR+/HER2−) Early Breast Cancer (EBC): Results from the GEICAM/2006–10 Study. Breast Cancer Res. Treat. 2019, 177, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Delbaldo, C.; Serin, D.; Mousseau, M.; Greget, S.; Audhuy, B.; Priou, F.; Berdah, J.F.; Teissier, E.; Laplaige, P.; Zelek, L.; et al. A Phase III Adjuvant Randomised Trial of 6 Cycles of 5-Fluorouracil–Epirubicine–Cyclophosphamide (FEC100) versus 4 FEC 100 Followed by 4 Taxol (FEC-T) in Node Positive Breast Cancer Patients (Trial B2000). Eur. J. Cancer 2014, 50, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Earl, H.M.; Vallier, A.-L.; Hiller, L.; Fenwick, N.; Young, J.; Iddawela, M.; Abraham, J.; Hughes-Davies, L.; Gounaris, I.; McAdam, K.; et al. Effects of the Addition of Gemcitabine, and Paclitaxel-First Sequencing, in Neoadjuvant Sequential Epirubicin, Cyclophosphamide, and Paclitaxel for Women with High-Risk Early Breast Cancer (Neo-tAnGo): An Open-Label, 2×2 Factorial Randomised Phase 3 Trial. Lancet Oncol. 2014, 15, 201–212. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Rezai, M.; Tesch, H.; Huober, J.; Gerber, B.; Zahm, D.M.; Hilfrich, J.; Costa, S.D.; Dubsky, P.; Blohmer, J.U.; et al. Zoledronate for Patients with Invasive Residual Disease after Anthracyclines-Taxane-Based Chemotherapy for Early Breast Cancer—The Phase III NeoAdjuvant Trial Add-oN (NaTaN) Study (GBG 36/ABCSG 29). Eur. J. Cancer 2016, 64, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Matikas, A.; Malamos, N.; Papakotoulas, P.; Kakolyris, S.; Boukovinas, I.; Athanasiadis, A.; Kentepozidis, N.; Ziras, N.; Katsaounis, P.; et al. Dose-Dense FEC Followed by Docetaxel versus Docetaxel plus Cyclophosphamide as Adjuvant Chemotherapy in Women with HER2−Negative, Axillary Lymph Node-Positive Early Breast Cancer: A Multicenter Randomized Study by the Hellenic Oncology Research Group (HORG). Ann. Oncol. 2016, 27, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Steger, G.G.; Greil, R.; Lang, A.; Rudas, M.; Fitzal, F.; Mlineritsch, B.; Hartmann, B.L.; Bartsch, R.; Melbinger, E.; Hubalek, M.; et al. Epirubicin and Docetaxel with or without Capecitabine as Neoadjuvant Treatment for Early Breast Cancer: Final Results of a Randomized Phase III Study (ABCSG-24). Ann. Oncol. 2014, 25, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Zielinski, C.; Ruiz-Borrego, M.; Carrasco, E.; Turner, N.; Ciruelos, E.M.; Muñoz, M.; Bermejo, B.; Margeli, M.; Anton, A.; et al. Palbociclib in Combination with Endocrine Therapy versus Capecitabine in Hormonal Receptor-Positive, Human Epidermal Growth Factor 2-Negative, Aromatase Inhibitor-Resistant Metastatic Breast Cancer: A Phase III Randomised Controlled Trial—PEARL. Ann. Oncol. 2021, 32, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant Carboplatin in Patients with Triple-Negative and HER2−Positive Early Breast Cancer (GeparSixto; GBG 66): A Randomised Phase 2 Trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Bedognetti, D.; Sertoli, M.R.; Pronzato, P.; Del Mastro, L.; Venturini, M.; Taveggia, P.; Zanardi, E.; Siffredi, G.; Pastorino, S.; Queirolo, P.; et al. Concurrent vs. Sequential Adjuvant Chemotherapy and Hormone Therapy in Breast Cancer: A Multicenter Randomized Phase III Trial. J. Natl. Cancer Inst. 2011, 103, 1529–1539. [Google Scholar] [CrossRef]
- Pérol, D.; Provençal, J.; Hardy-Bessard, A.; Coeffic, D.; Jacquin, J.-P.; Agostini, C.; Bachelot, T.; Guastalla, J.-P.; Pivot, X.; Martin, J.-P.; et al. Can Treatment with Cocculine Improve the Control of Chemotherapy-Induced Emesis in Early Breast Cancer Patients? A Randomized, Multi-Centered, Double-Blind, Placebo-Controlled Phase III Trial. BMC Cancer 2012, 12, 603. [Google Scholar] [CrossRef]
- Herrstedt, J.; Summers, Y.; Jordan, K.; von Pawel, J.; Jakobsen, A.H.; Ewertz, M.; Chan, S.; Naik, J.D.; Karthaus, M.; Dubey, S.; et al. Amisulpride Prevents Nausea and Vomiting Associated with Highly Emetogenic Chemotherapy: A Randomised, Double-Blind, Placebo-Controlled, Dose-Ranging Trial. Support. Care Cancer 2019, 27, 2699–2705. [Google Scholar] [CrossRef]
