Advancements in Image-Based Models for High-Grade Gliomas Might Be Accelerated
Abstract
Simple Summary
Abstract
1. Introduction
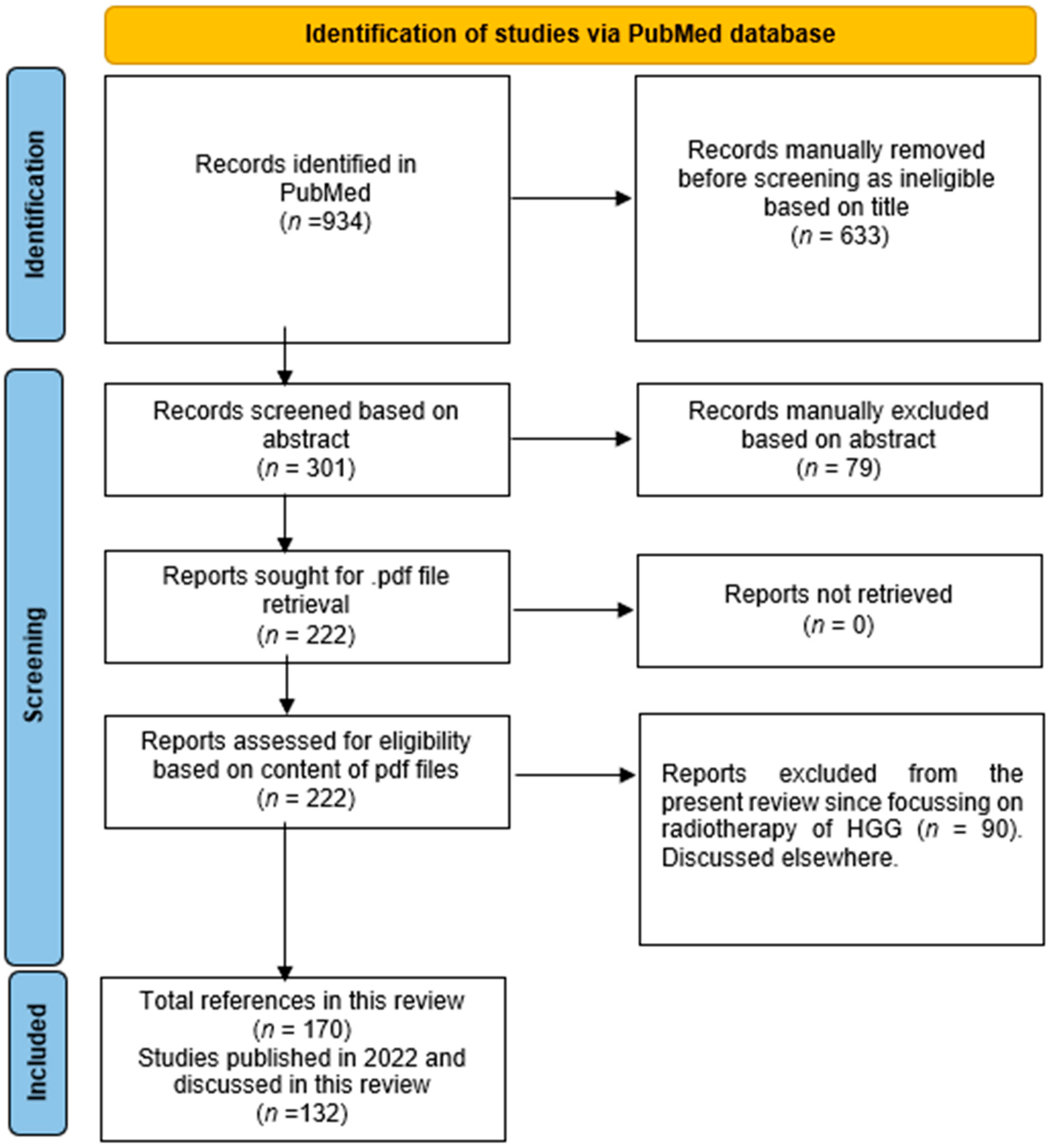
2. Materials and Methods
3. Models for the Diagnosis of HGG
3.1. MRI
3.1.1. Preclinical Studies
3.1.2. Clinical Studies

| Major Finding | Experimental System | Ref. |
|---|---|---|
| MRI | ||
| T1-relative CBV effectively diagnosed progressive lesions in patients with HGG, suggesting the potential role of T1-PWI as a valid alternative to the traditional T2*-PWI. | 45 MRIs of 34 patients with proved HGG. | [23] |
| Machine learning-based radiophysiomics might contribute to the clinical diagnosis of CE brain tumors. | A training cohort of 167 patients suffering from one of the five most common brain tumor entities (glioblastoma, anaplastic glioma, meningioma, primary CNS lymphoma, or brain metastasis). | [25] |
| The NeuroXAI software might be helpful in the detection and diagnosis of BTs. | NeuroXAI framework offering state-of-the-art XAI methods for classification and segmentation for both 2D and 3D medical image data. | [26] |
| Validation of MRI-only brain RT by a prospective clinical trial. | 21 glioma patients. | [27] |
| Proposal of a deep learning method for virtual CE T1 brain MRI prediction. | 200 multiparametric brain MRIs of a total of 145 patients. | [28] |
| A combination of radiomics and CNN features might improve the prediction performance of noninvasive genetic biomarkers. | Preoperative MRI data from 400 patients with GB who underwent resection and genetic testing. | [29] |
| Proposal of an an automated method to quantify the subtle deformations that occur in the peritumoral regions. | 229 MRI exams from 27 patients with histologically confirmed HGG. | [31] |
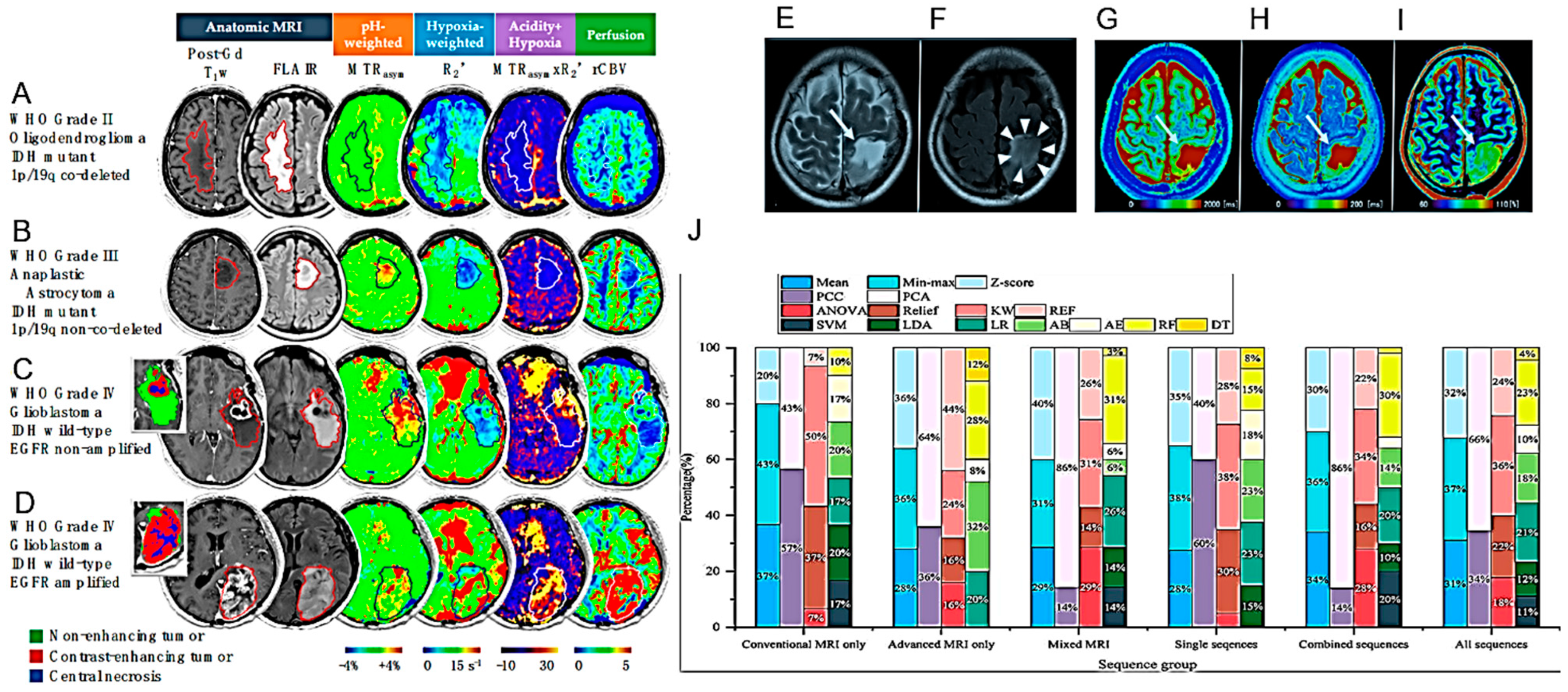
| APTw MRI imaging shows good scan–rescan reproducibility in healthy tissue and tumors. | 21 healthy volunteers and 6 glioma patients (4 GBs, 1 oligodendroglioma, 1 radiologically suspected LGG). | [32] |
| APTw MRI max values correlate positively with rCBVmax. | 40 adult patients, treated for histopathologically confirmed glioma (WHO grades II–IV). | [33] |
| APTw MRI mean values might be helpful in the differential diagnosis of HGGs and meningiomas or HGGs and LGGs. | Imaging data of 50 BTs confirmed by pathology. | [34] |
| The metabolite ratios and the results of glioma grading obtained by MRS are affected by the image quality. | 98 glioma patients confirmed by pathology. | [35] |
| ASL and DSC have similar diagnostic accuracy. | 115 BT patients who underwent both ASL and DSC perfusion in the same 3T MRI scanning session. | [36] |
| Semi-quantitative analysis using SWI may contribute to the differential diagnosis between HGG recurrence and radionecrosis, but cannot identify BM. | 56 patients with BM and 42 patients with HGG. | [37] |
| Study of an analytical qualitative algorithm to differentiate HGG from BM. | 36 patients with histologically proven HGG or solitary BM matched by size and location. | [38] |
| Study of 1H-MRS to differentiate primary and secondary brain neoplasms. | 61 MRI and 1H-MRS images of patients with histologically confirmed BTs. | [39] |
| CCA might help to distinguish PsP or RN from PD after RT. | 16 patients with a primary and 17 with a secondary BT. | [40] |
| SWI permits to identify haemorrhagic changes due to anti-VEGF drugs. | A case of pseudoprogression after ioRT and regorafenib therapy in a patient with anaplastic astrocytoma recurrence. | [41] |
| A radiomics approach useful to predict pseudoprogression. | 131 patients with HGG. | [42] |
| Use of the tissue permeability and microcirculation parameters Ktrans, Kep, IAUC to differentiate PT from TM. | 34 patients with HGG. | [43] |
| Features of conventional MRI and RT treatment such as radiation dose, marginal enhancement and isointense ADC-signal may be useful to distinguish between progressive disease and TIE. | HGG adult patients who were treated with chemo/RT and subsequently developed a new or increasing CE lesion on conventional follow-up MRI. | [44] |
| Unlike the quantitative measurements of DSC and DCE perfusion maps, their qualitative assessment has low inter-examinator agreement. | HGG patients who underwent re-resection of a new enhancing lesion on post-treatment 3T MR examination including DWI, DCE and DSC sequences. | [45] |
| Smaller than reported longitudinal changes in MD, FA, and RD after VMAT or tomotherapy RT treatment. | 27 patients newly diagnosed with GB and planned for VMAT or tomotherapy. | [46] |
| No significant improvement in therapy of DIPG in last decades. Post-RT necrosis is a frequent serious problem. | Medical records of 162 DIPG patients who underwent RT as an initial treatment. | [47] |
| LTS DIPG patients older at presentation compared to STS. ATRX mutation rates higher in this population than in the general DIPG population. | 152 patients ≥10 years of age at diagnosis with imaging confirmed DIPG. | [48] |
| Importance of central neuro-imaging review in the diagnosis of DIPG. | Cases submitted to the International DIPG registry (IDIPGR) with histopathologic and/or radiologic data. | [49] |
| Radiomics as prognostic tool to stratify DIPG patients. | 89 DIPG patients. | [50] |
| Variability of thalamic involvement of DMGs and its poor prognosis irrespective of H3 K27 subtype alterations. | 42 patients with radiologically evaluable thalamic-based DMG. | [51] |
| Quantitative 23Na MRI values in pediatric gliomas are higher than in normal tissues. | 26 pediatric patients with gliomas scanned with 23Na MRI. | [52] |
| PsP is frequent after RT of pediatric LGG and independent of the RT modality (IS vs. XRT vs. PBT). | Baseline and follow-up MRI of 136 LGG patients. | [53] |
| A non-GBCM-enhanced protocol was non-inferior to a GBCM-enhanced protocol for follow up of OPGs. | 42 children with isolated OPG. | [54] |
| After DKI of the peritumoral edema area, significant differences between grade III and IV gliomas. DKI parameters correlate with Ki-67. | 51 patients with gliomas undergoing DKI scans before surgery. | [55] |
| A machine learning model predicting the IDH mutation status of gliomas. | 69 patients with treatment-naïve diffuse glioma scanned with CEST MRI, DWI, FLAIR, and CE T1-weighted imaging at 3 T. | [56] |
| A radiomics model based on DCE-MRI and DWI predicting the IDH1 mutation and angiogenesis in gliomas. | 100 glioma patients examined with DCE-MRI and DWI. | [57] |
| The rCBV and PSR from DSC-MRI may predict the IDH mutation status in HGGs. | 58 patients with histopathologically proved HGGs. | [58] |
| Asynchrony in vascular dynamics determined by resting-state BOLD fMRI, correlates with tumor burden and permits to delineate tumor boundaries in IDH-mutated gliomas | 10 treatment-naïve patients with IDH-mutated gliomas who received standard-of-care preoperative imaging as well as echo-planar resting-state BOLD fMRI. | [59] |
| The pH- and oxygen-sensitive MRI is a feasible imaging technique for distinguishing glioma subtypes and determining their prognosis | 159 adult glioma patients scanned with pH- and oxygen-sensitive MRI at 3T. | [60] |
| Quantitative relaxometry using syMRI may differentiate astrocytomas from oligodendrogliomas with increased sensitivity and objectivity compared to T2-FLAIR | 13 patients with IDH-mutant diffuse gliomas, including 7 with astrocytomas and 6 with oligodendrogliomas. | [61] |
| The number of tumor blood vessels permits differentiating IDH1 mutations | 44 glioma patients [16 with IDH1 mutant-type (IDH1-MT), 28 with IDH1 wild-type (IDH1-WT)]. | [62] |
| DWI and PWI MRI features may help to predict the H3 K27M mutation status in DMGs | 94 DMG cases (mDMG = 48 and WT-DMG = 46). | [63] |
| The multiparametric MRI-based radiomic models may help to predict the H3 K27M mutation status in DMG | 102 patients with pathologically confirmed DMG (27 with H3 K27M-mutant and 75 with H3 WT status). | [64] |
| PET | ||
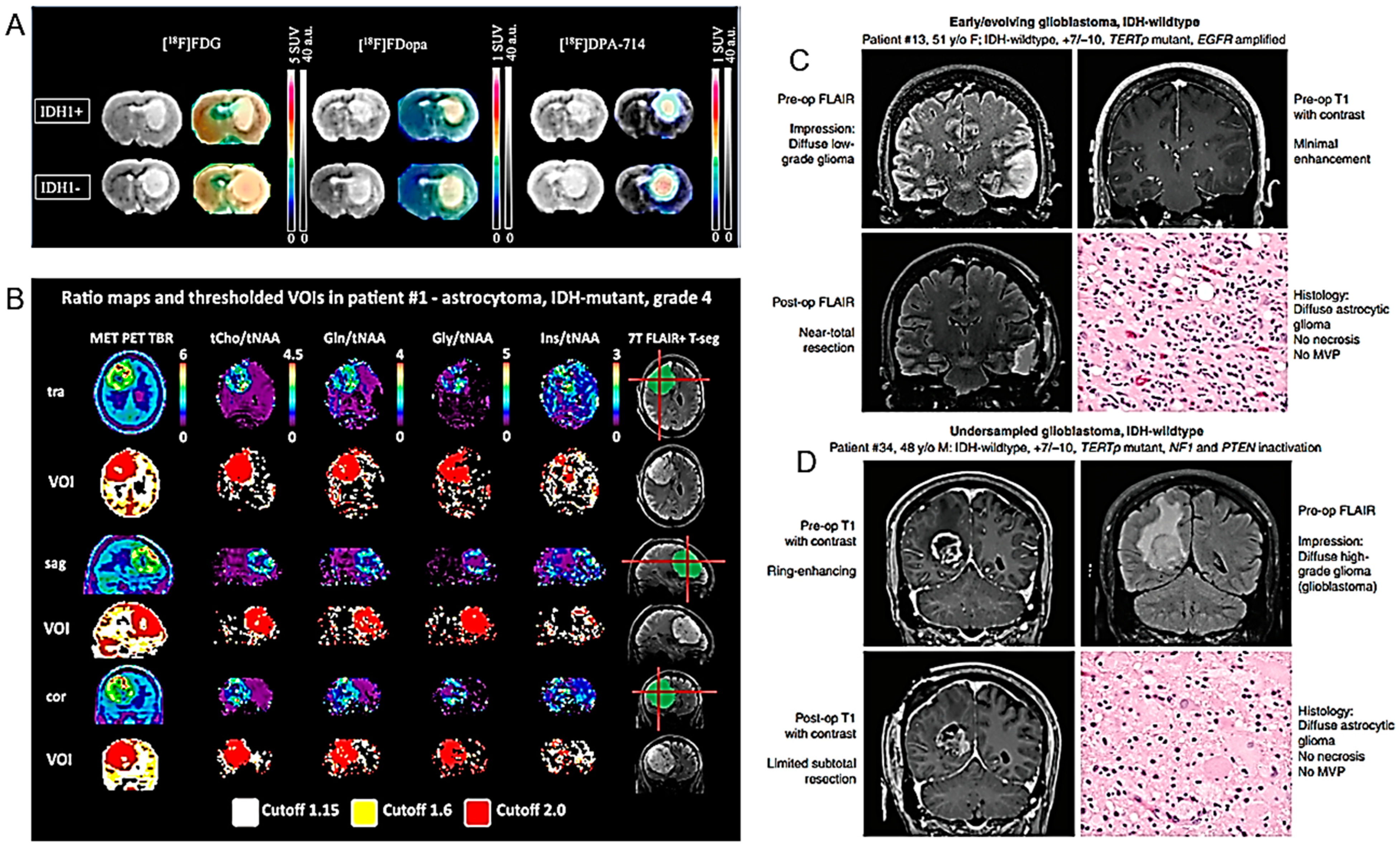
