Taking a BiTE out of Lymphoma: Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma
Abstract
Simple Summary
Abstract
1. Introduction
2. DLBCL and BsAbs
3. FL and BsABs
4. Mantle Cell Lymphoma (MCL) and BsABs
5. Resistance
6. Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalakonda, N.; Maerevoet, M.; Cavallo, F.; Follows, G.; Goy, A.; Vermaat, J.S.P.; Casasnovas, O.; Hamad, N.; Zijlstra, J.M.; Bakhshi, S.; et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): A single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020, 7, e511–e522. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Woyach, J.A.; Furman, R.R.; Martin, P.; O’brien, S.; Brown, J.R.; Stephens, D.M.; Barrientos, J.C.; Devereux, S.; Hillmen, P.; et al. Acalabrutinib in treatment-naive chronic lymphocytic leukemia. Blood 2021, 137, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2023, 388, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Hertzberg, M.; Opat, S.S.; Herrera, A.F.; Assouline, S.; Flowers, C.R.; Kim, T.M.; McMillan, A.K.; Ozcan, M.; Safar, V.; et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: Survival update and new extension cohort data. Blood Adv. 2022, 6, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.; Duell, J.; Barca, E.G.; Tournilhac, O.; Jurczak, W.; Liberati, A.M.; Nagy, Z.; Obr, A.; Gaidano, G.; André, M.; et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): A multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020, 21, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, E.; Hamadani, M.; Carlo-Stella, C. The antibody-drug conjugate loncastuximab tesirine for the treatment of diffuse large B-cell lymphoma. Blood 2022, 140, 303–308. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Hutchings, M.; Mous, R.; Clausen, M.R.; Johnson, P.; Linton, K.M.; Chamuleau, M.E.D.; Lewis, D.J.; Balari, A.S.; Cunningham, D.; Oliveri, R.S.; et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: An open-label, phase 1/2 study. Lancet 2021, 398, 1157–1169. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Budde, L.E.; Sehn, L.H.; Matasar, M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: A single-arm, multicentre, phase 2 study. Lancet Oncol. 2022, 23, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamadani, M.; Habermann, T.M.; Cerhan, J.R.; Macon, W.R.; Maurer, M.J.; Go, R.S. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am. J. Hematol. 