Profiling the Cardiovascular Toxicities of CDK4/6 Inhibitors: A Real-World Pharmacovigilance Study
Abstract
Simple Summary
Abstract
1. Introduction
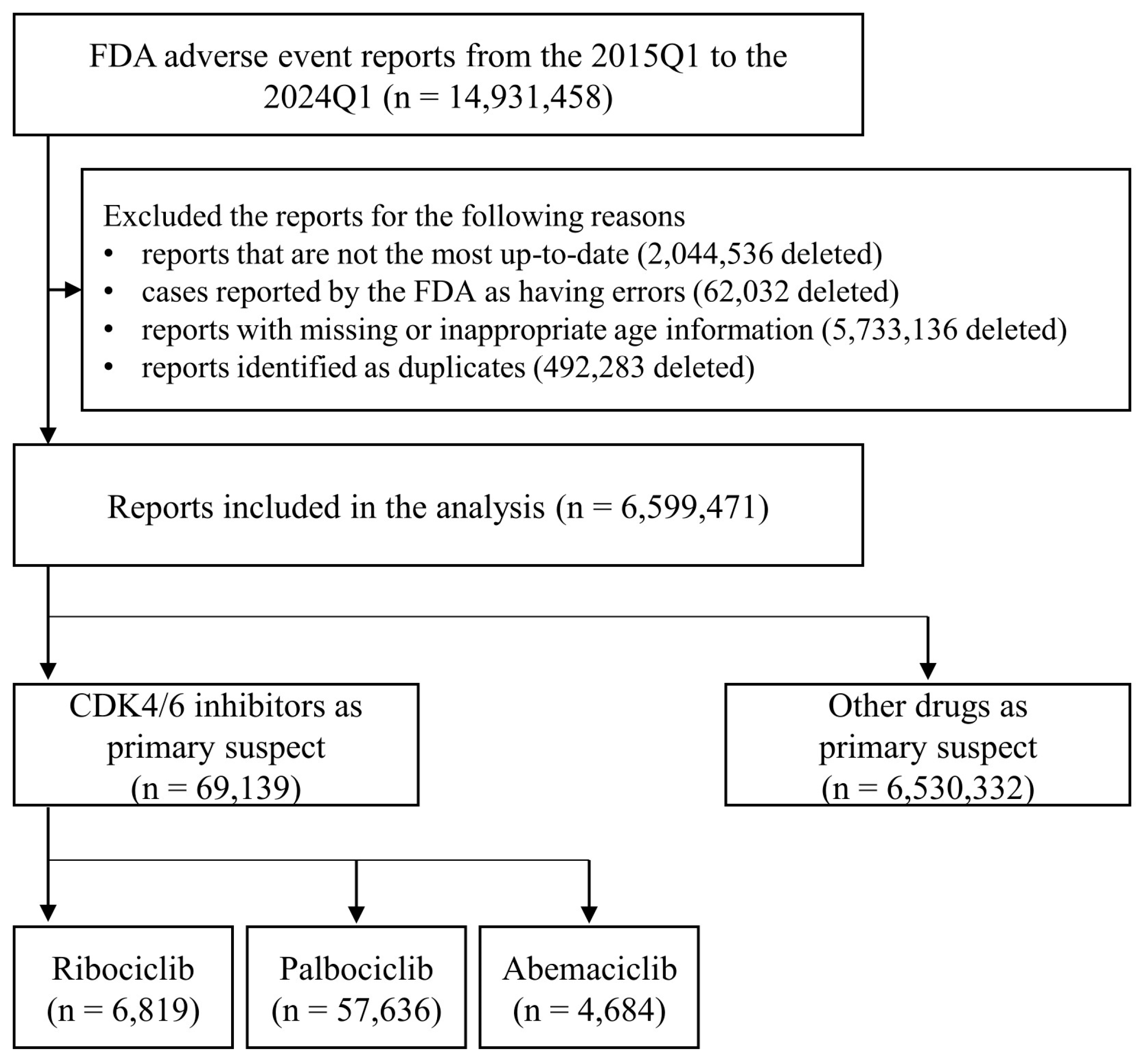
2. Materials and Methods
2.1. FAERS Data Preparation
2.2. Evaluation of Cardiovascular Toxicity
2.3. Statistical Analysis
2.4. Software
2.5. Ethics Approval
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Altekruse, S.F.; Li, C.I.; Chen, V.W.; Clarke, C.A.; Ries, L.A.; Cronin, K.A. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J. Natl. Cancer Inst. 2014, 106, dju055. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Abramson, V.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Bailey, J.; Burstein, H.J.; et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J. Systemic Therapy for Estrogen Receptor-Positive, HER2-Negative Breast Cancer. N. Engl. J. Med. 2020, 383, 2557–2570. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, K.; Marme, F. Current and emerging treatment approaches for hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. Cancer Treat. Rev. 2024, 123, 102670. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Huober, J.; Sohn, J.; Tredan, O.; Park, I.H.; Campone, M.; Chen, S.C.; Manso, L.M.; Paluch-Shimon, S.; et al. Abemaciclib plus a nonsteroidal aromatase inhibitor as initial therapy for HR+, HER2- advanced breast cancer: Final overall survival results of MONARCH 3. Ann. Oncol. 2024, 35, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Dieras, V.; Rugo, H.S.; Harbeck, N.; Im, S.A.; Gelmon, K.A.; Lipatov, O.N.; Walshe, J.M.; Martin, M.; Chavez-MacGregor, M.; et al. Overall Survival with Palbociclib Plus Letrozole in Advanced Breast Cancer. J. Clin. Oncol. 2024, 42, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.E.; Ciaccio, A.; Danesi, R.; Duhoux, F.P.; Girmenia, C.; Zaman, K.; Lindman, H.; Luppi, F.; Mavroudis, D.; Paris, I.; et al. Late onset toxicities associated with the use of CDK 4/6 inhibitors in hormone receptor positive (HR+), human epidermal growth factor receptor-2 negative (HER2-) metastatic breast cancer patients: A multidisciplinary, pan-EU position paper regarding their optimal management. The GIOCONDA project. Front. Oncol. 2023, 13, 1247270. [Google Scholar] [CrossRef]
- Papageorgiou, C.; Zagouri, F.; Tampakis, K.; Georgakopoulou, R.; Manios, E.; Kafouris, P.; Benetos, G.; Koutagiar, I.; Anagnostopoulos, C.; Dimopoulos, M.A.; et al. Vascular Inflammation and Cardiovascular Burden in Metastatic Breast Cancer Female Patients Receiving Hormonal Treatment and CDK 4/6 Inhibitors or Everolimus. Front. Cardiovasc. Med. 2021, 8, 638895. [Google Scholar] [CrossRef] [PubMed]