| 18F-DPA-714 and 18F-FDOPA correlate with the IDH1 mutation in HGG. | U87 human GB isogenic cell lines with or without the IDH1 mutation grafted into rat brains, and examined, in vitro, in vivo and ex vivo. PET imaging sessions, with radiotracers specific for glycolytic (18F-FDG), amino acid (18F-FDopa) and inflammation metabolism (18F-DPA-714). | [65] |
| 68Ga-DOTA-(Ser)3-LTVSPWY specifically recognizes HER2 receptors. | U87 GB cell line and xenografted U87 GB tumor-bearing mice. | [66] |
| Multiparametric 18F-FDG PET/MRI diagnostic model based on conventional MRI features and quantitative analysis of the enhancing tumors and peritumoral regions is superior to single parameter in the differentiation of HGG and PCNSL. | 45 patients with HGG and 20 patients with PCNSL undergoing simultaneous 18F-FDG PET, ASL PWI and DWI with hybrid PET/MRI before treatment. | [67] |
| 18F-FET PET can avoid the negative consequences of premature chemotherapy discontinuation. | Effectiveness and cost effectiveness of serial 18F-FET PET imaging determination by analysis of published clinical data. | [68] |
| 18F-FDOPA PET may contribute to prediction of glioma molecular parameters. | 72 retrospectively selected, newly diagnosed glioma patients with 18F-FDOPA PET dynamic acquisitions. | [69] |
| Evaluation of 18F-DOPA PET-guided re-irradiation for progressive HGG. | 20 adults with recurrent or progressive HGG previously treated with RT. | [70] |
| Elevated FBY activity found in primary GB, recurrent glioma and metastatic brain tumor which may suggest boron neutron capture therapy. | 35 patients with 36 lesions prospectively examined with FBY PET and MRI. | [71] |
| PSMA expression evaluated prospectively in recurrent HGG using Glu-NH-CO-NH-Lys-(Ahx)-[68Ga-68 (HBED-CC)]-(68Ga-68 PSMA) PET. | 49 lesions from 30 patients detected on MRI and further analyzed by fused PET/MRI with 68Ga-PSMA. | [72] |
| 7T MRS compared to PET. Gln and Gly suggested as possible PET tracers. | In 24 HGG patients, 7T MRS and routine PET were co-registered and hotspot volumes of interest (VOI) were compared. | [73] |
| Other diagnostic models | ||
| Elevated NADH is a metabolic consequence of TERT expression in cancer. [U-2H]-pyruvate is related to early response to therapy, prior to anatomic modifications. | RNAi, doxycycline-inducible expression systems, and pharmacologic inhibitors used in preclinical patient-derived tumor models. | [74] |
| Routine use of genomic and/or epigenomic profiling proposed to accurately classify gliomas. | 38 adult patients with IDH-wild-type diffuse astrocytic gliomas lacking necrosis or microvascular proliferation on histologic examination. | [75] |
| Hypothesis that molGBs are histological GBs diagnosed early. | 65 patients diagnosed with molGB. | [76] |
| Patients with DAG g experience clinical courses similar to GB. | 25 patients matching the DAG g diagnosis. | [77] |
| Proposed use of molecular profiling to guide enrolment in early phase trials. | Patients enrolled in early phase trials of cytotoxic therapies, small molecule inhibitors or monoclonal antibodies from 2008 to 2018. | [78] |
| The CT-based TA may help in differentiating between primary and secondary malignancies. | 36 patients with solitary BTs examined by CT. | [79] |
| Intramitochondrial heme biosynthesis factors as pharmacological targets to enhance intraoperative 5-ALA fluorescence visualization. | 19 strongly fluorescing and 21 non-fluorescing tissue samples from neurosurgical adult-type diffuse gliomas (WHO grades II–IV). | [80] |
| Grade-specific cerebrovascular dysregulation in the entire brain of glioma patients. | 96 patients with histologically confirmed cerebral glioma. | [81] |
| Systemic inflammatory biomarkers may contribute to the differential diagnosis of PCNSL from HGG. | 42 PCNSL versus 16 HGG patients. | [82] |
| Gyriform infiltration is a specific imaging marker of molecular GBs. | 426 patients: 31 molecular GB, 294 IDH-wild-type GB, 50 IDH-mutant astrocytoma, and 51 IDH-mutant 1p19q-codeleted oligodendroglioma. | [83] |
| Molecular investigations play an important role in the diagnosis and therapy of iHGG. | 11 children under five years of age with newly diagnosed HGG. | [84] |
| A specific diagnostic pathway proposed for patients with suspected TDL. | 41 TDLs and 91 HGG patients. | [85] |
3.1.3. Differentiating between HGG and Specific Pathologies
Lymphoma and other Primary Tumors
Metastases
Pseudoprogression and other Post-Treatment Effects
3.1.4. Specific Aspects
Pediatric Studies
Surgical Planning
IDH Mutation Identification
H3 K27M Mutation Identification
3.2. PET
3.2.1. Preclinical Studies
3.2.2. Clinical Studies
3.3. Other Modelling Advances
3.3.1. Preclinical Studies
3.3.2. Clinical Studies
4. Models for the Prognosis of HGG
4.1. Preclinical Studies
| Major Finding | Experimental System | Ref. |
|---|---|---|
| Increased accumulation of Fe and Se in tumor and Cu in peritumoral tissue in rodent models. | Orthotopic rat models of GB. | [103] |
| LAT1 as a new marker for GICs. | LAT1+ and LAT1- glioma cells sorted by flow cytometry. | [104] |
| Circulating miR-181a/b, miR-410 and miR-155 as diagnostic and prognostic biomarkers in HGG. | Determination of pre- and postoperative plasma levels of miR-181a/b, miR-410 and miR-155 in 114 HGG patients, 77 LGG patients and 85 healthy volunteers as control group. | [105] |
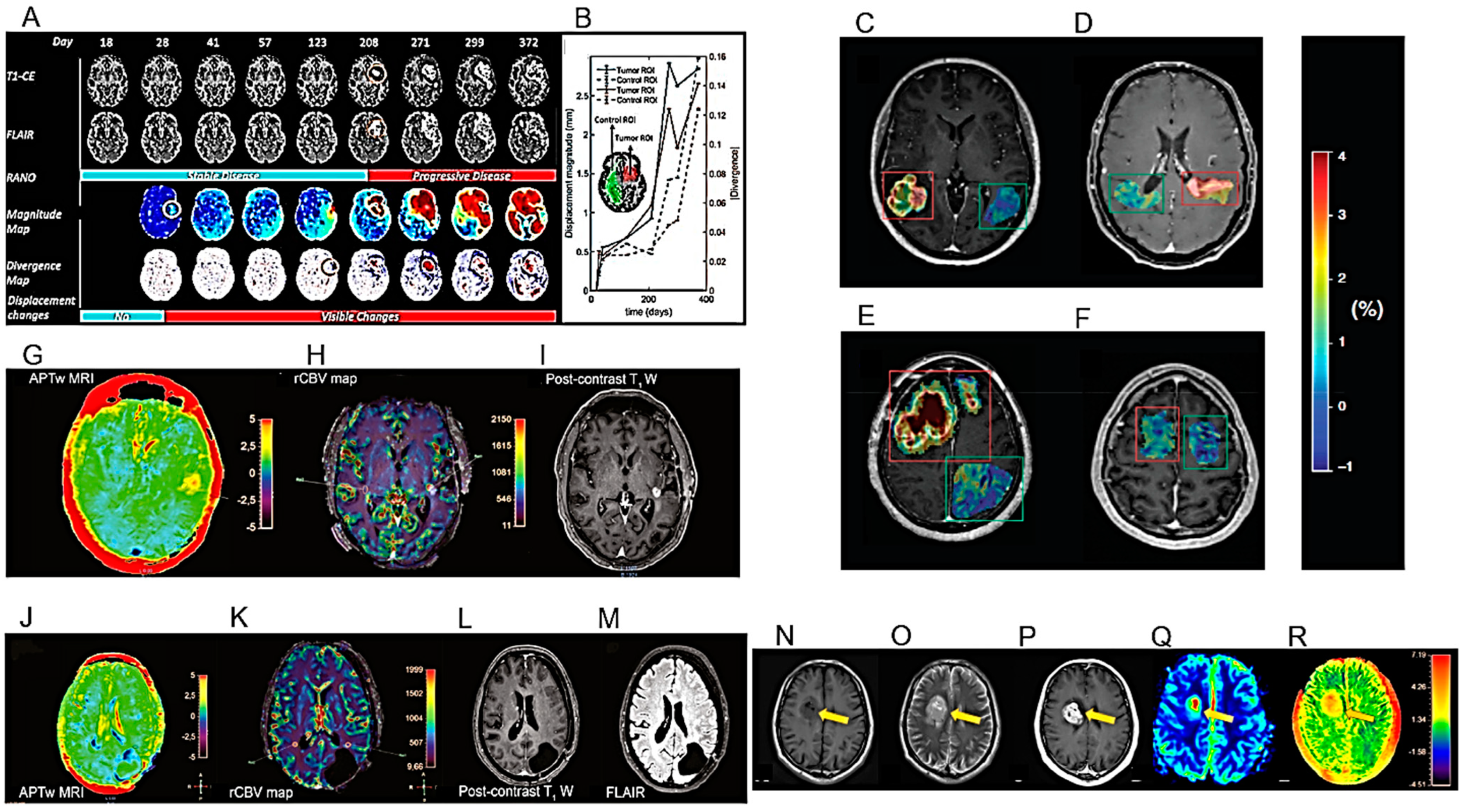
| Multi-parameter MRI as a non-invasive method for the prognosis of DMG. | 84 patients with DMG including 40 patients with OS > 12 months and 44 patients with OS < 12 months. | [106] |
| The S100 protein signature in the HGG patients’ prognosis. | Determination of the expression profiles of 17 S100 family genes in glioma. | [107] |
| The relationship between glioma angiogenesis and the malignant phenotype, immune characteristics, and prognosis. | An angiogenesis pathway score assessing the status of intra-glioma angiogenesis using public datasets. | [108] |
| Dismal prognosis in H3.3 G34-mutant glioma patients. | 30 adults with H3.3 G34-mutant diffuse gliomas. | [109] |
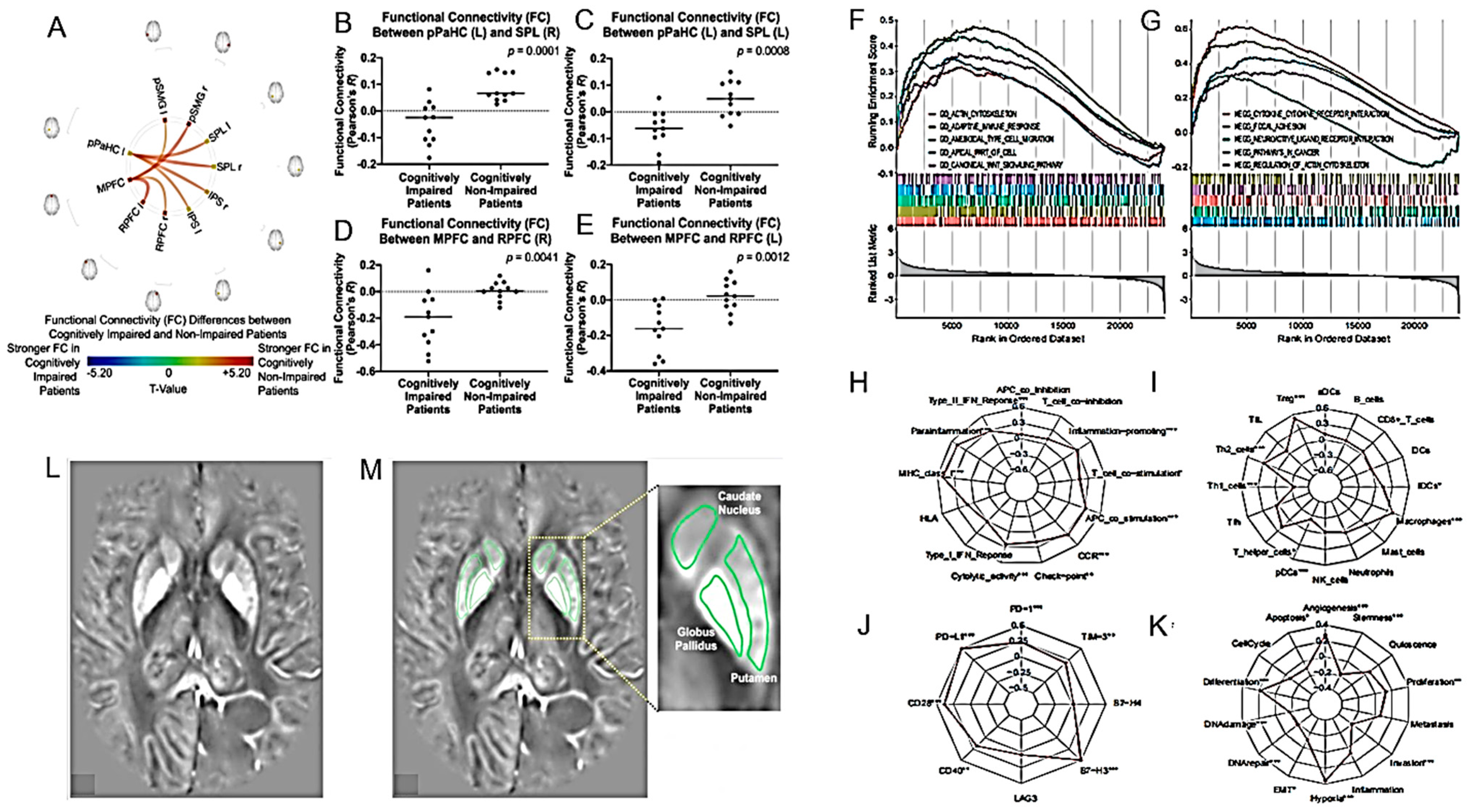
| rs-fMRI may identify neural correlates for cognitive and daily functioning in glioma patients. | 22 patients with diffuse gliomas who completed treatment within the past 10 years. | [110] |
| Assessment of side effects of RT should include depression. | 15 patients with HGG receiving standard radio(chemo)therapy. | [111] |
| The association between peripheral blood tests, cMRI and prognosis. | 131 GB patients. | [112] |
| Basal ganglia iron levels as a biomarker in glioma prognosis and treatment. | 59 patients with brain lesions. | [113] |
| A “DeepRisk” learning model predicting glioma survival from whole-brain MRI. | 1556 patients with diffuse gliomas. | [114] |
| A nomogram based on MRI radiomics and clinical features for predicting H3 K27M mutation in pediatric HGGs. | 107 patients with pHGGs with a midline location of the brain including 79 patients with H3 K27M mutation. | [115] |
| A nomogram based on clinical pathology, genetic factors, and MRI predicting early recurrence of HGG. | 154 patients with HGG classified into recurrence and nonrecurrence groups based on the pathological diagnosis and RANO criteria. | [116] |
| A novel ARG-related risk signature as a prognostic marker. | 1738 glioma patients collected from three public databases. | [117] |
| A 14 radiomic features-based prognostic model constructed from preoperative T2-weighted MRI images. | 652 glioma patients across three independent cohorts. | [118] |
| A combination of 18F-DOPA PET and MRI for distinguishing TP from TIE after RT. | 76 patients showing at least one gadolinium-enhanced lesion on the T1-w MRI sequence. | [119] |
| The relationship between CE in MRI and fluorescence during surgery in glioma patients. | 179 patients with newly diagnosedgrade II and grade III gliomas who received 5-ALA for resection. | [120] |
| Differences in survival between patients with primary and secondary GS. | 94 GS patients; 70 with primary disease and 24 with secondary. | [121] |