- Gennari, A.; Sun, Z.; Hasler-Strub, U.; Colleoni, M.; Kennedy, M.J.; Moos, R.V.; Cortés, J.; Vidal, M.J.; Hennessy, B.; Walshe, J.; et al. A Randomized Phase II Study Evaluating Different Maintenance Schedules of Nab-Paclitaxel in the First-Line Treatment of Metastatic Breast Cancer: Final Results of the IBCSG 42-12/BIG 2-12 SNAP Trial. Ann. Oncol. 2018, 29, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Bundred, N.; Porta, N.; Brunt, A.M.; Cramer, A.; Hanby, A.; Shaaban, A.M.; Rakha, E.A.; Armstrong, A.; Cutress, R.I.; Dodwell, D.; et al. Combined Perioperative Lapatinib and Trastuzumab in Early HER2−Positive Breast Cancer Identifies Early Responders: Randomized UK EPHOS-B Trial Long-Term Results. Clin. Cancer Res. 2022, 28, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Del Mastro, L.; Boni, L.; Michelotti, A.; Gamucci, T.; Olmeo, N.; Gori, S.; Giordano, M.; Garrone, O.; Pronzato, P.; Bighin, C.; et al. Effect of the Gonadotropin-Releasing Hormone Analogue Triptorelin on the Occurrence of Chemotherapy-Induced Early Menopause in Premenopausal Women with Breast Cancer: A Randomized Trial. JAMA 2011, 306, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Gladkov, O.; Moiseyenko, V.; Bondarenko, I.N.; Shparyk, Y.; Barash, S.; Adar, L.; Avisar, N. A Phase III Study of Balugrastim Versus Pegfilgrastim in Breast Cancer Patients Receiving Chemotherapy with Doxorubicin and Docetaxel. Oncologist 2016, 21, 7–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schneeweiss, A.; Marmé, F.; Ruiz, A.; Manikhas, A.G.; Bottini, A.; Wolf, M.; Sinn, H.-P.; Mansouri, K.; Kennedy, L.; Bauknecht, T. A Randomized Phase II Trial of Doxorubicin plus Pemetrexed Followed by Docetaxel versus Doxorubicin plus Cyclophosphamide Followed by Docetaxel as Neoadjuvant Treatment of Early Breast Cancer. Ann. Oncol. 2011, 22, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.F.; Semiglazov, V.F.; Tjulandin, S.; Bentsion, D.; Chan, S.; Challand, R. A Phase III Randomized Equivalence Study of Biosimilar Filgrastim versus Amgen Filgrastim in Patients Receiving Myelosuppressive Chemotherapy for Breast Cancer. Oncol. Res. Treat. 2010, 33, 504–511. [Google Scholar] [CrossRef]
- Tesch, H.; Stoetzer, O.; Decker, T.; Kurbacher, C.M.; Marmé, F.; Schneeweiss, A.; Mundhenke, C.; Distelrath, A.; Fasching, P.A.; Lux, M.P.; et al. Efficacy and Safety of Everolimus plus Exemestane in Postmenopausal Women with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Locally Advanced or Metastatic Breast Cancer: Results of the Single-Arm, Phase IIIB 4EVER Trial. Int. J. Cancer 2019, 144, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Hatschek, T.; Carlsson, L.; Einbeigi, Z.; Lidbrink, E.; Linderholm, B.; Lindh, B.; Loman, N.; Malmberg, M.; Rotstein, S.; Söderberg, M.; et al. Individually Tailored Treatment with Epirubicin and Paclitaxel with or without Capecitabine as First-Line Chemotherapy in Metastatic Breast Cancer: A Randomized Multicenter Trial. Breast Cancer Res. Treat. 2012, 131, 939–947. [Google Scholar] [CrossRef][Green Version]
- Zambetti, M.; Mansutti, M.; Gomez, P.; Lluch, A.; Dittrich, C.; Zamagni, C.; Ciruelos, E.; Pavesi, L.; Semiglazov, V.; De Benedictis, E.; et al. Pathological Complete Response Rates Following Different Neoadjuvant Chemotherapy Regimens for Operable Breast Cancer According to ER Status, in Two Parallel, Randomized Phase II Trials with an Adaptive Study Design (ECTO II). Breast Cancer Res. Treat. 2012, 132, 843–851. [Google Scholar] [CrossRef]
- Bartsch, R.; Singer, C.F.; Pfeiler, G.; Hubalek, M.; Stoeger, H.; Pichler, A.; Petru, E.; Bjelic-Radisic, V.; Greil, R.; Rudas, M.; et al. Conventional versus Reverse Sequence of Neoadjuvant Epirubicin/Cyclophosphamide and Docetaxel: Sequencing Results from ABCSG-34. Br. J. Cancer 2021, 124, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.W.; de Groot, S.M.; Honkoop, A.H.; Jager, A.; ten Tije, A.J.; Bos, M.M.E.M.; Linn, S.C.; van den Bosch, J.; Kroep, J.R.; Braun, J.J.; et al. Paclitaxel and Bevacizumab with or without Capecitabine as First-Line Treatment for HER2−Negative Locally Recurrent or Metastatic Breast Cancer: A Multicentre, Open-Label, Randomised Phase 2 Trial. Eur. J. Cancer 2014, 50, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Mariani, G.; Galli, G.; Cavalieri, S.; Valagussa, P.; Bianchi, G.V.; Capri, G.; Cresta, S.; Ferrari, L.; Damian, S.; Duca, M.; et al. Single Institution Trial of Anthracycline- and Taxane-Based Chemotherapy for Operable Breast Cancer: The ASTER Study. Breast J. 2019, 25, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Kümmel, S.; Paepke, S.; Huober, J.; Schem, C.; Untch, M.; Blohmer, J.U.; Eiermann, W.; Gerber, B.; Hanusch, C.; Hilfrich, J.; et al. Randomised, Open-Label, Phase II Study Comparing the Efficacy and the Safety of Cabazitaxel versus Weekly Paclitaxel given as Neoadjuvant Treatment in Patients with Operable Triple-Negative or Luminal B/HER2−Negative Breast Cancer (GENEVIEVE). Eur. J. Cancer 2017, 84, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Manikhas, A.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Vazquez, F.; Byakhow, M.; Lichinitser, M.; et al. Neoadjuvant Chemotherapy with Trastuzumab Followed by Adjuvant Trastuzumab versus Neoadjuvant Chemotherapy Alone, in Patients with HER2−Positive Locally Advanced Breast Cancer (the NOAH Trial): A Randomised Controlled Superiority Trial with a Parallel HER2−Negative Cohort. Lancet 2010, 375, 377–384. [Google Scholar] [CrossRef]