- Oyakawa, T.; Inagaki, L.; Hua, Z.; Ebihara, A.; Takano, T.; Ohno, S.; Shiga, T. Myocardial dysfunction caused by abemaciclib: A case report. Int. Cancer Conf. J. 2021, 10, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Cicini, M.P.; Ferretti, G.; Morace, N.; Nistico, C.; Cognetti, F.; Rulli, F. Second-Degree Type 2 Atrioventricular Block Requiring Permanent Cardiac Pacing in Patients on CDK4/6 Inhibitors: Report of Two Cases. Breast Care 2022, 17, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Fradley, M.G.; Nguyen, N.H.K.; Madnick, D.; Chen, Y.; DeMichele, A.; Makhlin, I.; Dent, S.; Lefebvre, B.; Carver, J.; Upshaw, J.N.; et al. Adverse Cardiovascular Events Associated With Cyclin-Dependent Kinase 4/6 Inhibitors in Patients With Metastatic Breast Cancer. J. Am. Heart Assoc. 2023, 12, e029361. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Niciforovic, D.; Papic, D.; Milojevic, K.; Markovic, M. CDK4/6 inhibitors: Basics, pros, and major cons in breast cancer treatment with specific regard to cardiotoxicity-a narrative review. Ther. Adv. Med. Oncol. 2023, 15, 17588359231205848. [Google Scholar] [CrossRef] [PubMed]
- Fiste, O.; Mavrothalassitis, E.; Apostolidou, K.; Trika, C.; Liontos, M.; Koutsoukos, K.; Kaparelou, M.; Dimitrakakis, C.; Gavriatopoulou, M.; Dimopoulos, M.A.; et al. Cardiovascular complications of ribociclib in breast cancer patients. Crit. Rev. Oncol. Hematol. 2024, 196, 104296. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Ryan, P.B.; Ta, C.N.; Kim, J.H.; Li, Z.; Weng, C. From clinical trials to clinical practice: How long are drugs tested and then used by patients? J. Am. Med. Inform. Assoc. 2021, 28, 2456–2460. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Y.; Papez, V.; Chang, W.H.; Mueller, S.H.; Denaxas, S.; Lai, A.G. Comparing clinical trial population representativeness to real-world populations: An external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longev. 2022, 3, e674–e689. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, M.L.; Celant, S.; Tomassini, L.; Arenare, L.; Caglio, A.; Canciello, A.; Salerno, F.; Olimpieri, P.P.; Di Segni, S.; Sferrazza, A.; et al. Comparison of baseline patient characteristics in Italian oncology drug monitoring registries and clinical trials: A real-world cross-sectional study. Lancet Reg. Health Eur. 2024, 41, 100912. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, M.A.; Khan, A.H.; Ghadzi, S.M.S.; Adnan, A.S.; Abdallah, Q.M. A Standardized Dataset of a Spontaneous Adverse Event Reporting System. Healthcare 2022, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- MedDRA. Introductory Guide for Standardised MedDRA Queries (SMQs) Version 27.0; International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2024. [Google Scholar]
- Evans, S.J.; Waller, P.C.; Davis, S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol. Drug Saf. 2001, 10, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef] [PubMed]