| The performance status of elderly patients is the most important prognostic factor. | 198 patients with grade IV glioma over 65 years at the time of diagnosis; grade III gliomas with nonmutated R132HIDH1 and radiographically only diagnosed gliomas. | [122] |
| The association between the Ki-67 index and edema. | MRI studies of 70 patients with GB acquired up to one week before surgery. | [123] |
| Elevated prognostic capacity of imaging-based risk stratification in patients with diffuse glioma, NOS. | 220 patients classified as diffuse glioma, NOS. | [124] |
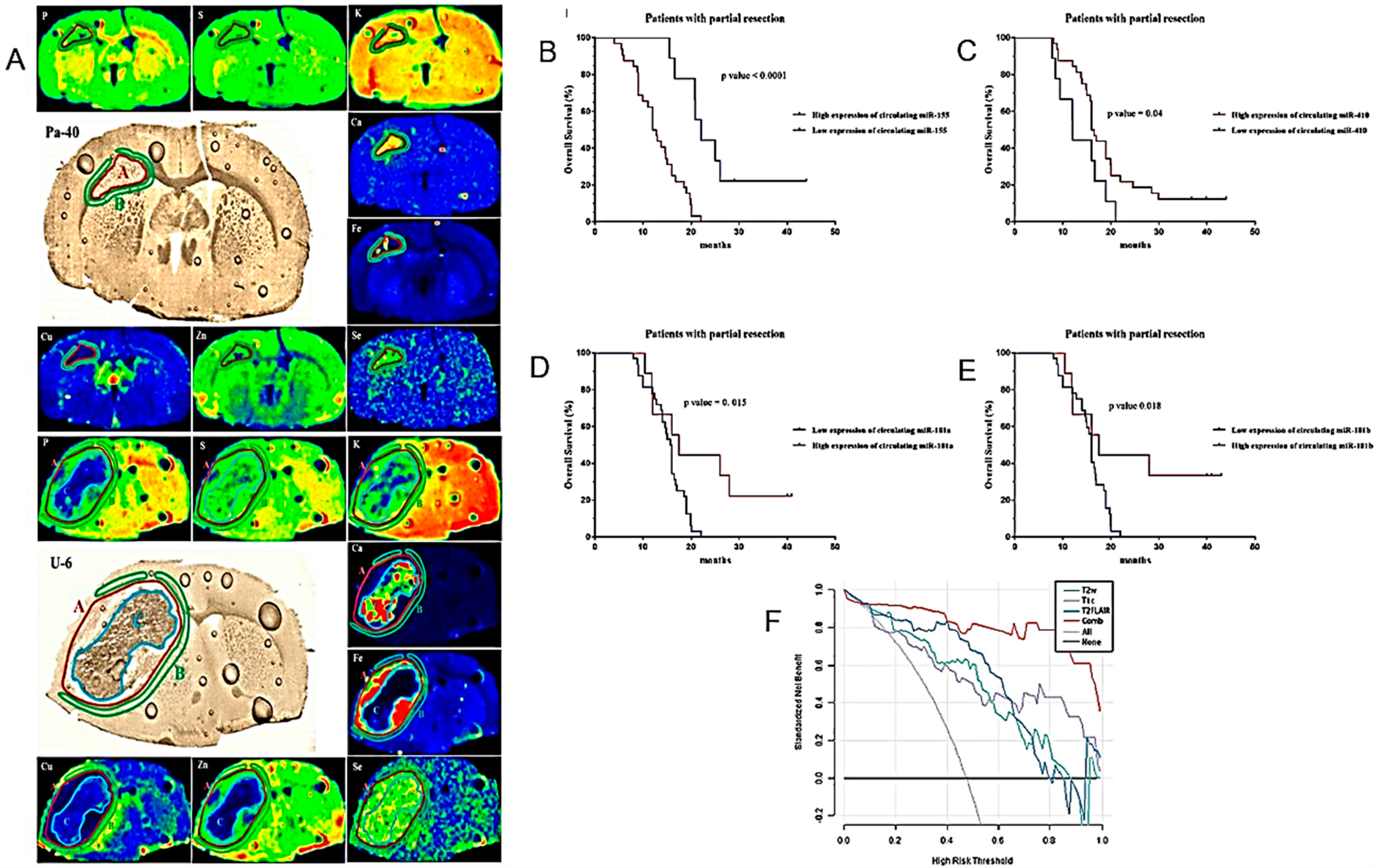
4.2. Clinical Studies
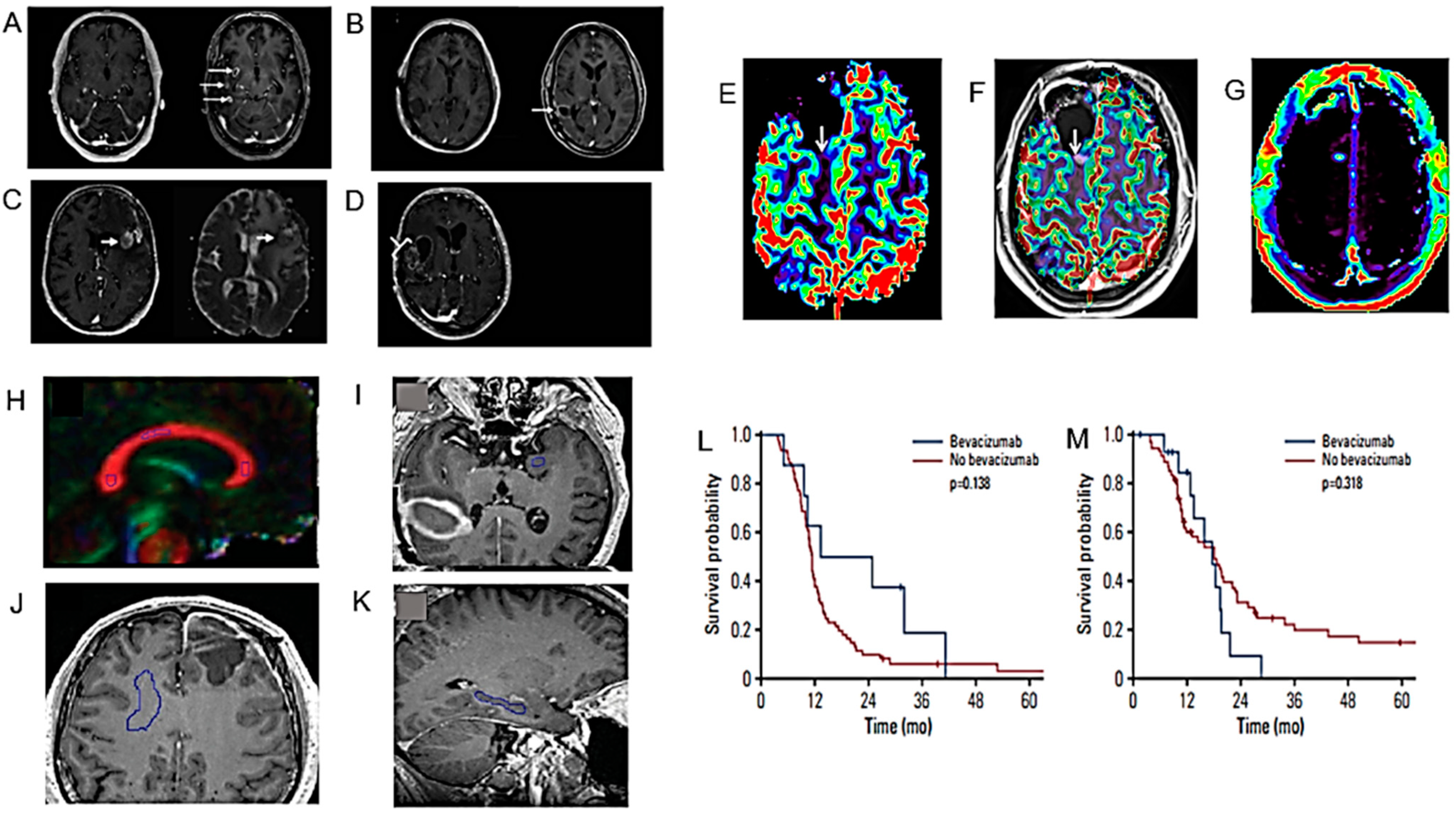
5. Discussion
5.1. Modelling the Diagnosis of HGG
5.2. Modelling the Prognosis of HGG
5.3. Regulating the End of Life
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Castaño-Vinyals, G.; Sadetzki, S.; Vermeulen, R.; Momoli, F.; Kundi, M.; Merletti, F.; Maslanyj, M.; Calderon, C.; Wiart, J.; Lee, A.K.; et al. Wireless Phone use in Childhood and Adolescence and Neuroepithelial Brain Tumours: Results from the International MOBI-Kids Study. Environ. Int. 2022, 160, 107069. [Google Scholar] [CrossRef]
- Schüz, J.; Pirie, K.; Reeves, G.K.; Floud, S.; Beral, V.; Million Women Study Collaborators. Cellular Telephone use and the Risk of Brain Tumors: Update of the UK Million Women Study. J. Natl. Cancer Inst. 2022, 114, 704–711. [Google Scholar] [CrossRef]
- Braganza, M.Z.; Kitahara, C.M.; Berrington de Gonzalez, A.; Inskip, P.D.; Johnson, K.J.; Rajaraman, P. Ionizing Radiation and the Risk of Brain and Central Nervous System Tumors: A Systematic Review. Neuro-Oncology 2012, 14, 1316–1324. [Google Scholar] [CrossRef]
- Auvinen, A.; Cardis, E.; Blettner, M.; Moissonnier, M.; Sadetzki, S.; Giles, G.; Johansen, C.; Swerdlow, A.; Cook, A.; Fleming, S.; et al. Diagnostic Radiological Examinations and Risk of Intracranial Tumours in Adults-Findings from the Interphone Study. Int. J. Epidemiol. 2022, 51, 537–546. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Montella, L.; Cuomo, M.; Del Gaudio, N.; Buonaiuto, M.; Costabile, D.; Visconti, R.; Di Risi, T.; Vinciguerra, R.; Trio, F.; Ferraro, S.; et al. Epigenetic Alterations in Glioblastomas: Diagnostic, Prognostic and Therapeutic Relevance. Int. J. Cancer 2023, 153, 476–488. [Google Scholar] [CrossRef]
- Wu, H.; Wei, M.; Li, Y.; Ma, Q.; Zhang, H. Research Progress on the Regulation Mechanism of Key Signal Pathways Affecting the Prognosis of Glioma. Front. Mol. Neurosci. 2022, 15, 910543. [Google Scholar] [CrossRef]
- Yu, J.; Lai, M.; Zhou, Z.; Zhou, J.; Hu, Q.; Li, J.; Li, H.; Chen, L.; Wen, L.; Zhou, M.; et al. The PTEN-Associated Immune Prognostic Signature Reveals the Landscape of the Tumor Microenvironment in Glioblastoma. J. Neuroimmunol. 2023, 376, 578034. [Google Scholar] [CrossRef]
- Mekala, J.R.; Adusumilli, K.; Chamarthy, S.; Angirekula, H.S.R. Novel Sights on Therapeutic, Prognostic, and Diagnostics Aspects of Non-Coding RNAs in Glioblastoma Multiforme. Metab. Brain Dis. 2023, 38, 1801–1829. [Google Scholar] [CrossRef]
- Di Nunno, V.; Gatto, L.; Tosoni, A.; Bartolini, S.; Franceschi, E. Implications of BRAF V600E Mutation in Gliomas: Molecular Considerations, Prognostic Value and Treatment Evolution. Front. Oncol. 2023, 12, 1067252. [Google Scholar] [CrossRef]
- Muniz, T.P.; Mason, W.P. BRAF Mutations in CNS Tumors-Prognostic Markers and Therapeutic Targets. CNS Drugs 2023, 37, 587–598. [Google Scholar] [CrossRef]
- Lhermitte, B.; Wolf, T.; Chenard, M.P.; Coca, A.; Todeschi, J.; Proust, F.; Hirsch, E.; Schott, R.; Noel, G.; Guerin, E.; et al. Molecular Heterogeneity in BRAF-Mutant Gliomas: Diagnostic, Prognostic, and Therapeutic Implications. Cancers 2023, 15, 1268. [Google Scholar] [CrossRef]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical Implications of the 2021 Edition of the WHO Classification of Central Nervous System Tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef]
- Frosina, G. Recapitulating the Key Advances in the Diagnosis and Prognosis of High-Grade Gliomas: Second Half of 2021 Update. Int. J. Mol. Sci. 2023, 24, 6375. [Google Scholar] [CrossRef]
- Frosina, G. Radiotherapy of High-Grade Gliomas: Dealing with a Stalemate. Crit. Rev. Oncol. Hematol. 2023, 190, 104110. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Carloni, A.; Bernardini, M.; Mattei, C.; De Magistris, A.V.; Llabres-Diaz, F.; Williams, J.; Gutierrez-Quintana, R.; Oevermann, A.; Schweizer-Gorgas, D.; Finck, C.; et al. Can MRI Differentiate between Ring-Enhancing Gliomas and Intra-Axial Abscesses? Vet. Radiol. Ultrasound 2022, 63, 563–572. [Google Scholar] [CrossRef]
- Ebrahimpour, A.; Tirgar, F.; Hajipour-Verdom, B.; Abbasi, A.; Hadjighassem, M.; Abdolmaleki, P.; Hosseindoost, S.; Javadi, S.A.H.; Hashemi, H.; Foroushani, A.R.; et al. Detection of Glioblastoma Multiforme using Quantitative Molecular Magnetic Resonance Imaging Based on 5-Aminolevulinic Acid: In Vitro and In Vivo Studies. Magn. Reson. Mater. Phys. Biol. Med. 2022, 35, 3–15. [Google Scholar] [CrossRef]
- Carrete, L.R.; Young, J.S.; Cha, S. Advanced Imaging Techniques for Newly Diagnosed and Recurrent Gliomas. Front. Neurosci. 2022, 16, 787755. [Google Scholar] [CrossRef]
- Muscas, G.; Orlandini, S.; Becattini, E.; Battista, F.; Staartjes, V.E.; Serra, C.; Della Puppa, A. Radiomic Features Associated with Extent of Resection in Glioma Surgery. Acta Neurochir. Suppl. 2022, 134, 341–347. [Google Scholar]
- Fatania, K.; Mohamud, F.; Clark, A.; Nix, M.; Short, S.C.; O’Connor, J.; Scarsbrook, A.F.; Currie, S. Intensity Standardization of MRI Prior to Radiomic Feature Extraction for Artificial Intelligence Research in Glioma-a Systematic Review. Eur. Radiol. 2022, 32, 7014–7025. [Google Scholar] [CrossRef]
- Ehret, F.; Kaul, D.; Clusmann, H.; Delev, D.; Kernbach, J.M. Machine Learning-Based Radiomics in Neuro-Oncology. Acta Neurochir. Suppl. 2022, 134, 139–151. [Google Scholar]
- Seo, M.; Ahn, K.J.; Choi, Y.; Shin, N.Y.; Jang, J.; Kim, B.S. Volumetric Measurement of Relative CBV using T1-Perfusion-Weighted MRI with High Temporal Resolution Compared with Traditional T2*-Perfusion-Weighted MRI in Postoperative Patients with High-Grade Gliomas. AJNR Am. J. Neuroradiol. 2022, 43, 864–871. [Google Scholar] [CrossRef]
- Scola, E.; Desideri, I.; Bianchi, A.; Gadda, D.; Busto, G.; Fiorenza, A.; Amadori, T.; Mancini, S.; Miele, V.; Fainardi, E. Assessment of Brain Tumors by Magnetic Resonance Dynamic Susceptibility Contrast Perfusion-Weighted Imaging and Computed Tomography Perfusion: A Comparison Study. Radiol. Med. 2022, 127, 664–672. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Marhold, F.; Oberndorfer, S.; Heinz, G.; Buchfelder, M.; Kinfe, T.M.; Meyer-Bäse, A. Radiophysiomics: Brain Tumors Classification by Machine Learning and Physiological MRI Data. Cancers 2022, 14, 2363. [Google Scholar] [CrossRef]
- Zeineldin, R.A.; Karar, M.E.; Elshaer, Z.; Coburger, J.; Wirtz, C.R.; Burgert, O.; Mathis-Ullrich, F. Explainability of Deep Neural Networks for MRI Analysis of Brain Tumors. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1673–1683. [Google Scholar] [CrossRef]
- Lerner, M.; Medin, J.; Jamtheim Gustafsson, C.; Alkner, S.; Olsson, L.E. Prospective Clinical Feasibility Study for MRI-Only Brain Radiotherapy. Front. Oncol. 2022, 11, 812643. [Google Scholar] [CrossRef]
- Ammari, S.; Bône, A.; Balleyguier, C.; Moulton, E.; Chouzenoux, É.; Volk, A.; Menu, Y.; Bidault, F.; Nicolas, F.; Robert, P.; et al. Can Deep Learning Replace Gadolinium in Neuro-Oncology?: A Reader Study. Investig. Radiol. 2022, 57, 99–107. [Google Scholar] [CrossRef]
- Calabrese, E.; Rudie, J.D.; Rauschecker, A.M.; Villanueva-Meyer, J.E.; Clarke, J.L.; Solomon, D.A.; Cha, S. Combining Radiomics and Deep Convolutional Neural Network Features from Preoperative MRI for Predicting Clinically Relevant Genetic Biomarkers in Glioblastoma. Neurooncol. Adv. 2022, 4, vdac060. [Google Scholar] [CrossRef]