2015, 90, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Tilly, H.; Morschhauser, F.; Sehn, L.H.; Friedberg, J.W.; Trněný, M.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; Matasar, M.; Rai, S.; et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Shahzad, M.; Shippey, E.; Bansal, R.; Mushtaq, M.U.; Mahmoudjafari, Z.; Faisal, M.S.; Hoffmann, M.; Abdallah, A.-O.; Divine, C.; et al. Socioeconomic and Racial Disparity in Chimeric Antigen Receptor T Cell Therapy Access. Transplant. Cell. Ther. 2022, 28, 358–364. [Google Scholar] [CrossRef]
- Ayyappan, S.; Kim, W.S.; Kim, T.M.; Walewski, J.; Cho, S.-G.; Jarque, I.; Iskierka-Jazdzewska, E.; Poon, M.; Oh, S.Y.; Lim, F.L.W.I.; et al. Final Analysis of the Phase 2 ELM-2 Study: Odronextamab in Patients with Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2023, 142 (Suppl. S1), 436. [Google Scholar] [CrossRef]
- Crombie, J.L.; Graff, T.; Falchi, L.; Karimi, Y.; Bannerji, R.; Nastoupil, L.; Thieblemont, C.; Ursu, R.; Bartlett, N.; Nachar, V.; et al. Consensus Recommendations on the Management of Toxicity Associated with CD3xCD20 Bispecific Antibody Therapy. Blood 2024, 143, 1565–1575. [Google Scholar] [CrossRef]
- Vose, J.M.; Feldman, T.; Chamuleau, M.E.; Kim, W.S.; Lugtenburg, P.; Kim, T.M.; Costa, P.A.; Cheah, C.Y.; Glimelius, I.C.; Hess, B.; et al. Mitigating the Risk of Cytokine Release Syndrome (CRS): Preliminary Results from a DLBCL Cohort of Epcore NHL-1. Blood 2023, 142 (Suppl. S1), 1729. [Google Scholar] [CrossRef]
- Hutchings, M.; Carlo-Stella, C.; Morschhauser, F.; Falchi, L.; Bachy, E.; Cartron, G.; Khan, C.; Tani, M.; Martinez-Lopez, J.; Bartlett, N.L.; et al. Glofitamab Monotherapy in Relapsed or Refractory Large B-Cell Lymphoma: Extended Follow-Up from a Pivotal Phase II Study and Subgroup Analyses in Patients with Prior Chimeric Antigen Receptor T-Cell Therapy and by Baseline Total Metabolic Tumor Volume. Blood 2023, 142 (Suppl. S1), 433. [Google Scholar] [CrossRef]
- Falchi, L.; Carlo-Stella, C.; Morschhauser, F.; Dickinson, M.; Bachy, E.; Cartron, G.; Khan, C.; Tani, M.; Martinez-Lopez, J.; Bartlett, N.L.; et al. Dexamethasone is Associated with a Lower Incidence and Severity of Cytokine Release Syndrome Compared with Other Corticosteroid Regimens When Given as Premedication for Glofitamab Monotherapy in Patients with Relapsed/Refractory (R/R) Large B-Cell Lymphoma (LBCL). Blood 2023, 142 (Suppl. S1), 3130. [Google Scholar]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar] [PubMed]