- van Puijenbroek, E.P.; Bate, A.; Leufkens, H.G.; Lindquist, M.; Orre, R.; Egberts, A.C. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol. Drug Saf. 2002, 11, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Song, Y.K. Utilizing temporal pattern of adverse event reports to identify potential late-onset adverse events. Expert. Opin. Drug Saf. 2024, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.M.; Shi, X.; Ramachandran, R.; Chen, E.M.; Wallach, J.D.; Ross, J.S. Characterization and corroboration of safety signals identified from the US Food and Drug Administration Adverse Event Reporting System, 2008–2019: Cross sectional study. BMJ 2022, 379, e071752. [Google Scholar] [CrossRef] [PubMed]
- Novartis Pharmaceuticals Corporation. Kisqali (Ribociclib) [Package Insert]. U.S. Food and Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/209092s016lbl.pdf (accessed on 22 July 2024).
- Zhou, Y.; Li, Y.; Shen, J.; Li, J.; Li, X. Abemaciclib induces apoptosis in cardiomyocytes by activating the Hippo signaling pathway. Acta Biochim. Biophys. Sin. 2020, 52, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Tredan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | CDK4/6 Inhibitors | Palbociclib | Ribociclib | Abemaciclib |
|---|---|---|---|---|
| Total | 69,139 | 57,636 | 6819 | 4684 |
| Age (years) | ||||
| Median (IQR) | 65 (57–74) | 66 (57–74) | 61 (50–71) | 63 (55–72) |
| Sex | ||||
| Female | 66,783 (96.6) | 55,757 (96.7) | 6518 (95.6) | 4508 (96.2) |
| Male | 1749 (2.5) | 1491 (2.6) | 150 (2.2) | 108 (2.3) |
| Unknown | 607 (0.9) | 388 (0.7) | 151 (2.2) | 68 (1.5) |
| Reporter occupation | ||||
| Healthcare professional | 28,939 (41.9) | 24,424 (42.4) | 2905 (42.6) | 1610 (34.4) |
| Consumer | 30,832 (44.6) | 25,349 (44.0) | 3316 (48.6) | 2167 (46.3) |
| Other | 9368 (13.5) | 7863 (13.6) | 598 (8.8) | 907 (19.4) |
| Time-to-event (days) | ||||
| No. of reports | 18,370 (26.6) | 12,701 (22.0) | 3623 (53.1) | 2046 (43.7) |
| Median (IQR) | 69 (18–260) | 83 (20–300) | 63 (16–241) | 33 (11–109) |
| Serious outcomes | ||||
| Death | 9038 (13.1) | 6770 (11.7) | 1815 (26.6) | 453 (9.7) |
| Life-threatening | 729 (1.1) | 378 (0.7) | 250 (3.7) | 101 (2.2) |
| Hospitalization | 11,728 (17) | 8470 (14.7) | 1978 (29) | 1206 (25.7) |
| Disability | 339 (0.5) | 201 (0.3) | 91 (1.3) | 47 (1) |
| Required intervention | 107 (0.2) | 70 (0.1) | 16 (0.2) | 21 (0.4) |
| Characteristics | Total | Palbociclib | Ribociclib | Abemaciclib |
|---|---|---|---|---|
| Total number of reports | 2065 | 1304 | 598 | 163 |
| Top 5 Preferred Terms, n (%) | ||||
| 1 | Myocardial infarction, 266 (12.9) | Myocardial infarction, 194 (14.9) | Atrial fibrillation, 67 (11.2) | Cardiac failure, 24 (14.7) |
| 2 | Cardiac disorder, 250 (12.1) | Cardiac disorder, 192 (14.7) | Myocardial infarction, 58 (9.7) | Atrial fibrillation, 21 (12.9) |