- Duran-Peña, A.; Ducray, F.; Ramirez, C.; Bauchet, L.; Constans, J.M.; Grand, S.; Guillamo, J.S.; Larrieu-Ciron, D.; Frappaz, D.; Pyatigorskaya, N.; et al. Adult Brainstem Glioma Differential Diagnoses: An MRI-Based Approach in a Series of 68 Patients. J. Neurol. 2022, 269, 4349–4362. [Google Scholar] [CrossRef]
- Fuster-Garcia, E.; Thokle Hovden, I.; Fløgstad Svensson, S.; Larsson, C.; Vardal, J.; Bjørnerud, A.; Emblem, K.E. Quantification of Tissue Compression Identifies High-Grade Glioma Patients with Reduced Survival. Cancers 2022, 14, 1725. [Google Scholar] [CrossRef]
- Wamelink, I.J.H.G.; Kuijer, J.P.A.; Padrela, B.E.; Zhang, Y.; Barkhof, F.; Mutsaerts, H.J.M.M.; Petr, J.; van de Giessen, E.; Keil, V.C. Reproducibility of 3 T APT-CEST in Healthy Volunteers and Patients with Brain Glioma. J. Magn. Reson. Imaging 2022, 57, 206–215. [Google Scholar] [CrossRef]
- Friismose, A.I.; Markovic, L.; Nguyen, N.; Gerke, O.; Schulz, M.K.; Mussmann, B.R. Amide Proton Transfer-Weighted MRI in the Clinical Setting—Correlation with Dynamic Susceptibility Contrast Perfusion in the Post-Treatment Imaging of Adult Glioma Patients at 3T. Radiography 2022, 28, 95–101. [Google Scholar] [CrossRef]
- Zhang, H.W.; Liu, X.L.; Zhang, H.B.; Li, Y.Q.; Wang, Y.L.; Feng, Y.N.; Deng, K.; Lei, Y.; Huang, B.; Lin, F. Differentiation of Meningiomas and Gliomas by Amide Proton Transfer Imaging: A Preliminary Study of Brain Tumour Infiltration. Front. Oncol. 2022, 12, 886968. [Google Scholar] [CrossRef]
- Shakir, T.M.; Fengli, L.; Chenguang, G.; Chen, N.; Zhang, M.; Shaohui, M. 1H-MR Spectroscopy in Grading of Cerebral Glioma: A New View Point, MRS Image Quality Assessment. Acta Radiol. Open 2022, 11, 20584601221077068. [Google Scholar] [CrossRef]
- Lavrova, A.; Teunissen, W.H.T.; Warnert, E.A.H.; van den Bent, M.; Smits, M. Diagnostic Accuracy of Arterial Spin Labeling in Comparison with Dynamic Susceptibility Contrast-Enhanced Perfusion for Brain Tumor Surveillance at 3T MRI. Front. Oncol. 2022, 12, 849657. [Google Scholar] [CrossRef]
- Qin, J.; Yu, Z.; Yao, Y.; Liang, Y.; Tang, Y.; Wang, B. Susceptibility-Weighted Imaging Cannot Distinguish Radionecrosis from Recurrence in Brain Metastases After Radiotherapy: A Comparison with High-Grade Gliomas. Clin. Radiol. 2022, 77, e585–e591. [Google Scholar] [CrossRef]
- Voicu, I.P.; Pravatà, E.; Panara, V.; Navarra, R.; Mattei, P.A.; Caulo, M. Differentiating Solitary Brain Metastases from High-Grade Gliomas with MR: Comparing Qualitative Versus Quantitative Diagnostic Strategies. Radiol. Med. 2022, 127, 891–898. [Google Scholar] [CrossRef]
- Farche, M.K.; Fachinetti, N.O.; da Silva, L.R.; Matos, L.A.; Appenzeller, S.; Cendes, F.; Reis, F. Revisiting the use of Proton Magnetic Resonance Spectroscopy in Distinguishing between Primary and Secondary Malignant Tumors of the Central Nervous System. Neuroradiol. J. 2022, 35, 619–626. [Google Scholar] [CrossRef]
- Bodensohn, R.; Forbrig, R.; Quach, S.; Reis, J.; Boulesteix, A.L.; Mansmann, U.; Hadi, I.; Fleischmann, D.F.; Mücke, J.; Holzgreve, A.; et al. MRI-Based Contrast Clearance Analysis shows High Differentiation Accuracy between Radiation-Induced Reactions and Progressive Disease After Cranial Radiotherapy. ESMO Open 2022, 7, 100424. [Google Scholar] [CrossRef]
- Mansour, M.; Vitale, V.; Lombardi, G.; Riva, G.; Pancheri, F.; Zanusso, M. Modification of MRI Pattern of High-Grade Glioma Pseudoprogression in Regorafenib Therapy. J. Med. Imaging Radiat. Oncol. 2022, 66, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Ari, A.P.; Akkurt, B.H.; Musigmann, M.; Mammadov, O.; Blömer, D.A.; Kasap, D.N.G.; Henssen, D.J.H.A.; Nacul, N.G.; Sartoretti, E.; Sartoretti, T.; et al. Pseudoprogression Prediction in High Grade Primary CNS Tumors by use of Radiomics. Sci. Rep. 2022, 12, 5915. [Google Scholar] [CrossRef] [PubMed]
- Dündar, T.T.; Cetinkaya, E.; Yurtsever, İ.; Uysal, Ö.; Aralaşmak, A. Follow-Up of High-Grade Glial Tumor; Differentiation of Posttreatment Enhancement and Tumoral Enhancement by DCE-MR Perfusion. Contrast Media Mol. Imaging 2022, 2022, 6948422. [Google Scholar] [CrossRef] [PubMed]
- Flies, C.M.; van Leuken, K.H.; Voorde, M.T.; Verhoeff, J.J.C.; De Vos, F.Y.F.; Seute, T.; Robe, P.A.; Witkamp, T.D.; Hendrikse, J.; Dankbaar, J.W.; et al. Conventional MRI Criteria to Differentiate Progressive Disease from Treatment-Induced Effects in High-Grade (WHO Grade 3–4) Gliomas. Neurology 2022, 99, e77–e88. [Google Scholar] [CrossRef] [PubMed]
- Zakhari, N.; Taccone, M.; Torres, C.; Chakraborty, S.; Sinclair, J.; Woulfe, J.; Jansen, G.; Cron, G.; Nguyen, T.B. Qualitative Assessment of Advanced MRI in Post-Treatment High Grade Gliomas Follow Up: Do we Agree? Can. Assoc. Radiol. J. 2022, 73, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Rydelius, A.; Lampinen, B.; Rundcrantz, A.; Bengzon, J.; Engelholm, S.; van Westen, D.; Kinhult, S.; Knutsson, L.; Lätt, J.; Nilsson, M.; et al. Diffusion Tensor Imaging in Glioblastoma Patients Treated with Volumetric Modulated Arc Radiotherapy: A Longitudinal Study. Acta Oncol. 2022, 61, 680–687. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H.; Kim, Y.; Lim, D.H.; Park, S.H.; Ahn, S.D.; Kim, I.A.; Im, J.H.; Chung, J.W.; Kim, J.Y.; et al. Suggestions for Escaping the Dark Ages for Pediatric Diffuse Intrinsic Pontine Glioma Treated with Radiotherapy: Analysis of Prognostic Factors from the National Multicenter Study. Cancer. Res. Treat. 2023, 55, 41–49. [Google Scholar] [CrossRef]
- Erker, C.; Lane, A.; Chaney, B.; Leary, S.; Minturn, J.E.; Bartels, U.; Packer, R.J.; Dorris, K.; Gottardo, N.G.; Warren, K.E.; et al. Characteristics of Patients ≥10 Years of Age with Diffuse Intrinsic Pontine Glioma: A Report from the International DIPG/DMG Registry. Neuro-Oncology 2022, 24, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Lazow, M.A.; Fuller, C.; DeWire, M.; Lane, A.; Bandopadhayay, P.; Bartels, U.; Bouffet, E.; Cheng, S.; Cohen, K.J.; Cooney, T.M.; et al. Accuracy of Central Neuro-Imaging Review of DIPG Compared with Histopathology in the International DIPG Registry. Neuro-Oncology 2022, 24, 821–833. [Google Scholar] [CrossRef]
- Wagner, M.W.; Namdar, K.; Napoleone, M.; Hainc, N.; Amirabadi, A.; Fonseca, A.; Laughlin, S.; Shroff, M.M.; Bouffet, E.; Hawkins, C.; et al. Radiomic Features Based on MRI Predict Progression-Free Survival in Pediatric Diffuse Midline Glioma/Diffuse Intrinsic Pontine Glioma. Can. Assoc. Radiol. J. 2022, 74, 119–126. [Google Scholar] [CrossRef]
- Rodriguez, D.; Calmon, R.; Aliaga, E.S.; Warren, D.; Warmuth-Metz, M.; Jones, C.; Mackay, A.; Varlet, P.; Le Deley, M.C.; Hargrave, D.; et al. MRI and Molecular Characterization of Pediatric High-Grade Midline Thalamic Gliomas: The HERBY Phase II Trial. Radiology 2022, 304, 174–182. [Google Scholar] [CrossRef]
- Bhatia, A.; Lee, V.K.; Qian, Y.; Paldino, M.J.; Ceschin, R.; Hect, J.; Mountz, J.M.; Sun, D.; Kohanbash, G.; Pollack, I.F.; et al. Quantitative Sodium ((23)Na) MRI in Pediatric Gliomas: Initial Experience. Diagnostics 2022, 12, 1223. [Google Scholar] [CrossRef]
- Stock, A.; Hancken, C.V.; Kandels, D.; Kortmann, R.D.; Dietzsch, S.; Timmermann, B.; Pietsch, T.; Bison, B.; Schmidt, R.; Pham, M.; et al. Pseudoprogression is Frequent After Front-Line Radiation Therapy in Pediatric Low-Grade Glioma: Results from the German Low-Grade Glioma Cohort. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 1190–1202. [Google Scholar] [CrossRef]
- Maloney, E.; Perez, F.A.; Iyer, R.S.; Otto, R.K.; Wright, J.N.; Menashe, S.J.; Hippe, D.S.; Shaw, D.W.W.; Stanescu, A.L. Non-Inferiority of a Non-Gadolinium-Enhanced Magnetic Resonance Imaging Follow-Up Protocol for Isolated Optic Pathway Gliomas. Pediatr. Radiol. 2022, 52, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Deng, K.; Wang, P.; Chen, C.; Luo, Y.; Yuan, S.; Wen, J. Application of Diffusion Kurtosis Imaging to the Study of Edema in Solid and Peritumoral Areas of Glioma. Magn. Reson. Imaging 2022, 86, 10–16. [Google Scholar] [CrossRef]