- Smith, A.; Crouch, S.; Lax, S.; Li, J.; Painter, D.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Lymphoma incidence, survival and prevalence 2004–2014: Sub-type analyses from the UK’s Haematological Malignancy Research Network. Br. J. Cancer 2015, 112, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Flinn, I.W.; van der Jagt, R.; Kahl, B.S.; Wood, P.; Hawkins, T.E.; MacDonald, D.; Hertzberg, M.; Kwan, Y.-L.; Simpson, D.; Craig, M.; et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: The BRIGHT study. Blood 2014, 123, 2944–2952. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Fowler, N.H.; Feugier, P.; Bouabdallah, R.; Tilly, H.; Palomba, M.L.; Fruchart, C.; Libby, E.N.; Casasnovas, R.-O.; Flinn, I.W.; et al. Rituximab plus Lenalidomide in Advanced Untreated Follicular Lymphoma. N. Engl. J. Med. 2018, 379, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Fowler, N.H.; Dickinson, M.; Dreyling, M.; Martinez-Lopez, J.; Kolstad, A.; Butler, J.; Ghosh, M.; Popplewell, L.; Chavez, J.C.; Bachy, E.; et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: The phase 2 ELARA trial. Nat. Med. 2022, 28, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, C.A.; Chavez, J.C.; Sehgal, A.R.; William, B.M.; Munoz, J.; Salles, G.; Munshi, P.N.; Casulo, C.; Maloney, D.G.; de Vos, S.; et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022, 23, 91–103. [Google Scholar] [CrossRef]
- Morschhauser, F.; Tilly, H.; Chaidos, A.; McKay, P.; Phillips, T.; Assouline, S.; Batlevi, C.L.; Campbell, P.; Ribrag, V.; Damaj, G.L.; et al. Tazemetostat for patients with relapsed or refractory follicular lymphoma: An open-label, single-arm, multicentre, phase 2 trial. Lancet Oncol. 2020, 21, 1433–1442. [Google Scholar] [CrossRef]
- Linton, K.; Jurczak, W.; Lugtenburg, P.; Gyan, E.; Balari, A.M.S.; Christensen, J.H.; Hess, B.; Tilly, H.; Cordoba, R.; Lewis, D.; et al. Epcoritamab SC Monotherapy Leads to Deep and Durable Responses in Patients with Relapsed or Refractory Follicular Lymphoma: First Data Disclosure from the Epcore NHL-1 Follicular Lymphoma Dose-Expansion Cohort. Blood 2023, 142 (Suppl. S1), 1655. [Google Scholar] [CrossRef]