| 3 | Atrial fibrillation, 234 (11.3) | Palpitations, 150 (11.5) | Arrhythmia, 55 (9.2) | Tachycardia, 18 (11.0) |
| 4 | Palpitations, 208 (10.1) | Atrial fibrillation, 146 (11.2) | Tachycardia, 50 (8.4) | Palpitations, 17 (10.4) |
| 5 | Cardiac failure, 175 (8.5) | Cardiac failure, 108 (8.3) | Cardiac disorder, 46 (7.7) | Myocardial infarction, 14 (8.6) |
| Drug | SMQ | N | PRR | ROR | Chi-Square | IC | IC025 |
|---|---|---|---|---|---|---|---|
| Ribociclib | TdP/QT prolongation | 190 | 8.43 | 8.65 | 1237.99 | 3.07 | 2.86 |
| Arrhythmia-related investigations, signs, and symptoms | 11 | 8.19 | 8.2 | 68.83 | 3.15 | 2.33 | |
| Cardiac failure | 166 | 1.8 | 1.82 | 60.04 | 0.86 | 0.64 | |
| Hypertension | 164 | 1.08 | 1.08 | 0.9 | 0.11 | −0.11 | |
| Cardiomyopathy | 19 | 0.99 | 0.99 | 0 | 0.06 | −0.57 | |
| Noninfectious myocarditis/pericarditis | 4 | 0.34 | 0.34 | 5.25 | −1.25 | −2.52 | |
| Myocardial infarction | 79 | 1.12 | 1.12 | 1.08 | 0.19 | −0.13 | |
| Palbociclib | TdP/QT prolongation | 31 | 0.16 | 0.16 | 136.57 | −2.58 | −3.08 |
| Arrhythmia-related investigations, signs, and symptoms | 0 | - | - | 11.54 | −3.52 | −6.35 | |
| Cardiac failure | 587 | 0.75 | 0.75 | 48.36 | −0.41 | −0.52 | |
| Hypertension | 766 | 0.59 | 0.59 | 218.41 | −0.75 | −0.85 | |
| Cardiomyopathy | 43 | 0.26 | 0.26 | 88.52 | −1.88 | −2.31 | |
| Noninfectious myocarditis/pericarditis | 6 | 0.06 | 0.06 | 89.83 | −3.84 | −4.91 | |
| Myocardial infarction | 224 | 0.37 | 0.37 | 235.30 | −1.40 | −1.59 | |
| Abemaciclib | TdP/QT prolongation | 1 | 0.06 | 0.06 | 13.72 | −2.96 | −4.96 |
| Arrhythmia-related investigations, signs, and symptoms | 0 | - | - | 0.93 | 0.1 | −2.73 | |
| Cardiac failure | 53 | 0.84 | 0.84 | 1.7 | −0.23 | −0.62 | |
| Hypertension | 16 | 0.15 | 0.15 | 76.91 | −2.62 | −3.31 | |
| Cardiomyopathy | 10 | 0.76 | 0.76 | 0.77 | −0.26 | −1.11 | |
| Noninfectious myocarditis/pericarditis | 2 | 0.24 | 0.24 | 4.67 | −1.45 | −3.08 | |
| Myocardial infarction | 23 | 0.48 | 0.47 | 13.41 | −1.01 | −1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H. Profiling the Cardiovascular Toxicities of CDK4/6 Inhibitors: A Real-World Pharmacovigilance Study. Cancers 2024, 16, 2869. https://doi.org/10.3390/cancers16162869
Kim JH. Profiling the Cardiovascular Toxicities of CDK4/6 Inhibitors: A Real-World Pharmacovigilance Study. Cancers. 2024; 16(16):2869. https://doi.org/10.3390/cancers16162869
Chicago/Turabian StyleKim, Jae Hyun. 2024. "Profiling the Cardiovascular Toxicities of CDK4/6 Inhibitors: A Real-World Pharmacovigilance Study" Cancers 16, no. 16: 2869. https://doi.org/10.3390/cancers16162869
APA StyleKim, J. H. (2024). Profiling the Cardiovascular Toxicities of CDK4/6 Inhibitors: A Real-World Pharmacovigilance Study. Cancers, 16(16), 2869. https://doi.org/10.3390/cancers16162869