- Hagiwara, A.; Tatekawa, H.; Yao, J.; Raymond, C.; Everson, R.; Patel, K.; Mareninov, S.; Yong, W.H.; Salamon, N.; Pope, W.B.; et al. Visualization of Tumor Heterogeneity and Prediction of Isocitrate Dehydrogenase Mutation Status for Human Gliomas using Multiparametric Physiologic and Metabolic MRI. Sci. Rep. 2022, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Y.; Zhou, X.; Bao, S.; Chen, Y.; Ge, M.; Jia, Z. A Radiomics Model Based on DCE-MRI and DWI may Improve the Prediction of Estimating IDH1 Mutation and Angiogenesis in Gliomas. Eur. J. Radiol. 2022, 147, 110141. [Google Scholar] [CrossRef] [PubMed]
- Cindil, E.; Sendur, H.N.; Cerit, M.N.; Erdogan, N.; Celebi, F.; Dag, N.; Celtikci, E.; Inan, A.; Oner, Y.; Tali, T. Prediction of IDH Mutation Status in High-Grade Gliomas using DWI and High T1-Weight DSC-MRI. Acad. Radiol. 2022, 29 (Suppl. S3), S52–S62. [Google Scholar] [CrossRef]
- Petridis, P.D.; Horenstein, C.I.; Pereira, B.; Wu, P.B.; Samanamud, J.; Marie, T.; Boyett, D.; Sudhakar, T.D.; Sheth, S.A.; McKhann, G.M.; et al. BOLD Asynchrony Elucidates Tumor Burden in IDH-Mutated Gliomas. Neuro-Oncology 2022, 24, 78–87. [Google Scholar] [CrossRef]
- Yao, J.; Hagiwara, A.; Oughourlian, T.C.; Wang, C.; Raymond, C.; Pope, W.B.; Salamon, N.; Lai, A.; Ji, M.; Nghiemphu, P.L.; et al. Diagnostic and Prognostic Value of pH- and Oxygen-Sensitive Magnetic Resonance Imaging in Glioma: A Retrospective Study. Cancers 2022, 14, 2520. [Google Scholar] [CrossRef]
- Kikuchi, K.; Togao, O.; Yamashita, K.; Momosaka, D.; Kikuchi, Y.; Kuga, D.; Hata, N.; Mizoguchi, M.; Yamamoto, H.; Iwaki, T.; et al. Quantitative Relaxometry using Synthetic MRI could be Better than T2-FLAIR Mismatch Sign for Differentiation of IDH-Mutant Gliomas: A Pilot Study. Sci. Rep. 2022, 12, 9197. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Shen, N.X.; Wu, D.; Zhang, J.; Zhang, J.X.; Jiang, J.J.; Zhu, W.Z. A Comparative Study between Tumor Blood Vessels and Dynamic Contrast-Enhanced MRI for Identifying Isocitrate Dehydrogenase Gene 1 (IDH1) Mutation Status in Glioma. Curr. Med. Sci. 2022, 42, 650–657. [Google Scholar] [CrossRef]
- Kathrani, N.; Chauhan, R.S.; Kotwal, A.; Kulanthaivelu, K.; Bhat, M.D.; Saini, J.; Prasad, C.; Chakrabarti, D.; Santosh, V.; Uppar, A.M.; et al. Diffusion and Perfusion Imaging Biomarkers of H3 K27M Mutation Status in Diffuse Midline Gliomas. Neuroradiology 2022, 64, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; She, D.; Xing, Z.; Lin, X.; Wang, F.; Song, Y.; Cao, D. Multiparametric MRI-Based Radiomics Model for Predicting H3 K27M Mutant Status in Diffuse Midline Glioma: A Comparative Study Across Different Sequences and Machine Learning Techniques. Front. Oncol. 2022, 12, 796583. [Google Scholar] [CrossRef] [PubMed]
- Clément, A.; Zaragori, T.; Filosa, R.; Ovdiichuk, O.; Beaumont, M.; Collet, C.; Roeder, E.; Martin, B.; Maskali, F.; Barberi-Heyob, M.; et al. Multi-Tracer and Multiparametric PET Imaging to Detect the IDH Mutation in Glioma: A Preclinical Translational in Vitro, in Vivo, and Ex Vivo Study. Cancer Imaging 2022, 22, 16. [Google Scholar] [CrossRef]
- Ranjbar, V.; Molavipordanjani, S.; Biabani Ardakani, J.; Akhlaghi, M.; Nikkholgh, B.; Hosseinimehr, S.J. Initial Preclinical Evaluation of 68Ga-DOTA-(Ser)3-LTVSPWY Peptide as a PET Radiotracer for Glioblastoma Targeting and Imaging. Nucl. Med. Commun. 2022, 43, 945–951. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Wang, K.; Li, X.; Zhao, X.; Chen, Q.; Zhang, W.; Ai, L. Differentiation of High-Grade Glioma and Primary Central Nervous System Lymphoma: Multiparametric Imaging of the Enhancing Tumor and Peritumoral Regions Based on Hybrid (18)F-FDG PET/MRI. Eur. J. Radiol. 2022, 150, 110235. [Google Scholar] [CrossRef]
- Rosen, J.; Ceccon, G.; Bauer, E.K.; Werner, J.M.; Tscherpel, C.; Dunkl, V.; Rapp, M.; Sabel, M.; Herrlinger, U.; Heinzel, A.; et al. Cost-Effectiveness of (18)F-FET PET for Early Treatment Response Assessment in Glioma Patients Following Adjuvant Temozolomide Chemotherapy. J. Nucl. Med. 2022, 63, 1677–1682. [Google Scholar]
- Zaragori, T.; Oster, J.; Roch, V.; Hossu, G.; Chawki, M.B.; Grignon, R.; Pouget, C.; Gauchotte, G.; Rech, F.; Blonski, M.; et al. (18)F-FDOPA PET for the Noninvasive Prediction of Glioma Molecular Parameters: A Radiomics Study. J. Nucl. Med. 2022, 63, 147–157. [Google Scholar] [CrossRef]
- Breen, W.G.; Youland, R.S.; Giri, S.; Jacobson, S.B.; Pafundi, D.H.; Brown, P.D.; Hunt, C.H.; Mahajan, A.; Ruff, M.W.; Kizilbash, S.H.; et al. Initial Results of a Phase II Trial of (18)F-DOPA PET-Guided Re-Irradiation for Recurrent High-Grade Glioma. J. Neurooncol. 2022, 158, 323–330. [Google Scholar] [CrossRef]
- Kong, Z.; Li, Z.; Chen, J.; Liu, S.; Liu, D.; Li, J.; Li, N.; Ma, W.; Feng, F.; Wang, Y.; et al. Metabolic Characteristics of [(18)F]Fluoroboronotyrosine (FBY) PET in Malignant Brain Tumors. Nucl. Med. Biol. 2022, 106–107, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; ArunRaj, S.T.; Bhullar, K.; Haresh, K.P.; Gupta, S.; Ballal, S.; Yadav, M.; Singh, M.; Damle, N.A.; Garg, A.; et al. Ga-68 PSMA PET/CT in Recurrent High-Grade Gliomas: Evaluating PSMA Expression In Vivo. Neuroradiology 2022, 64, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Hangel, G.; Lazen, P.; Sharma, S.; Hristoska, B.; Cadrien, C.; Furtner, J.; Rausch, I.; Lipka, A.; Niess, E.; Hingerl, L.; et al. 7T HR FID-MRSI Compared to Amino Acid PET: Glutamine and Glycine as Promising Biomarkers in Brain Tumors. Cancers 2022, 14, 2163. [Google Scholar] [CrossRef] [PubMed]
- Batsios, G.; Taglang, C.; Tran, M.; Stevers, N.; Barger, C.; Gillespie, A.M.; Ronen, S.M.; Costello, J.F.; Viswanath, P. Deuterium Metabolic Imaging Reports on TERT Expression and Early Response to Therapy in Cancer. Clin. Cancer Res. 2022, 28, 3526–3536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lucas, C.G.; Young, J.S.; Morshed, R.A.; McCoy, L.; Oberheim Bush, N.A.; Taylor, J.W.; Daras, M.; Butowski, N.A.; Villanueva-Meyer, J.E.; et al. Prospective Genomically-Guided Identification of ’Early/Evolving’ and ’Undersampled’ IDH-Wildtype Glioblastoma Leads to Improved Clinical Outcomes. Neuro-Oncology 2022, 24, 1749–1762. [Google Scholar] [CrossRef]
- Ramos-Fresnedo, A.; Domingo, R.A.; Perez-Vega, C.; Pullen, M.W.; Akinduro, O.O.; Almeida, J.P.; Jentoft, M.E.; Bendok, B.R.; Chaichana, K.L.; Trifiletti, D.M.; et al. The Early Infiltrative Phase of GBM Hypothesis: Are Molecular Glioblastomas Histological Glioblastomas in the Making? A Preliminary Multicenter Study. J. Neurooncol. 2022, 158, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Grogan, D.; Bray, D.P.; Cosgrove, M.; Boucher, A.; Erwood, A.; Linder, D.F.; Mendoza, P.; Morales, B.; Pradilla, G.; Nduom, E.K.; et al. Clinical and Radiographic Characteristics of Diffuse Astrocytic Glioma, IDH-Wildtype, with Molecular Features of Glioblastoma: A Single Institution Review. J. Neurooncol. 2022, 157, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Younan, N.; Castanon Alvarez, E.; Ammari, S.; Alentorn, A.; Dumont, S.; Frenel, J.S.; Di Stefano, A.L.; Louvel, G.; Michot, J.M.; et al. Genome-Driven Medicine for Patients with Recurrent Glioma Enrolled in Early Phase Trials. Eur. J. Cancer 2022, 163, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Mărginean, L.; Ștefan, P.A.; Lebovici, A.; Opincariu, I.; Csutak, C.; Lupean, R.A.; Coroian, P.A.; Suciu, B.A. CT in the Differentiation of Gliomas from Brain Metastases: The Radiomics Analysis of the Peritumoral Zone. Brain Sci. 2022, 12, 109. [Google Scholar] [CrossRef]
- Mischkulnig, M.; Roetzer-Pejrimovsky, T.; Lötsch-Gojo, D.; Kastner, N.; Bruckner, K.; Prihoda, R.; Lang, A.; Martinez-Moreno, M.; Furtner, J.; Berghoff, A.; et al. Heme Biosynthesis Factors and 5-ALA Induced Fluorescence: Analysis of mRNA and Protein Expression in Fluorescing and Non-Fluorescing Gliomas. Front. Med. 2022, 9, 907442. [Google Scholar] [CrossRef]
- Cai, S.; Shi, Z.; Zhou, S.; Liang, Y.; Wang, L.; Wang, K.; Zhang, L. Cerebrovascular Dysregulation in Patients with Glioma Assessed with Time-Shifted BOLD fMRI. Radiology 2022, 304, 155–163. [Google Scholar] [CrossRef]