- Kim, T.M.; Taszner, M.; Cho, S.-G.; Novelli, S.; Le Gouill, S.; Poon, M.L.; Villasboas, J.C.; Champion, R.; Bachy, E.; Guidez, S.; et al. Odronextamab in Patients with Relapsed/Refractory (R/R) Follicular Lymphoma (FL) Grade 1-3a: Results from a Prespecified Analysis of the Pivotal Phase II Study ELM-2. Blood 2022, 140 (Suppl. S1), 2280–2282. [Google Scholar] [CrossRef]
- Budde, L.E.; Assouline, S.; Sehn, L.H.; Schuster, S.J.; Yoon, S.-S.; Yoon, D.H.; Matasar, M.J.; Bosch, F.; Kim, W.S.; Nastoupil, L.J.; et al. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients With Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J. Clin. Oncol. 2022, 40, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Assouline, S.; Kamdar, M.; Ghosh, N.; Naik, S.; Nakhoda, S.K.; Chavez, J.C.; Jia, T.; Pham, S.; Huw, L.-Y.; et al. Fixed Duration Mosunetuzumab Plus Polatuzumab Vedotin Has Promising Efficacy and a Manageable Safety Profile in Patients with BTKi Relapsed/Refractory Mantle Cell Lymphoma: Initial Results from a Phase Ib/II Study. Blood 2023, 142 (Suppl. S1), 734. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Iraola-Truchuelo, J.; Iacoboni, G.; Palomo, L.; Garces, V.N.; Castellvi, J.; Mas, A.; Nonell, L.; A Mayor, L.; Dourdil, V.; Cerecedo, T.G.; et al. Resistance Mechanisms Impacting Bispecific Antibody (BsAbs) and Chimeric Antigen Receptor (CAR) T-Cell Therapy Outcomes in Large B Cell Lymphoma (LBCL) Patients. Blood 2023, 142 (Suppl. S1), 1635. [Google Scholar] [CrossRef]
- Brouwer-Visser, J.; Fiaschi, N.; Deering, R.P.; Dhanik, A.; Cygan, K.J.; Zhang, W.; Jeong, S.; Pourpe, S.; Boucher, L.; Hamon, S.; et al. Baseline Biomarkers of T-Cell Function Correlate with Clinical Responses to Odronextamab (REGN1979), and Loss of CD20 Target Antigen Expression Identified As a Mechanism of Treatment Resistance. Blood 2020, 136, 10–11. [Google Scholar] [CrossRef]
- Piccione, E.C.; Belousov, A.; Hamidi, H.; Carlo-Stella, C.; Dickinson, M.; Morschhauser, F.; Lundberg, L.; Humphrey, K.; Tracy, S.; Hutchings, M. P1210: Immune Correlates of Response to Glofitamab: Biomarker Findings from a Pivotal Phase Ii Expansion Study in Patients with Relapsed or Refractory (R/R) Diffuse Large B-Cell Lymphoma (Dlbcl). HemaSphere 2022, 6, 1096–1097. [Google Scholar] [CrossRef]