- Nielsen, D.L.; Bjerre, K.D.; Jakobsen, E.H.; Cold, S.; Stenbygaard, L.; Sørensen, P.G.; Kamby, C.; Møller, S.; Jørgensen, C.L.T.; Andersson, M. Gemcitabine Plus Docetaxel Versus Docetaxel in Patients with Predominantly Human Epidermal Growth Factor Receptor 2–Negative Locally Advanced or Metastatic Breast Cancer: A Randomized, Phase III Study by the Danish Breast Cancer Cooperative Group. J. Clin. Oncol. 2011, 29, 4748–4754. [Google Scholar] [CrossRef]
- Pierga, J.-Y.; Delaloge, S.; Espié, M.; Brain, E.; Sigal-Zafrani, B.; Mathieu, M.-C.; Bertheau, P.; Guinebretière, J.M.; Spielmann, M.; Savignoni, A.; et al. A Multicenter Randomized Phase II Study of Sequential Epirubicin/Cyclophosphamide Followed by Docetaxel with or without Celecoxib or Trastuzumab According to HER2 Status, as Primary Chemotherapy for Localized Invasive Breast Cancer Patients. Breast Cancer Res. Treat. 2010, 122, 429–437. [Google Scholar] [CrossRef]
- Sirohi, B.; A’Hern, R.; Coombes, G.; Bliss, J.M.; Hickish, T.; Perren, T.; Crawford, M.; O’Brien, M.; Iveson, T.; Ebbs, S.; et al. A Randomised Comparative Trial of Infusional ECisF versus Conventional FEC as Adjuvant Chemotherapy in Early Breast Cancer: The TRAFIC Trial. Ann. Oncol. 2010, 21, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.; Loibl, S.; von Minckwitz, G.; Morales, S.; Martinez, N.; Guerrero, A.; Anton, A.; Aktas, B.; Schoenegg, W.; Muñoz, M.; et al. Phase III Trial Evaluating the Addition of Bevacizumab to Endocrine Therapy As First-Line Treatment for Advanced Breast Cancer: The Letrozole/Fulvestrant and Avastin (LEA) Study. J. Clin. Oncol. 2015, 33, 1045–1052. [Google Scholar] [CrossRef]
- Harbeck, N.; Gluz, O.; Christgen, M.; Kates, R.E.; Braun, M.; Küemmel, S.; Schumacher, C.; Potenberg, J.; Kraemer, S.; Kleine-Tebbe, A.; et al. De-Escalation Strategies in Human Epidermal Growth Factor Receptor 2 (HER2)–Positive Early Breast Cancer (BC): Final Analysis of the West German Study Group Adjuvant Dynamic Marker-Adjusted Personalized Therapy Trial Optimizing Risk Assessment and Therapy Response Prediction in Early BC HER2− and Hormone Receptor–Positive Phase II Randomized Trial—Efficacy, Safety, and Predictive Markers for 12 Weeks of Neoadjuvant Trastuzumab Emtansine with or without Endocrine Therapy (ET) Versus Trastuzumab Plus ET. J. Clin. Oncol. 2017, 35, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Volovat, C.; Gladkov, O.A.; Bondarenko, I.M.; Barash, S.; Buchner, A.; Bias, P.; Adar, L.; Avisar, N. Efficacy and Safety of Balugrastim Compared with Pegfilgrastim in Patients with Breast Cancer Receiving Chemotherapy. Clin. Breast Cancer 2014, 14, 101–108. [Google Scholar] [CrossRef]
- Claessens, A.K.M.; Bos, M.E.M.M.; Lopez-Yurda, M.; Bouma, J.M.; Rademaker-Lakhai, J.M.; Honkoop, A.H.; de Graaf, H.; van Druten, E.; van Warmerdam, L.J.C.; van der Sangen, M.J.C.; et al. Intermittent versus Continuous First-Line Treatment for HER2−Negative Metastatic Breast Cancer: The Stop & Go Study of the Dutch Breast Cancer Research Group (BOOG). Breast Cancer Res. Treat. 2018, 172, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Amadori, D.; Aglietta, M.; Alessi, B.; Gianni, L.; Ibrahim, T.; Farina, G.; Gaion, F.; Bertoldo, F.; Santini, D.; Rondena, R.; et al. Efficacy and Safety of 12-Weekly versus 4-Weekly Zoledronic Acid for Prolonged Treatment of Patients with Bone Metastases from Breast Cancer (ZOOM): A Phase 3, Open-Label, Randomised, Non-Inferiority Trial. Lancet Oncol. 2013, 14, 663–670. [Google Scholar] [CrossRef]
- van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentjé, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant Chemotherapy with or without Anthracyclines in the Presence of Dual HER2 Blockade for HER2−Positive Breast Cancer (TRAIN-2): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Saloustros, E.; Malamos, N.; Kakolyris, S.; Boukovinas, I.; Papakotoulas, P.; Kentepozidis, N.; Ziras, N.; Georgoulias, V. Six versus 12 Months of Adjuvant Trastuzumab in Combination with Dose-Dense Chemotherapy for Women with HER2−Positive Breast Cancer: A Multicenter Randomized Study by the Hellenic Oncology Research Group (HORG). Ann. Oncol. 2015, 26, 1333–1340. [Google Scholar] [CrossRef]