- Gupta, T.; Nayak, P.; Baviskar, Y.; Gupta, M.; Moiyadi, A.; Epari, S.; Janu, A.; Purandare, N.; Rangarajan, V.; Bagal, B.; et al. Systemic Inflammatory Biomarkers in Primary Central Nervous System Lymphoma versus High-Grade Glioma: Exploratory, Comparative and Correlative Analysis. CNS Oncol. 2022, 11, CNS83-0004. [Google Scholar] [CrossRef]
- Mesny, E.; Barritault, M.; Izquierdo, C.; Poncet, D.; d’Hombres, A.; Guyotat, J.; Jouanneau, E.; Ameli, R.; Honnorat, J.; Meyronet, D.; et al. Gyriform Infiltration as Imaging Biomarker for Molecular Glioblastomas. J. Neurooncol. 2022, 157, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Di Ruscio, V.; Carai, A.; Del Baldo, G.; Vinci, M.; Cacchione, A.; Miele, E.; Rossi, S.; Antonelli, M.; Barresi, S.; Caulo, M.; et al. Molecular Landscape in Infant High-Grade Gliomas: A Single Center Experience. Diagnostics 2022, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- French, H.; Fontes-Villalba, A.; Maharaj, M.; Naidoo, C.S.Y.; Bhatia, K.; Paterson, A.; Cook, R.; Parratt, J. Tumefactive Multiple Sclerosis Versus High Grade Glioma: A Diagnostic Dilemma. Surg. Neurol. Int. 2022, 13, 146. [Google Scholar] [CrossRef]
- Pasqualetti, F.; Malfatti, G.; Cantarella, M.; Gonnelli, A.; Montrone, S.; Montemurro, N.; Gadducci, G.; Giannini, N.; Pesaresi, I.; Perrini, P.; et al. Role of Magnetic Resonance Imaging Following Postoperative Radiotherapy in Clinical Decision-Making of Patients with High-Grade Glioma. Radiol. Med. 2022, 127, 803–808. [Google Scholar] [CrossRef]
- Zhou, J.; Tryggestad, E.; Wen, Z.; Lal, B.; Zhou, T.; Grossman, R.; Wang, S.; Yan, K.; Fu, D.X.; Ford, E.; et al. Differentiation between Glioma and Radiation Necrosis using Molecular Magnetic Resonance Imaging of Endogenous Proteins and Peptides. Nat. Med. 2011, 17, 130–134. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, L.; Verma, G.; Hangel, G.; Neal, A.; Moffat, B.A.; Stockmann, J.P.; Andronesi, O.C.; Balchandani, P.; Hadjipanayis, C.G. Application of 7T MRS to High-Grade Gliomas. AJNR Am. J. Neuroradiol. 2022, 43, 1378–1395. [Google Scholar] [CrossRef]
- Cassinelli Petersen, G.I.; Shatalov, J.; Verma, T.; Brim, W.R.; Subramanian, H.; Brackett, A.; Bahar, R.C.; Merkaj, S.; Zeevi, T.; Staib, L.H.; et al. Machine Learning in Differentiating Gliomas from Primary CNS Lymphomas: A Systematic Review, Reporting Quality, and Risk of Bias Assessment. AJNR Am. J. Neuroradiol. 2022, 43, 526–533. [Google Scholar] [CrossRef]
- Kurokawa, R.; Baba, A.; Kurokawa, M.; Ota, Y.; Hassan, O.; Capizzano, A.; Kim, J.; Johnson, T.; Srinivasan, A.; Moritani, T. Neuroimaging of Astroblastomas: A Case Series and Systematic Review. J. Neuroimaging 2022, 32, 201–212. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liang, Y.; Wei, R.; Zhang, W.; Yao, W.; Luo, S.; Pang, X.; Wang, Y.; Jiang, X.; et al. Radiomics can Differentiate High-Grade Glioma from Brain Metastasis: A Systematic Review and Meta-Analysis. Eur. Radiol. 2022, 32, 8039–8051. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.; Dai, K. Hydrogen Proton Magnetic Resonance Spectroscopy (MRS) in Differential Diagnosis of Intracranial Tumors: A Systematic Review. Contrast Media Mol. Imaging 2022, 2022, 7242192. [Google Scholar] [CrossRef] [PubMed]
- Matsumae, M.; Nishiyama, J.; Kuroda, K. Intraoperative MR Imaging during Glioma Resection. Magn. Reson. Med. Sci. 2022, 21, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Guo, P.; Wang, P.; Liu, P.; Lin, D.D.M.; Fan, H.; Li, Y.; Wei, Z.; Lin, Z.; Jiang, D.; et al. Deep-Learning-Enabled Brain Hemodynamic Mapping using Resting-State fMRI. NPJ Digit. Med. 2023, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Borja, A.J.; Saini, J.; Raynor, W.Y.; Ayubcha, C.; Werner, T.J.; Alavi, A.; Revheim, M.E.; Nagaraj, C. Role of Molecular Imaging with PET/MR Imaging in the Diagnosis and Management of Brain Tumors. PET Clin. 2022, 17, 431–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang-Yin, J.T.; Girard, A.; Bertaux, M. What does PET Imaging Bring to Neuro-Oncology in 2022? A Review. Cancers 2022, 14, 879. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Kas, A.; Darcourt, J.; Guedj, E. PET Imaging in Neuro-Oncology: An Update and Overview of a Rapidly Growing Area. Cancers 2022, 14, 1103. [Google Scholar] [CrossRef] [PubMed]
- van de Weijer, T.; Broen, M.P.G.; Moonen, R.P.M.; Hoeben, A.; Anten, M.; Hovinga, K.; Compter, I.; van der Pol, J.A.J.; Mitea, C.; Lodewick, T.M.; et al. The use of (18)F-FET-PET-MRI in Neuro-Oncology: The Best of both Worlds-A Narrative Review. Diagnostics 2022, 12, 1202. [Google Scholar] [CrossRef] [PubMed]
- Piccardo, A.; Albert, N.L.; Borgwardt, L.; Fahey, F.H.; Hargrave, D.; Galldiks, N.; Jehanno, N.; Kurch, L.; Law, I.; Lim, R.; et al. Joint EANM/SIOPE/RAPNO Practice Guidelines/SNMMI Procedure Standards for Imaging of Paediatric Gliomas using PET with Radiolabelled Amino Acids and [(18)F]FDG: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3852–3869. [Google Scholar] [CrossRef]
- García Vicente, A.M.; Pérez-Beteta, J.; Bosque, J.J.; Soriano Castrejón, Á.; Pérez-García, V.M. Multiple and Diffuse Gliomas by 18F-Fluorocholine PET/CT: Two Sides of the Same Coin. Clin. Nucl. Med. 2022, 47, e457–e465. [Google Scholar] [CrossRef]
- Ricciardi, L.; Sturiale, C.L.; Scerrati, A.; Stifano, V.; Somma, T.; Ius, T.; Trungu, S.; Acqui, M.; Raco, A.; Miscusi, M.; et al. 5-Aminolevulinic Acid False-Positive Rates in Newly Diagnosed and Recurrent Glioblastoma: Do Pseudoprogression and Radionecrosis Play a Role? A Meta-Analysis. Front. Oncol. 2022, 12, 848036. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.F.J.; Schwartz, C.; Machegger, L.; Zellinger, B.; Hölzl, D.; Schlicker, H.U.; Pöppe, J.; Ladisich, B.; Spendel, M.; Kral, M.; et al. A Patient with Two Gliomas with Independent Oligodendroglioma and Glioblastoma Biology Proved by DNA-Methylation Profiling: A Case Report and Review of the Literature. Brain Tumor Pathol. 2022, 39, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Planeta, K.; Setkowicz, Z.; Czyzycki, M.; Janik-Olchawa, N.; Ryszawy, D.; Janeczko, K.; Simon, R.; Baumbach, T.; Chwiej, J. Altered Elemental Distribution in Male Rat Brain Tissue as a Predictor of Glioblastoma Multiforme Growth-Studies using SR-XRF Microscopy. Int. J. Mol. Sci. 2022, 23, 703. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Liu, X.H.; Zeng, X.Y.; Long, Q.Y.; Liu, Y.H.; Mao, Q. Evaluation of CD98 Light Chain-LAT1 as a Potential Marker of Cancer Stem-Like Cells in Glioblastoma. Biochim. Biophys. Acta Mol. Cell. Res. 2022, 1869, 119303. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Al-Zahrani, A.; Beylerli, O.; Sufianov, R.; Talybov, R.; Meshcheryakova, S.; Sufianova, G.; Gareev, I.; Sufianov, A. Circulating miRNAs as Diagnostic and Prognostic Biomarkers in High-Grade Gliomas. Front. Oncol. 2022, 12, 898537. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.B.; Liao, Y.T.; Zhou, J.F.; Cheng, L.N.; He, P.; Wu, S.N.; Wang, W.S.; Zhou, Q. Non-Invasive Prediction of Survival Time of Midline Glioma Patients using Machine Learning on Multiparametric MRI Radiomics Features. Front. Neurol. 2022, 13, 866274. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Lv, P.; Lou, Y. Alarm Signal S100-Related Signature is Correlated with Tumor Microenvironment and Predicts Prognosis in Glioma. Dis. Markers 2022, 2022, 4968555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, Y.X.; Zhang, W.L.; Lian, H.Y.; Iranzad, N.; Wang, E.; Li, Y.C.; Tong, H.C.; Li, L.Y.; Dong, L.Y.; et al. Intra-Tumoral Angiogenesis Correlates with Immune Features and Prognosis in Glioma. Aging 2022, 14, 4402–4424. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shao, L.; Li, H.; Yao, K.; Duan, Z.; Zhi, C.; Song, S.; Cheng, Y.; Wang, F.; Wang, W.; et al. Histone H3.3 G34-Mutant Diffuse Gliomas in Adults. Am. J. Surg. Pathol. 2022, 46, 249–257. [Google Scholar] [CrossRef]