- Falchi, L.; Okwali, M.; Ghione, P.; Owens, C.; Hamlin, P.A.; Lue, J.K.; Epstein-Peterson, Z.D.; Palomba, M.L.; Kumar, A.; Torka, P.; et al. Subcutaneous (SC) Mosunetuzumab (mosun) As First-Line Therapy for Patients (pts) with High Tumor-Burden Follicular Lymphoma (FL): First Results of a Multicenter Phase 2 Study. Blood 2023, 142 (Suppl. S1), 604. [Google Scholar] [CrossRef]
- Falchi, L.; Leslie, L.A.; Belada, D.; Kopeckova, K.; Offner, F.; Brody, J.; Canales, M.; Martin Garcia-Sancho, A.; Nijland, M.; Andersson, P.O.; et al. Subcutaneous Epcoritamab in Combination with Rituximab + Lenalidomide (R2) for First-Line Treatment of Follicular Lymphoma: Initial Results from Phase 1/2 Trial. Blood 2022, 140 (Suppl. S1), 1471–1473. [Google Scholar] [CrossRef]
- Michael Dickinson, A.V.; Marks, R.; Topp, M.S.; Morschhauser, F.; Jacobs, B.; Tani, M.; Bosch, F.; Esteban, D.; Cordoba, R.; Kaufman, D.; et al. Glofitamab + Pola-R-CHP in patients with previously untreated diffuse large B-cell lymphoma (DLBCL): Results from a phase Ib study. J. Clin. Oncol. 2023, 41, 7549. [Google Scholar] [CrossRef]
- Topp, M.S.; Tani, M.; Dickinson, M.; Ghosh, N.; Santoro, A.; Pinto, A.; Bosch, F.; Fox, C.P.; Lopez-Guillermo, A.; Gastinne, T.; et al. Glofitamab Plus R-CHOP Induces High Response Rates with a Manageable Safety Profile in Patients with Previously Untreated Diffuse Large B-Cell Lymphoma (DLBCL): A 12-Month Analysis from a Phase Ib Study. Blood 2023, 142 (Suppl. S1), 3085. [Google Scholar] [CrossRef]
- Falchi, L.; Offner, F.; Belada, D.; Brody, J.; Linton, K.M.; Karimi, Y.; Cordoba, R.; Snauwaert, S.; Abbas, A.; Wang, L.; et al. First-line treatment (Tx) with subcutaneous (SC) epcoritamab (epco) + R-CHOP in patients (pts) with high-risk diffuse large B-cell lymphoma (DLBCL): Phase 1/2 data update. J. Clin.Oncol. 2022, 40 (Suppl. S16), 7523. [Google Scholar] [CrossRef]
- Budde, L.E.; Olszewski, A.J.; Assouline, S.; Lossos, I.S.; Diefenbach, C.; Kamdar, M.; Ghosh, N.; Modi, D.; Sabry, W.; Naik, S.; et al. Mosunetuzumab Plus Polatuzumab Vedotin Demonstrates a Favorable Safety Profile and Efficacy in Patients (Pts) with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL): Primary Analysis of a Phase Ib/II Study. Blood 2023, 142 (Suppl. S1), 613. [Google Scholar] [CrossRef]
- Hutchings, M.; Sureda, A.; Terol, M.J.; Albareda, F.B.; Corradini, P.; Larsen, T.S.; Dominguez, A.R.; Panchal, A.; Bottos, A.; Carlile, D.; et al. Glofitamab (Glofit) in Combination with Polatuzumab Vedotin (Pola): Phase Ib/II Preliminary Data Support Manageable Safety and Encouraging Efficacy in Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL). Blood 2021, 138 (Suppl. S1), 525. [Google Scholar] [CrossRef]