- Llombart-Cussac, A.; Pérez-García, J.M.; Bellet, M.; Dalenc, F.; Gil-Gil, M.; Ruíz-Borrego, M.; Gavilá, J.; Sampayo-Cordero, M.; Aguirre, E.; Schmid, P.; et al. Fulvestrant-Palbociclib vs. Letrozole-Palbociclib as Initial Therapy for Endocrine-Sensitive, Hormone Receptor–Positive, ERBB2-Negative Advanced Breast Cancer. JAMA Oncol. 2021, 7, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Boccardo, F.; Guglielmini, P.; Parodi, A.; Rubagotti, A. Chemotherapy versus Tamoxifen versus Chemotherapy plus Tamoxifen in Node-Positive, Oestrogen Receptor-Positive Breast Cancer Patients. Very Late Results of the ‘Gruppo Di Ricerca per La Chemio-Ormonoterapia Adiuvante (GROCTA)’ 01-Trial in Early Breast Cancer. Breast Cancer Res. Treat. 2011, 126, 653–661. [Google Scholar] [CrossRef]
- De Laurentiis, M.; Caputo, R.; Mazza, M.; Mansutti, M.; Masetti, R.; Ballatore, Z.; Torrisi, R.; Michelotti, A.; Zambelli, A.; Ferro, A.; et al. Safety and Efficacy of Ribociclib in Combination with Letrozole in Patients with HR+, HER2− Advanced Breast Cancer: Results from the Italian Subpopulation of Phase 3b CompLEEment-1 Study. Target. Oncol. 2022, 17, 615–625. [Google Scholar] [CrossRef]
- Lang, I.; Brodowicz, T.; Ryvo, L.; Kahan, Z.; Greil, R.; Beslija, S.; Stemmer, S.M.; Kaufman, B.; Zvirbule, Z.; Steger, G.G.; et al. Bevacizumab plus Paclitaxel versus Bevacizumab plus Capecitabine as First-Line Treatment for HER2−Negative Metastatic Breast Cancer: Interim Efficacy Results of the Randomised, Open-Label, Non-Inferiority, Phase 3 TURANDOT Trial. Lancet Oncol. 2013, 14, 125–133. [Google Scholar] [CrossRef]
- Ekholm, M.; Bendahl, P.-O.; Fernö, M.; Nordenskjöld, B.; Stål, O.; Rydén, L. Two Years of Adjuvant Tamoxifen Provides a Survival Benefit Compared with No Systemic Treatment in Premenopausal Patients with Primary Breast Cancer: Long-Term Follow-Up (>25 Years) of the Phase III SBII:2pre Trial. J. Clin. Oncol. 2016, 34, 2232–2238. [Google Scholar] [CrossRef]
- Lück, H.-J.; Du Bois, A.; Loibl, S.; Schrader, I.; Huober, J.; Heilmann, V.; Beckmann, M.; Stähler, A.; Jackisch, C.; Hubalek, M.; et al. Capecitabine plus Paclitaxel versus Epirubicin plus Paclitaxel as First-Line Treatment for Metastatic Breast Cancer: Efficacy and Safety Results of a Randomized, Phase III Trial by the AGO Breast Cancer Study Group. Breast Cancer Res. Treat. 2013, 139, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Welt, A.; Marschner, N.; Lerchenmueller, C.; Decker, T.; Steffens, C.-C.; Koehler, A.; Depenbusch, R.; Busies, S.; Hegewisch-Becker, S. Capecitabine and Bevacizumab with or without Vinorelbine in First-Line Treatment of HER2/Neu-Negative Metastatic or Locally Advanced Breast Cancer: Final Efficacy and Safety Data of the Randomised, Open-Label Superiority Phase 3 CARIN Trial. Breast Cancer Res. Treat. 2016, 156, 97–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mavroudis, D.; Saloustros, E.; Boukovinas, I.; Papakotoulas, P.; Kakolyris, S.; Ziras, N.; Christophylakis, C.; Kentepozidis, N.; Fountzilas, G.; Rigas, G.; et al. Sequential vs. Concurrent Epirubicin and Docetaxel as Adjuvant Chemotherapy for High-Risk, Node-Negative, Early Breast Cancer: An Interim Analysis of a Randomised Phase III Study from the Hellenic Oncology Research Group. Br. J. Cancer 2017, 117, 164–170. [Google Scholar] [CrossRef] [PubMed]
- van Rossum, A.G.J.; Kok, M.; van Werkhoven, E.; Opdam, M.; Mandjes, I.A.M.; van Leeuwen-Stok, A.E.; van Tinteren, H.; Imholz, A.L.T.; Portielje, J.E.A.; Bos, M.M.E.M.; et al. Adjuvant Dose-Dense Doxorubicin-Cyclophosphamide versus Docetaxel-Doxorubicin-Cyclophosphamide for High-Risk Breast Cancer: First Results of the Randomised MATADOR Trial (BOOG 2004-04). Eur. J. Cancer 2018, 102, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Untch, M.; von Minckwitz, G.; Konecny, G.E.; Conrad, U.; Fett, W.; Kurzeder, C.; Lück, H.-J.; Stickeler, E.; Urbaczyk, H.; Liedtke, B.; et al. PREPARE Trial: A Randomized Phase III Trial Comparing Preoperative, Dose-Dense, Dose-Intensified Chemotherapy with Epirubicin, Paclitaxel, and CMF versus a Standard-Dosed Epirubicin–Cyclophosphamide Followed by Paclitaxel with or without Darbepoetin Alfa in Primary Breast Cancer—Outcome on Prognosis. Ann. Oncol. 