- Wang, C.; Van Dyk, K.; Cho, N.; Raymond, C.; Choi, J.; Salamon, N.; Pope, W.B.; Lai, A.; Cloughesy, T.F.; Nghiemphu, P.L.; et al. Characterization of Cognitive Function in Survivors of Diffuse Gliomas using Resting-State Functional MRI (Rs-fMRI). Brain Imaging Behav. 2022, 16, 239–251. [Google Scholar] [CrossRef]
- Donix, M.; Seidlitz, A.; Buthut, M.; Löck, S.; Meissner, G.; Matthes, C.; Troost, E.G.C.; Baumann, M.; Raschke, F.; Linn, J.; et al. Subjective Memory Impairment in Glioma Patients with Curative Radiotherapy. Radiother. Oncol. 2022, 171, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Jin, J.; Lu, J.; Wang, C.; Wu, Z.; Zhu, Z.; Tu, M.; Su, Z.; Li, Q. A Multielement Prognostic Nomogram Based on a Peripheral Blood Test, Conventional MRI and Clinical Factors for Glioblastoma. Front. Neurol. 2022, 13, 822735. [Google Scholar] [CrossRef] [PubMed]
- Reith, T.P.; Prah, M.A.; Choi, E.J.; Lee, J.; Wujek, R.; Al-Gizawiy, M.; Chitambar, C.R.; Connelly, J.M.; Schmainda, K.M. Basal Ganglia Iron Content Increases with Glioma Severity using Quantitative Susceptibility Mapping: A Potential Biomarker of Tumor Severity. Tomography 2022, 8, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Yan, J.; Zhang, S.; Liang, C.; Lv, X.; Zou, Y.; Zhang, H.; Liang, D.; Zhang, Z.; Chen, Y. Glioma Survival Prediction from Whole-Brain MRI without Tumor Segmentation using Deep Attention Network: A Multicenter Study. Eur. Radiol. 2022, 32, 5719–5729. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zheng, H.; Li, J.; Zhang, Y.; Duan, S.; Li, Y.; Wang, D. MRI-Based Radiomics Signature and Clinical Factor for Predicting H3K27M Mutation in Pediatric High-Grade Gliomas Located in the Midline of the Brain. Eur. Radiol. 2022, 32, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ke, X.; Xue, C.; Li, S.; Huang, X.; Zhang, B.; Zhou, J. A Nomogram for Predicting Early Recurrence in Patients with High-Grade Gliomas. World Neurosurg. 2022, 164, e619–e628. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Hong, L.; Gong, H.; Kan, G.; Zhang, P.; Cui, T.T.; Fan, G.; Si, X.; Zhu, J. Identification of a Nomogram with an Autophagy-Related Risk Signature for Survival Prediction in Patients with Glioma. Int. J. Gen. Med. 2022, 15, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, L.; Li, Y.; Qian, Z.; Wu, F.; He, Y.; Jiang, H.; Li, R.; Wang, D.; Zhai, Y.; et al. An MRI Radiomics Approach to Predict Survival and Tumour-Infiltrating Macrophages in Gliomas. Brain 2022, 145, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Bertaux, M.; Berenbaum, A.; Di Stefano, A.L.; Rozenblum, L.; Soret, M.; Bergeret, S.; Hoang-Xuan, K.; Tainturier, L.E.; Sgard, B.; Habert, M.O.; et al. Hybrid [(18)F]-F-DOPA PET/MRI Interpretation Criteria and Scores for Glioma Follow-Up After Radiotherapy. Clin. Neuroradiol. 2022, 32, 735–747. [Google Scholar] [CrossRef]
- Müther, M.; Jaber, M.; Johnson, T.D.; Orringer, D.A.; Stummer, W. A Data-Driven Approach to Predicting 5-Aminolevulinic Acid-Induced Fluorescence and World Health Organization Grade in Newly Diagnosed Diffuse Gliomas. Neurosurgery 2022, 90, 800–806. [Google Scholar] [CrossRef]
- Amer, A.; Khose, S.; Alhasan, H.; Pokhylevych, H.; Fuller, G.; Chasen, N.; de Groot, J.; Johnson, J.M. Clinical and Survival Characteristics of Primary and Secondary Gliosarcoma Patients. Clin. Neurol. Neurosurg. 2022, 214, 107146. [Google Scholar] [CrossRef]
- Pirkkalainen, J.M.; Jääskeläinen, A.S.; Halonen, P. Retrospective Single-Center Study on Elderly Patients with Glioblastoma between 2014 and 2018 Evaluating the Effect of Age and Performance Status on Survival. Neurooncol. Pract. 2022, 9, 142–148. [Google Scholar] [CrossRef]
- Caramanti, R.; Aprígio, R.M.; D Aglio Rocha, C.E.; Morais, D.F.; Góes, M.J.; Chaddad-Neto, F.; Tognola, W.A. Is Edema Zone Volume Associated with Ki-67 Index in Glioblastoma Patients? Cureus 2022, 14, e24246. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.B.; Kim, H.S.; Park, J.E.; Park, S.Y.; Nam, Y.K.; Nam, S.J.; Kim, Y.H.; Kim, J.H. Diffuse Glioma, Not Otherwise Specified: Imaging-Based Risk Stratification Achieves Histomolecular-Level Prognostication. Eur. Radiol. 2022, 32, 7780–7788. [Google Scholar] [CrossRef]
- De Luca, C.; Virtuoso, A.; Papa, M.; Certo, F.; Barbagallo, G.M.V.; Altieri, R. Regional Development of Glioblastoma: The Anatomical Conundrum of Cancer Biology and its Surgical Implication. Cells 2022, 11, 1349. [Google Scholar] [CrossRef]
- Jian, A.; Liu, S.; Di Ieva, A. Artificial Intelligence for Survival Prediction in Brain Tumors on Neuroimaging. Neurosurgery 2022, 91, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Zander, E.; Ardeleanu, A.; Singleton, R.; Bede, B.; Wu, Y.; Zheng, S. A Functional Artificial Neural Network for Noninvasive Pretreatment Evaluation of Glioblastoma Patients. Neurooncol. Adv. 2021, 4, vdab167. [Google Scholar] [CrossRef]
- Schiavolin, S.; Mariniello, A.; Broggi, M.; Abete-Fornara, G.; Bollani, A.; Palmas, G.G.; Bottini, G.; Querzola, M.; Scarpa, P.; Casarotti, A.; et al. Patient-Reported Outcome and Cognitive Measures to be used in Vascular and Brain Tumor Surgery: Proposal for a Minimum Set. Neurol. Sci. 2022, 43, 5143–5151. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yi, L.; Kang, Q.M.; Zhou, J.; Chen, T.Q.; Hugnot, J.P.; Yu, S.C. Glioma Invasion Along White Matter Tracts: A Dilemma for Neurosurgeons. Cancer Lett. 2022, 526, 103–111. [Google Scholar] [CrossRef]
- Alamer, O.B.; Jimenez, A.E.; Azad, T.D.; Bettegowda, C.; Mukherjee, D. H3K27M-Altered Diffuse Midline Gliomas among Adult Patients: A Systematic Review of Clinical Features and Survival Analysis. World Neurosurg. 2022, 165, e251–e264. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, C.; Ke, X.; Zhou, J. Treatment Response and Prognosis Evaluation in High-Grade Glioma: An Imaging Review Based on MRI. J. Magn. Reson. Imaging 2022, 56, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Leibetseder, A.; Leitner, J.; Mair, M.J.; Meckel, S.; Hainfellner, J.A.; Aichholzer, M.; Widhalm, G.; Dieckmann, K.; Weis, S.; Furtner, J.; et al. Prognostic Factors in Adult Brainstem Glioma: A Tertiary Care Center Analysis and Review of the Literature. J. Neurol. 2022, 269, 1574–1590. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.C.; Ma, C.; Yip, S. From Theory to Practice: Implementing the WHO 2021 Classification of Adult Diffuse Gliomas in Neuropathology Diagnosis. Brain Sci. 2023, 13, 817. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.L. 2021 Updates to the World Health Organization Classification of Adult-Type and Pediatric-Type Diffuse Gliomas: A Clinical Practice Review. Chin. Clin. Oncol. 2023, 12, 7–120. [Google Scholar] [CrossRef] [PubMed]
- Eraky, A.M.; Beck, R.T.; Treffy, R.W.; Aaronson, D.M.; Hedayat, H. Role of Advanced MR Imaging in Diagnosis of Neurological Malignancies: Current Status and Future Perspective. J. Integr. Neurosci. 2023, 22, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huo, X.; Wang, H.; Wang, C. Diagnostic Performance of Dynamic Susceptibility Contrast-Enhanced Perfusion-Weighted Imaging in Differentiating Recurrence from Radiation Injury in Postoperative Glioma: A Meta-Analysis. J. Comput. Assist. Tomogr. 2022, 46, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Duan, Y.; Liu, N.; Dong, J.; Liang, Y.; Ju, R. Value of DWI Combined with Magnetic Resonance Spectroscopy in the Differential Diagnosis between Recurrent Glioma and Radiation Injury: A Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 1629570. [Google Scholar] [CrossRef] [PubMed]
- Van Hese, L.; De Vleeschouwer, S.; Theys, T.; Rex, S.; Heeren, R.M.A.; Cuypers, E. The Diagnostic Accuracy of Intraoperative Differentiation and Delineation Techniques in Brain Tumours. Discov. Oncol. 2022, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, D.; Kong, Z.; Liu, Q.; Xing, H.; Wang, Y.; Wang, Y.; Ma, W. Prognostic Value of Choline and Other Metabolites Measured using (1)H-Magnetic Resonance Spectroscopy in Gliomas: A Meta-Analysis and Systemic Review. Metabolites 2022, 12, 1219. [Google Scholar] [CrossRef]