- Brody, J.; Wahlin, B.E.; Phillips, T.J.; Costello, R.; Lugtenburg, P.; Cordoba, R.; Wang, L.; Wu, J.; Elliott, B.; Abbas, A.; et al. Epcoritamab (epco) with gemcitabine + oxaliplatin (GemOx) in patients (pts) with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) ineligible for autologous stem cell transplant (ASCT) induces high response rate even in pts failing CAR T therapy. J. Clin.Oncol. 2022, 40 (Suppl. S16), 7527. [Google Scholar]
- Cordoba, R.; Falchi, L.; Phillips, T.; de Vos, S.; Nijland, M.; Offner, F.; Bykhovski, I.; Wu, J.; Wang, L.; Rana, A.; et al. P1215: Preliminary Phase 1/2 Results of Subcutaneous Epcoritamab + R-Dhax/C in Patients with Relapsed or Refractory Diffuse Large B-Cell Lymphoma Eligible for Autologous Stem Cell Transplant. HemaSphere 2022, 6, 1101–1102. [Google Scholar] [CrossRef]
- Avivi Mazza, I.; Kim, W.S.; Ko, P.-S.; Grande, C.; Lavie, D.; Chism, D.; Seliem, M.; Jeng, E.E.; Joshi, N.; Siddani, S.; et al. Subcutaneous Epcoritamab Plus Lenalidomide in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma from EPCORE NHL-5. Blood 2023, 142 (Suppl. S1), 438. [Google Scholar] [CrossRef]
- Izhak, L.; Cullen, D.E.; Elgawly, M.; Luistro, L.; Johnson, S.; Bald, J.; Sasser, A.K.; Balasubramanian, S. Abstract 3636: Potent antitumor activity of duvortuxizumab, a CD19 x CD3 DART® molecule, in lymphoma models. Cancer Res. 2017, 77, 3636. [Google Scholar] [CrossRef][Green Version]
- Zhao, X.; Zhu, J.; Wu, Z.; Li, J.; Zhou, Y.; Nie, L.; Chen, G. Abstract LB218: Developing a bispecific anti-ROR1 antibody drug conjugate for hematological and solid tumor treatment. Cancer Res. 2023, 83 (Suppl. S8), LB218. [Google Scholar] [CrossRef]
- Oostindie, S.C.; Alemdehy, M.F.; Janmaat, M.L.; Meerding, J.M.; Kemper, K.; Engelberts, P.J.; De Goeij, B.E.; Satijn, D.; Sasser, A.K.; Breij, E.C. Duobody-CD3xCD30 Demonstrates Potent Anti-Tumor Activity in Preclinical Models of CD30+ Hematologic Malignancies. Blood 2022, 140 (Suppl. S1), 3153–3154. [Google Scholar] [CrossRef]
- Nieto, Y.; Banerjee, P.; Kaur, I.; Griffin, L.; Barnett, M.; Ganesh, C.; Borneo, Z.; Bassett, R.L.; Kerbauy, L.N.; Basar, R.; et al. Innate Cell Engager (ICE®) AFM13 Combined with Preactivated and Expanded (P+E) Cord Blood (CB)-Derived Natural Killer (NK) Cells for Patients with Refractory CD30-Positive Lymphomas: Final Results. Blood 2023, 142 (Suppl. S1), 774. [Google Scholar] [CrossRef]