2011, 22, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Vici, P.; Brandi, M.; Giotta, F.; Foggi, P.; Schittulli, F.; Di Lauro, L.; Gebbia, N.; Massidda, B.; Filippelli, G.; Giannarelli, D.; et al. A Multicenter Phase III Prospective Randomized Trial of High-Dose Epirubicin in Combination with Cyclophosphamide (EC) versus Docetaxel Followed by EC in Node-Positive Breast Cancer. GOIM (Gruppo Oncologico Italia Meridionale) 9902 Study. Ann. Oncol. 2012, 23, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Coombes, R.C.; Kilburn, L.S.; Tubiana-Mathieu, N.; Olmos, T.; Van Bochove, A.; Perez-Lopez, F.R.; Palmieri, C.; Stebbing, J.; Bliss, J.M. Epirubicin Dose and Sequential Hormonal Therapy—Mature Results of the HMFEC Randomised Phase III Trial in Premenopausal Patients with Node Positive Early Breast Cancer. Eur. J. Cancer 2016, 60, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Earl, H.M.; Hiller, L.; Dunn, J.A.; Blenkinsop, C.; Grybowicz, L.; Vallier, A.-L.; Abraham, J.; Thomas, J.; Provenzano, E.; Hughes-Davies, L.; et al. Efficacy of Neoadjuvant Bevacizumab Added to Docetaxel Followed by Fluorouracil, Epirubicin, and Cyclophosphamide, for Women with HER2−Negative Early Breast Cancer (ARTemis): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2015, 16, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Coombes, R.C.; Bliss, J.M.; Espie, M.; Erdkamp, F.; Wals, J.; Tres, A.; Marty, M.; Coleman, R.E.; Tubiana-Mathieu, N.; den Boer, M.O.; et al. Randomized, Phase III Trial of Sequential Epirubicin and Docetaxel Versus Epirubicin Alone in Postmenopausal Patients with Node-Positive Breast Cancer. J. Clin. Oncol. 2011, 29, 3247–3254. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Möbus, V.; Tesch, H.; Hanusch, C.; Denkert, C.; Lübbe, K.; Huober, J.; Klare, P.; Kümmel, S.; Untch, M.; et al. Intense Dose-Dense Epirubicin, Paclitaxel, Cyclophosphamide versus Weekly Paclitaxel, Liposomal Doxorubicin (plus Carboplatin in Triple-Negative Breast Cancer) for Neoadjuvant Treatment of High-Risk Early Breast Cancer (GeparOcto-GBG 84): A Randomised Phase III Trial. Eur. J. Cancer 2019, 106, 181–192. [Google Scholar] [CrossRef]
- Fountzilas, G.; Dafni, U.; Papadimitriou, C.; Timotheadou, E.; Gogas, H.; Eleftheraki, A.G.; Xanthakis, I.; Christodoulou, C.; Koutras, A.; Papandreou, C.N.; et al. Dose-Dense Sequential Adjuvant Chemotherapy Followed, as Indicated, by Trastuzumab for One Year in Patients with Early Breast Cancer: First Report at 5-Year Median Follow-up of a Hellenic Cooperative Oncology Group Randomized Phase III Trial. BMC Cancer 2014, 14, 515. [Google Scholar] [CrossRef]
- Del Mastro, L.; De Placido, S.; Bruzzi, P.; De Laurentiis, M.; Boni, C.; Cavazzini, G.; Durando, A.; Turletti, A.; Nisticò, C.; Valle, E.; et al. Fluorouracil and Dose-Dense Chemotherapy in Adjuvant Treatment of Patients with Early-Stage Breast Cancer: An Open-Label, 2 × 2 Factorial, Randomised Phase 3 Trial. Lancet 2015, 385, 1863–1872. [Google Scholar] [CrossRef]
- Perrone, F.; De Laurentiis, M.; De Placido, S.; Orditura, M.; Cinieri, S.; Riccardi, F.; Ribecco, A.S.; Putzu, C.; Del Mastro, L.; Rossi, E.; et al. Adjuvant Zoledronic Acid and Letrozole plus Ovarian Function Suppression in Premenopausal Breast Cancer: HOBOE Phase 3 Randomised Trial. Eur. J. Cancer 2019, 118, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Amadori, D.; Silvestrini, R.; De Lena, M.; Boccardo, F.; Rocca, A.; Scarpi, E.; Schittulli, F.; Brandi, M.; Maltoni, R.; Serra, P.; et al. Randomized Phase III Trial of Adjuvant Epirubicin Followed by Cyclophosphamide, Methotrexate, and 5-Fluorouracil (CMF) versus CMF Followed by Epirubicin in Patients with Node-Negative or 1–3 Node-Positive Rapidly Proliferating Breast Cancer. Breast Cancer Res. Treat. 2011, 125, 775–784. [Google Scholar] [CrossRef]
- Bidard, F.-C.; Hardy-Bessard, A.-C.; Dalenc, F.; Bachelot, T.; Pierga, J.-Y.; de la Motte Rouge, T.; Sabatier, R.; Dubot, C.; Frenel, J.-S.; Ferrero, J.M.; et al. Switch to Fulvestrant and Palbociclib versus No Switch in Advanced Breast Cancer with Rising ESR1 Mutation during Aromatase Inhibitor and Palbociclib Therapy (PADA-1): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Ejlertsen, B.; Mouridsen, H.T.; Jensen, M.-B.; Andersen, J.; Andersson, M.; Kamby, C.; Knoop, A.S.; Danish Breast Cancer Cooperative Group. Cyclophosphamide, Methotrexate, and Fluorouracil; Oral Cyclophosphamide; Levamisole; or No Adjuvant Therapy for Patients with High-Risk, Premenopausal Breast Cancer. Cancer 2010, 116, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Untch, M.; Jackisch, C.; Schneeweiss, A.; Conrad, B.; Aktas, B.; Denkert, C.; Eidtmann, H.; Wiebringhaus, H.; Kümmel, S.; Hilfrich, J.; et al. Nab-Paclitaxel versus Solvent-Based Paclitaxel in Neoadjuvant Chemotherapy for Early Breast Cancer (GeparSepto—GBG 69): A Randomised, Phase 3 Trial. Lancet Oncol. 2016, 17, 345–356. [Google Scholar] [CrossRef]