- Chekhonin, I.V.; Cohen, O.; Otazo, R.; Young, R.J.; Holodny, A.I.; Pronin, I.N. Magnetic Resonance Relaxometry in Quantitative Imaging of Brain Gliomas: A Literature Review. Neuroradiol. J. 2023, 19714009231173100. [Google Scholar] [CrossRef]
- Zhang, G.; Li, J.; Hui, X. Use of 18F-FDG-PET/CT in Differential Diagnosis of Primary Central Nervous System Lymphoma and High-Grade Gliomas: A Meta-Analysis. Front. Neurol. 2022, 13, 935459. [Google Scholar] [CrossRef]
- Schlürmann, T.; Waschulzik, B.; Combs, S.; Gempt, J.; Wiestler, B.; Weber, W.; Yakushev, I. Utility of Amino Acid PET in the Differential Diagnosis of Recurrent Brain Metastases and Treatment-Related Changes: A Meta-Analysis. J. Nucl. Med. 2023, 64, 816–821. [Google Scholar] [CrossRef]
- Fioni, F.; Chen, S.J.; Lister, I.N.E.; Ghalwash, A.A.; Long, M.Z. Differentiation of High Grade Glioma and Solitary Brain Metastases by Measuring Relative Cerebral Blood Volume and Fractional Anisotropy: A Systematic Review and Meta-Analysis of MRI Diagnostic Test Accuracy Studies. Br. J. Radiol. 2023, 96, 20220052. [Google Scholar] [CrossRef]
- Ninatti, G.; Pini, C.; Gelardi, F.; Sollini, M.; Chiti, A. The Role of PET Imaging in the Differential Diagnosis between Radiation Necrosis and Recurrent Disease in Irradiated Adult-Type Diffuse Gliomas: A Systematic Review. Cancers 2023, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- Xiaoxue, T.; Yinzhong, W.; Meng, Q.; Lu, X.; Lei, J. Diagnostic Value of PET with Different Radiotracers and MRI for Recurrent Glioma: A Bayesian Network Meta-Analysis. BMJ Open 2023, 13, e062555. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallières, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The Image Biomarker Standardization Initiative: Standardized Quantitative Radiomics for High-Throughput Image-Based Phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Wu, Z.; Xie, R.; Zeng, F.; Cai, H.; Lui, S.; Song, B.; Chen, L.; Wu, M. Advanced Imaging Techniques for Differentiating Pseudoprogression and Tumor Recurrence After Immunotherapy for Glioblastoma. Front. Immunol. 2021, 12, 790674. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Pope, W.B. Imaging Advances for Central Nervous System Tumors. Hematol. Oncol. Clin. N. Am. 2022, 36, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, F.; Rizzo, M.; Franceschi, S.; Lessi, F.; Paiar, F.; Buffa, F.M. New Perspectives in Liquid Biopsy for Glioma Patients. Curr. Opin. Oncol. 2022, 34, 705–712. [Google Scholar] [CrossRef]
- Andrews, L.J.; Davies, P.; Herbert, C.; Kurian, K.M. Pre-Diagnostic Blood Biomarkers for Adult Glioma. Front. Oncol. 2023, 13, 1163289. [Google Scholar] [CrossRef]
- Tűzesi, Á.; Hallal, S.; Satgunaseelan, L.; Buckland, M.E.; Alexander, K.L. Understanding the Epitranscriptome for Avant-Garde Brain Tumour Diagnostics. Cancers 2023, 15, 1232. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.N.; Whaley, L.A.; Norton, E.S.; Zarco, N.; Guerrero-Cázares, H. Extracellular Vesicles in the Glioblastoma Microenvironment: A Diagnostic and Therapeutic Perspective. Mol. Asp. Med. 2023, 91, 101167. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, Y.; Jiao, H.; Miao, X. The Prognostic Significance of PD-L1 Expression in Patients with Glioblastoma: A Meta-Analysis. Front. Oncol. 2022, 12, 925560. [Google Scholar] [CrossRef]
- Das, S.; Mishra, R.K.; Agrawal, A. Prognostic Factors Affecting Outcome of Multifocal Or Multicentric Glioblastoma: A Scoping Review. J. Neurosci. Rural Pract. 2023, 14, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, D.; Ohno, M.; Honda-Kitahara, M.; Miyakita, Y.; Takahashi, M.; Yanagisawa, S.; Tamura, Y.; Kikuchi, M.; Ichimura, K.; Narita, Y. Clinical Characteristics and Prognosis of Glioblastoma Patients with Infratentorial Recurrence. BMC Neurol. 2023, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Gorenflo, M.P.; Shen, A.; Murphy, E.S.; Cullen, J.; Yu, J.S. Area-Level Socioeconomic Status is Positively Correlated with Glioblastoma Incidence and Prognosis in the United States. Front. Oncol. 2023, 13, 1110473. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, X.; Huang, M.; Ma, M.; Huang, Q.; Huang, N.; Cheng, Y. Prognostic Value of Prognostic Nutritional Index Score and Controlling Nutritional Status Score in Patients with Glioblastoma: A Comprehensive Meta-Analysis. Front. Oncol. 2023, 13, 1117764. [Google Scholar] [CrossRef] [PubMed]
- Sadhwani, N.; Aggarwal, A.; Mishra, A.; Garg, K. Temporal Muscle Thickness as an Independent Prognostic Marker in Glioblastoma Patients-a Systematic Review and Meta-Analysis. Neurosurg. Rev. 2022, 45, 3619–3628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ni, Q. Prognostic Role of the Pretreatment Systemic Immune-Inflammation Index in Patients with Glioma: A Meta-Analysis. Front. Neurol. 2023, 14, 1094364. [Google Scholar] [CrossRef]
- Montemurro, N.; Pahwa, B.; Tayal, A.; Shukla, A.; De Jesus Encarnacion, M.; Ramirez, I.; Nurmukhametov, R.; Chavda, V.; De Carlo, A. Macrophages in Recurrent Glioblastoma as a Prognostic Factor in the Synergistic System of the Tumor Microenvironment. Neurol. Int. 2023, 15, 595–608. [Google Scholar] [CrossRef]
- Lin, L.; Li, X.; Zhu, S.; Long, Q.; Hu, Y.; Zhang, L.; Liu, Z.; Li, B.; Li, X. Ferroptosis-Related NFE2L2 and NOX4 Genes are Potential Risk Prognostic Biomarkers and Correlated with Immunogenic Features in Glioma. Cell Biochem. Biophys. 2023, 81, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Bispo, R.G.; Bastos Siqueira, I.F.; de Oliveira, B.F.S.; Moreira Fernandes, C.E.; Figueiredo, L.A.; Cintra, L.P.; de Oliveira, A.J.M. Prognostic Value of the Platelet-Lymphocyte Ratio for Glioblastoma: A Systematic Review. World Neurosurg. 2023, 175, 137–141.e1. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Torrens-Burton, A.; Sivell, S.; Moraes, F.Y.; Bulbeck, H.; Bernstein, M.; Nelson, A.; Fielding, H. Early Palliative Interventions for Improving Outcomes in People with a Primary Malignant Brain Tumour and their Carers. Cochrane Database Syst. Rev. 2022, 1, CD013440. [Google Scholar] [CrossRef] [PubMed]
- Walbert, T.; Stec, N.E. Palliative Care in Brain Tumors. Handb. Clin. Neurol. 2023, 191, 69–80. [Google Scholar] [PubMed]
- Di Paolo, M.; Gori, F.; Papi, L.; Turillazzi, E. A Review and Analysis of New Italian Law 219/2017: ‘Provisions for Informed Consent and Advance Directives Treatment’. BMC Med. Ethics 2019, 20, 17. [Google Scholar] [CrossRef]
- Pace, A.; Tanzilli, A.; Benincasa, D. Prognostication in Brain Tumors. Handb. Clin. Neurol. 2022, 190, 149–161. [Google Scholar]
- Li, Y.; Qin, Q.; Zhang, Y.; Cao, Y. Noninvasive Determination of the IDH Status of Gliomas using MRI and MRI-Based Radiomics: Impact on Diagnosis and Prognosis. Curr. Oncol. 2022, 29, 6893–6907. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Pan, M.; Mo, K.; Mao, Y.; Zou, D. Emerging Role of Artificial Intelligence in Diagnosis, Classification and Clinical Management of Glioma. Semin. Cancer Biol. 2023, 91, 110–123. [Google Scholar] [CrossRef]
- Chieffo, D.P.R.; Lino, F.; Ferrarese, D.; Belella, D.; Della Pepa, G.M.; Doglietto, F. Brain Tumor at Diagnosis: From Cognition and Behavior to Quality of Life. Diagnostics 2023, 13, 541. [Google Scholar] [CrossRef]
- Hu, Y.; Deng, F.; Zhang, L.; Hu, K.; Liu, S.; Zhong, S.; Yang, J.; Zeng, X.; Peng, X. Depression and Quality of Life in Patients with Gliomas: A Narrative Review. J. Clin. Med. 2022, 11, 4811. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frosina, G. Advancements in Image-Based Models for High-Grade Gliomas Might Be Accelerated. Cancers 2024, 16, 1566. https://doi.org/10.3390/cancers16081566
Frosina G. Advancements in Image-Based Models for High-Grade Gliomas Might Be Accelerated. Cancers. 2024; 16(8):1566. https://doi.org/10.3390/cancers16081566
Chicago/Turabian StyleFrosina, Guido. 2024. "Advancements in Image-Based Models for High-Grade Gliomas Might Be Accelerated" Cancers 16, no. 8: 1566. https://doi.org/10.3390/cancers16081566
APA StyleFrosina, G. (2024). Advancements in Image-Based Models for High-Grade Gliomas Might Be Accelerated. Cancers, 16(8), 1566. https://doi.org/10.3390/cancers16081566