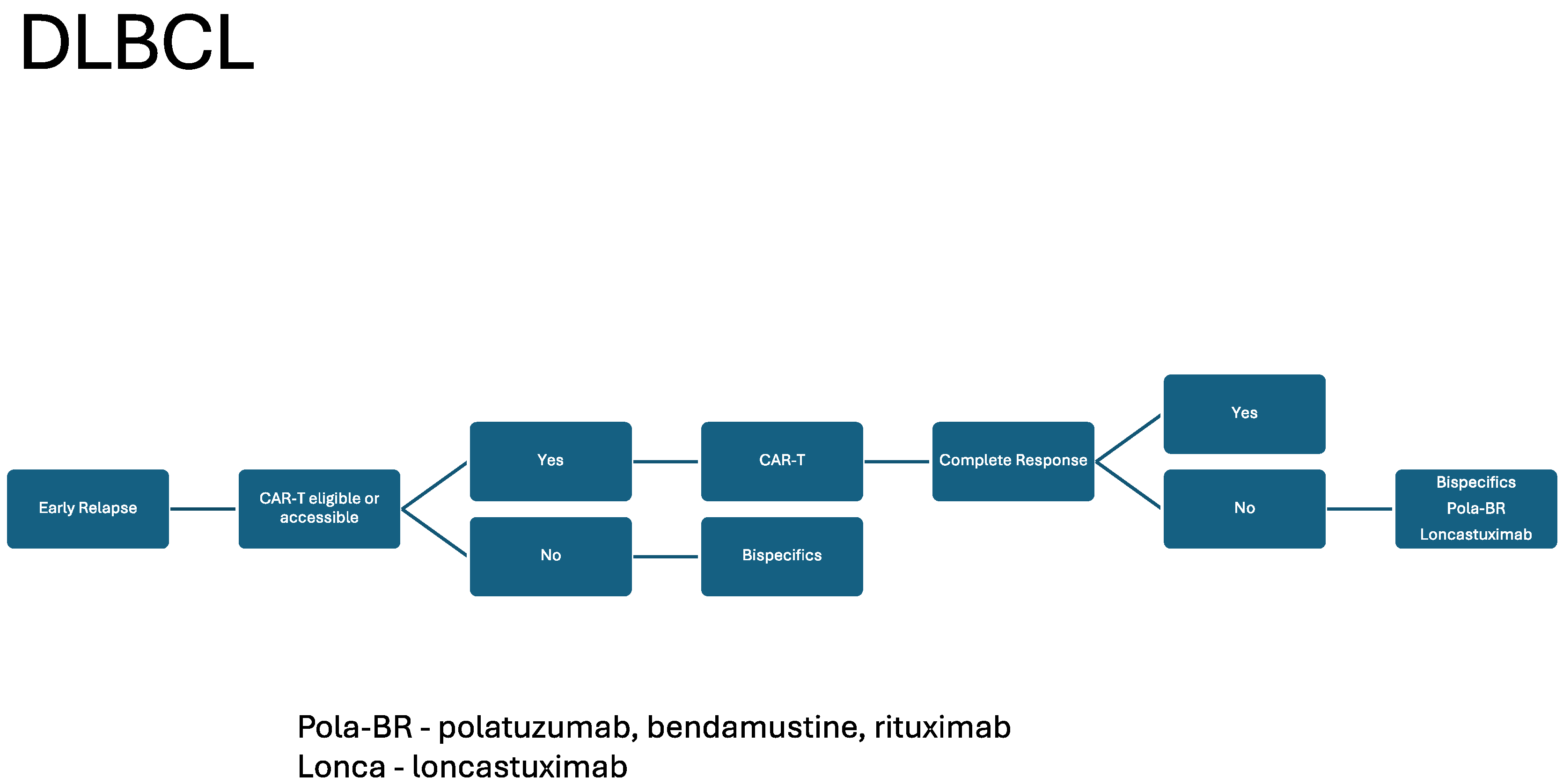
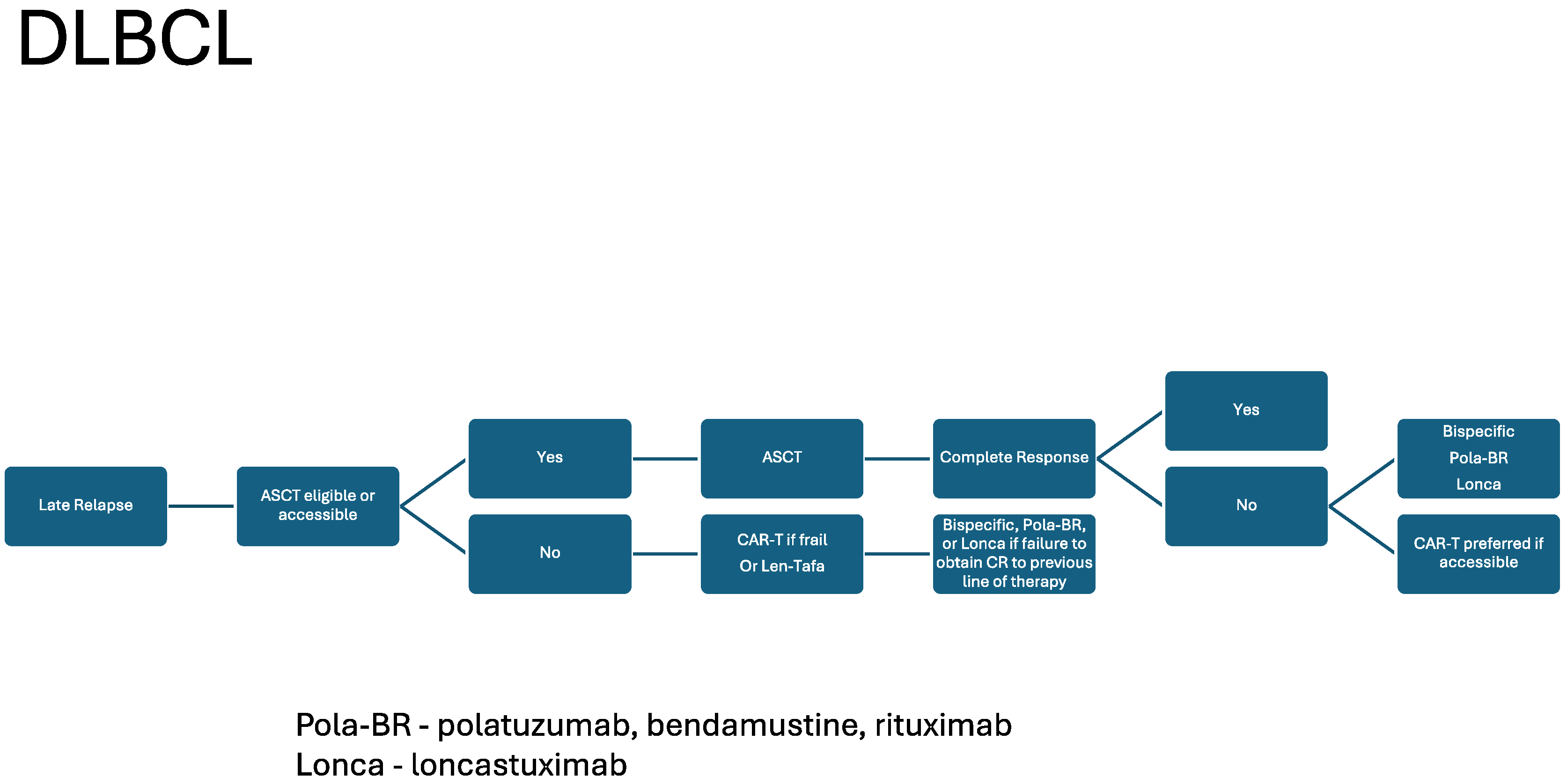

| BsAB | Disease Type Approval | ORR | CRR | Median DOR | Post CAR T-Cell CRR |
|---|---|---|---|---|---|
| Epcoritamab [12] | DLBCL | 63% (99/157) | 39% (61/157) | 15.6 months | 34.4% (21/61) |
| Glofitamab [21] | DLBCL | 52% (80/155) | 39% (61/155) | 18.4 months | 32% (12/38) |
| Mosunetuzumab [13] | FL | 80% (72/90) | 60% (54/90) | 22.8 months | NA |
| DLBCL | Clinicaltrials.gov ID | Line of Therapy |
|---|---|---|
| Glofitamab + Pola-R-CHP | NCT06050694, NCT03467373 | Front |
| Glofitamab + RICE | NCT05364424 | R/R |
| Epcoritamab + R-DHAOx | NCT06287398 | R/R |
| Epcoritamab + lenalidomide | NCT05283720 | R/R |
| Epcoritamab + ibrutinib | NCT05283720 | R/R |
| Epcoritamab + Pola-R-CHP | NCT05283720 | Front |
| Mosunetuzumab + Pola–lenalidomide | NCT06015880 | R/R |
| FL | Clinicaltrials.gov ID | Line of Therapy |
| Mosunetuzumab + Pola | NCT05410418 | Front |
| Mosunetuzumab + tazemetostat | NCT05994235 | Front |
| Mosunetuzumab + Pola + Obin | NCT05169658 | Front |
| Mosunetuzumab + lenalidomide | NCT06284122, NCT04792502 | Front |
| Mosunetuzumab + lenalidomide | NCT04712097 | R/R |
| Epcoritamab + lenalidomide | NCT06112847 | Front |
| Epcoritamab + lenalidomide + rituximab | NCT06191744 | Front |
| Odronextamab + R-CHOP | NCT06097364 | Front |
| Odronextamab + lenalidomide | NCT06149286 | R/R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, J.M.; Phillips, T.J. Taking a BiTE out of Lymphoma: Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma. Cancers 2024, 16, 1724. https://doi.org/10.3390/cancers16091724
Weiss JM, Phillips TJ. Taking a BiTE out of Lymphoma: Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma. Cancers. 2024; 16(9):1724. https://doi.org/10.3390/cancers16091724
Chicago/Turabian StyleWeiss, Jonathan M., and Tycel J. Phillips. 2024. "Taking a BiTE out of Lymphoma: Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma" Cancers 16, no. 9: 1724. https://doi.org/10.3390/cancers16091724
APA StyleWeiss, J. M., & Phillips, T. J. (2024). Taking a BiTE out of Lymphoma: Bispecific Antibodies in B-Cell Non-Hodgkin Lymphoma. Cancers, 16(9), 1724. https://doi.org/10.3390/cancers16091724