- Conte, P.; Frassoldati, A.; Bisagni, G.; Brandes, A.A.; Donadio, M.; Garrone, O.; Piacentini, F.; Cavanna, L.; Giotta, F.; Aieta, M.; et al. Nine Weeks versus 1 Year Adjuvant Trastuzumab in Combination with Chemotherapy: Final Results of the Phase III Randomized Short-HER Study‡. Ann. Oncol. 2018, 29, 2328–2333. [Google Scholar] [CrossRef]
- Möbus, V.; Jackisch, C.; Lück, H.J.; du Bois, A.; Thomssen, C.; Kuhn, W.; Nitz, U.; Schneeweiss, A.; Huober, J.; Harbeck, N.; et al. Ten-Year Results of Intense Dose-Dense Chemotherapy Show Superior Survival Compared with a Conventional Schedule in High-Risk Primary Breast Cancer: Final Results of AGO Phase III iddEPC Trial. Ann. Oncol. 2018, 29, 178–185. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Rezai, M.; Loibl, S.; Fasching, P.A.; Huober, J.; Tesch, H.; Bauerfeind, I.; Hilfrich, J.; Eidtmann, H.; Gerber, B.; et al. Capecitabine in Addition to Anthracycline- and Taxane-Based Neoadjuvant Treatment in Patients with Primary Breast Cancer: Phase III GeparQuattro Study. J. Clin. Oncol. 2010, 28, 2015–2023. [Google Scholar] [CrossRef]
- Ejlertsen, B.; Jensen, M.-B.; Elversang, J.; Rasmussen, B.B.; Andersson, M.; Andersen, J.; Nielsen, D.L.; Cold, S.; Mouridsen, H.T. One Year of Adjuvant Tamoxifen Compared with Chemotherapy and Tamoxifen in Postmenopausal Patients with Stage II Breast Cancer. Eur. J. Cancer 2013, 49, 2986–2994. [Google Scholar] [CrossRef] [PubMed]
- Kerbrat, P.; Desmoulins, I.; Roca, L.; Levy, C.; Lortholary, A.; Marre, A.; Delva, R.; Rios, M.; Viens, P.; Brain, É.; et al. Optimal Duration of Adjuvant Chemotherapy for High-Risk Node-Negative (N-) Breast Cancer Patients: 6-Year Results of the Prospective Randomised Multicentre Phase III UNICANCER-PACS 05 Trial (UCBG-0106). Eur. J. Cancer 2017, 79, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Tjan-Heijnen, V.C.G.; van Hellemond, I.E.G.; Peer, P.G.M.; Swinkels, A.C.P.; Smorenburg, C.H.; van der Sangen, M.J.C.; Kroep, J.R.; De Graaf, H.; Honkoop, A.H.; Erdkamp, F.L.G.; et al. Extended Adjuvant Aromatase Inhibition after Sequential Endocrine Therapy (DATA): A Randomised, Phase 3 Trial. Lancet Oncol. 2017, 18, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Schramm, A.; Schochter, F.; Friedl, T.W.P.; de Gregorio, N.; Andergassen, U.; Alunni-Fabbroni, M.; Trapp, E.; Jaeger, B.; Heinrich, G.; Camara, O.; et al. Prevalence of Circulating Tumor Cells After Adjuvant Chemotherapy with or without Anthracyclines in Patients with HER2−Negative, Hormone Receptor-Positive Early Breast Cancer. Clin. Breast Cancer 2017, 17, 279–285. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Eidtmann, H.; Rezai, M.; Fasching, P.A.; Tesch, H.; Eggemann, H.; Schrader, I.; Kittel, K.; Hanusch, C.; Kreienberg, R.; et al. Neoadjuvant Chemotherapy and Bevacizumab for HER2−Negative Breast Cancer. N. Engl. J. Med. 2012, 366, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Nitz, U.; Gluz, O.; Huober, J.; Kreipe, H.H.; Kates, R.E.; Hartmann, A.; Erber, R.; Scholz, M.; Lisboa, B.; Mohrmann, S.; et al. Final Analysis of the Prospective WSG-AGO EC-Doc versus FEC Phase III Trial in Intermediate-Risk (pN1) Early Breast Cancer: Efficacy and Predictive Value of Ki67 Expression†. Ann. Oncol. 2014, 25, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Coudert, B.; Asselain, B.; Campone, M.; Spielmann, M.; Machiels, J.-P.; Pénault-Llorca, F.; Serin, D.; Lévy, C.; Romieu, G.; Canon, J.-L.; et al. Extended Benefit from Sequential Administration of Docetaxel after Standard Fluorouracil, Epirubicin, and Cyclophosphamide Regimen for Node-Positive Breast Cancer: The 8-Year Follow-Up Results of the UNICANCER-PACS01 Trial. Oncologist 2012, 17, 900–909. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foukakis, T.; von Minckwitz, G.; Bengtsson, N.-O.; Brandberg, Y.; Wallberg, B.; Fornander, T.; Mlineritsch, B.; Schmatloch, S.; Singer, C.F.; Steger, G.; et al. Effect of Tailored Dose-Dense Chemotherapy vs. Standard 3-Weekly Adjuvant Chemotherapy on Recurrence-Free Survival Among Women with High-Risk Early Breast Cancer: A Randomized Clinical Trial. JAMA 2016, 316, 1888–1896. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jerusalem, G.; Mariani, G.; Ciruelos, E.M.; Martin, M.; Tjan-Heijnen, V.C.G.; Neven, P.; Gavila, J.G.; Michelotti, A.; Montemurro, F.; Generali, D.; et al. Safety of Everolimus plus Exemestane in Patients with Hormone-Receptor–Positive, HER2–Negative Locally Advanced or Metastatic Breast Cancer Progressing on Prior Non-Steroidal Aromatase Inhibitors: Primary Results of a Phase IIIb, Open-Label, Single-Arm, Expanded-Access Multicenter Trial (BALLET). Ann. Oncol. 2016, 27, 1719–1725. [Google Scholar] [CrossRef]
- Fernando, I.N.; Bowden, S.J.; Herring, K.; Brookes, C.L.; Ahmed, I.; Marshall, A.; Grieve, R.; Churn, M.; Spooner, D.; Latief, T.N.; et al. Synchronous versus Sequential Chemo-Radiotherapy in Patients with Early Stage Breast Cancer (SECRAB): A Randomised, Phase III, Trial. Radiother. Oncol. 2020, 142, 52–61. [Google Scholar] [CrossRef]
- Nitz, U.; Gluz, O.; Clemens, M.; Malter, W.; Reimer, T.; Nuding, B.; Aktas, B.; Stefek, A.; Pollmanns, A.; Lorenz-Salehi, F.; et al. West German Study PlanB Trial: Adjuvant Four Cycles of Epirubicin and Cyclophosphamide Plus Docetaxel Versus Six Cycles of Docetaxel and Cyclophosphamide in HER2−Negative Early Breast Cancer. J. Clin. Oncol. 2019, 37, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Coombes, R.C.; Tovey, H.; Kilburn, L.; Mansi, J.; Palmieri, C.; Bartlett, J.; Hicks, J.; Makris, A.; Evans, A.; Loibl, S.; et al. Effect of Celecoxib vs. Placebo as Adjuvant Therapy on Disease-Free Survival Among Patients with Breast Cancer: The REACT Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1291–1301. [Google Scholar] [CrossRef]
- Möbus, V.; Lück, H.-J.; Ladda, E.; Klare, P.; Schmidt, M.; Schneeweiss, A.; Grischke, E.-M.; Wachsmann, G.; Forstbauer, H.; Untch, M.; et al. Phase III Randomised Trial Comparing Intense Dose-Dense Chemotherapy to Tailored Dose-Dense Chemotherapy in High-Risk Early Breast Cancer (GAIN-2). Eur. J. Cancer 2021, 156, 138–148. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Möbus, V.; Schneeweiss, A.; Huober, J.; Thomssen, C.; Untch, M.; Jackisch, C.; Diel, I.J.; Elling, D.; Conrad, B.; et al. German Adjuvant Intergroup Node-Positive Study: A Phase III Trial to Compare Oral Ibandronate Versus Observation in Patients with High-Risk Early Breast Cancer. J. Clin. Oncol. 2013, 31, 3531–3539. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant Denosumab in Breast Cancer (ABCSG-18): A Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef] [PubMed]
- De Placido, S.; Gallo, C.; De Laurentiis, M.; Bisagni, G.; Arpino, G.; Sarobba, M.G.; Riccardi, F.; Russo, A.; Del Mastro, L.; Cogoni, A.A.; et al. Adjuvant Anastrozole versus Exemestane versus Letrozole, Upfront or after 2 Years of Tamoxifen, in Endocrine-Sensitive Breast Cancer (FATA-GIM3): A Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 474–485. [Google Scholar] [CrossRef] [PubMed]
- de Gregorio, A.; Häberle, L.; Fasching, P.A.; Müller, V.; Schrader, I.; Lorenz, R.; Forstbauer, H.; Friedl, T.W.P.; Bauer, E.; de Gregorio, N.; et al. Gemcitabine as Adjuvant Chemotherapy in Patients with High-Risk Early Breast Cancer—Results from the Randomized Phase III SUCCESS-A Trial. Breast Cancer Res. 2020, 22, 111. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Vallier, A.-L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Harnett, A.N.; Ah-See, M.-L.; Simcock, R.; Rea, D.; et al. 6 versus 12 Months of Adjuvant Trastuzumab for HER2−Positive Early Breast Cancer (PERSEPHONE): 4-Year Disease-Free Survival Results of a Randomised Phase 3 Non-Inferiority Trial. Lancet 2019, 393, 2599–2612. [Google Scholar] [CrossRef]
- GOV.UK Ethnicity Facts and Figures Population of England and Wales. Available online: https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/national-and-regional-populations/population-of-england-and-wales/latest/ (accessed on 2 January 2024).
- Statistiche Demografiche Popolazione Italia (2001–2022) Grafici Su Dati ISTAT. Available online: https://www.tuttitalia.it/statistiche/popolazione-andamento-demografico/ (accessed on 2 January 2024).
- Statistiche Demografiche Cittadini Stranieri in Italia—2022. Available online: https://www.tuttitalia.it/statistiche/cittadini-stranieri-2022/ (accessed on 2 January 2024).
- Turner, B.E.; Steinberg, J.R.; Weeks, B.T.; Rodriguez, F.; Cullen, M.R. Race/Ethnicity Reporting and Representation in US Clinical Trials: A Cohort Study. Lancet Reg. Health-Am. 2022, 11, 100252. [Google Scholar] [CrossRef]
- Lee, L.K.; Narang, C.; Rees, C.A.; Thiagarajan, R.R.; Melvin, P.; Ward, V.; Bourgeois, F.T. Reporting and Representation of Participant Race and Ethnicity in National Institutes of Health–Funded Pediatric Clinical Trials. JAMA Netw. Open 2023, 6, e2331316. [Google Scholar] [CrossRef]
- Fain, K.M.; Nelson, J.; Tse, T.; Williams, R.J. Race and Ethnicity Reporting for Clinical Trials in ClinicalTrials.Gov and Publications. Contemp. Clin. Trials 2021, 101, 106237. [Google Scholar] [CrossRef] [PubMed]
- Nanavati, H.D.; Andrabi, M.; Arevalo, Y.A.; Liu, E.; Shen, J.; Lin, C. Disparities in Race and Ethnicity Reporting and Representation for Clinical Trials in Stroke: 2010 to 2020. J. Am. Heart Assoc. 2024, 13, e033467. [Google Scholar] [CrossRef] [PubMed]
- Candelario, N.M.; Major, J.; Dreyfus, B.; Sattler, D.; Paulucci, D.; Misra, S.; Micsinai, M.; Kuri, L. Diversity in Clinical Trials in Europe and the USA: A Review of a Pharmaceutical Company’s Data Collection, Reporting, and Interpretation of Race and Ethnicity. Ann. Oncol. 2023, 34, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- US Census Bureau about the Topic of Race. Available online: https://www.census.gov/topics/population/race/about.html (accessed on 2 January 2024).
- GOV.UK Ethnicity Facts and Figures List of Ethnic Groups. Available online: https://www.ethnicity-facts-figures.service.gov.uk/style-guide/ethnic-groups/ (accessed on 2 January 2024).
- Missouri Census Data Center All about Race and Ethnicity in the Census—MCDC. Available online: https://mcdc.missouri.edu/help/race-ethnicity.html (accessed on 2 January 2024).
- Mulinari, S.; Bredström, A. Race in Clinical Trials in Sweden: How Regulatory and Medical Standards in Clinical Research Trump the Post-Racial Discourse. Sociol. Health Illn. 2024, 46, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, C.; Browne, A.J.; Wong, S.; Smye, V.L. Harms and Benefits: Collecting Ethnicity Data in a Clinical Context. Soc. Sci. Med. 2009, 68, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- European Comission Roma Equality, Inclusion and Participation in the EU. Available online: https://commission.europa.eu/strategy-and-policy/policies/justice-and-fundamental-rights/combatting-discrimination/roma-eu/roma-equality-inclusion-and-participation-eu_en (accessed on 2 January 2024).
- Albain, K.S.; Unger, J.M.; Crowley, J.J.; Coltman, C.A.; Hershman, D.L. Racial Disparities in Cancer Survival Among Randomized Clinical Trials Patients of the Southwest Oncology Group. J. Natl. Cancer Inst. 2009, 101, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.P.; Shen, F.; Jiang, G.; O’Neill, A.; Radovich, M.; Li, L.; Gardner, L.; Lai, D.; Foroud, T.; Sparano, J.A.; et al. Impact of Genetic Ancestry on Outcomes in ECOG-ACRIN-5103. JCO Precis. Oncol. 2017, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.; Moy, B.; Mroz, E.A.; Ross, K.; Niemierko, A.; Rocco, J.W.; Isakoff, S.; Ellisen, L.W.; Bardia, A. Comparison of the Genomic Landscape Between Primary Breast Cancer in African American Versus White Women and the Association of Racial Differences with Tumor Recurrence. J. Clin. Oncol. 2015, 33, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S., Jr.; Lara, P.N.; Dang, J.H.T.; Paterniti, D.A.; Kelly, K. Twenty Years Post-NIH Revitalization Act: Enhancing Minority Participation in Clinical Trials (EMPaCT): Laying the Groundwork for Improving Minority Clinical Trial Accrual. Cancer 2014, 120, 1091–1096. [Google Scholar] [CrossRef]
- Hernandez, N.D.; Durant, R.; Lisovicz, N.; Nweke, C.; Belizaire, C.; Cooper, D.; Soiro, F.; Rivers, D.; Sodeke, S.; Rivers, B.M. African American Cancer Survivors’ Perspectives on Cancer Clinical Trial Participation in a Safety-Net Hospital: Considering the Role of the Social Determinants of Health. J. Cancer Educ. 2022, 37, 1589–1597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bania, A.; Adamou, A.; Saloustros, E. Racial and Ethnic Disparities in European Breast Cancer Clinical Trials. Cancers 2024, 16, 1726. https://doi.org/10.3390/cancers16091726
Bania A, Adamou A, Saloustros E. Racial and Ethnic Disparities in European Breast Cancer Clinical Trials. Cancers. 2024; 16(9):1726. https://doi.org/10.3390/cancers16091726
Chicago/Turabian StyleBania, Angelina, Antonis Adamou, and Emmanouil Saloustros. 2024. "Racial and Ethnic Disparities in European Breast Cancer Clinical Trials" Cancers 16, no. 9: 1726. https://doi.org/10.3390/cancers16091726
APA StyleBania, A., Adamou, A., & Saloustros, E. (2024). Racial and Ethnic Disparities in European Breast Cancer Clinical Trials. Cancers, 16(9), 1726. https://doi.org/10.3390/cancers16091726





