CD10 Expression Correlates with Earlier Tumour Stages and Left-Sided Tumour Location in Colorectal Cancer but Has No Prognostic Impact in a European Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Tissue Microarray Manufacturing
2.3. Immunohistochemistry
2.4. Statistics
3. Results
3.1. Technical Implications
3.2. Clinicopathological Parameters
3.3. CD10 Expression in Colorectal Cancer Cells
3.4. Association of CD10 Expression and Molecular Marker Expression
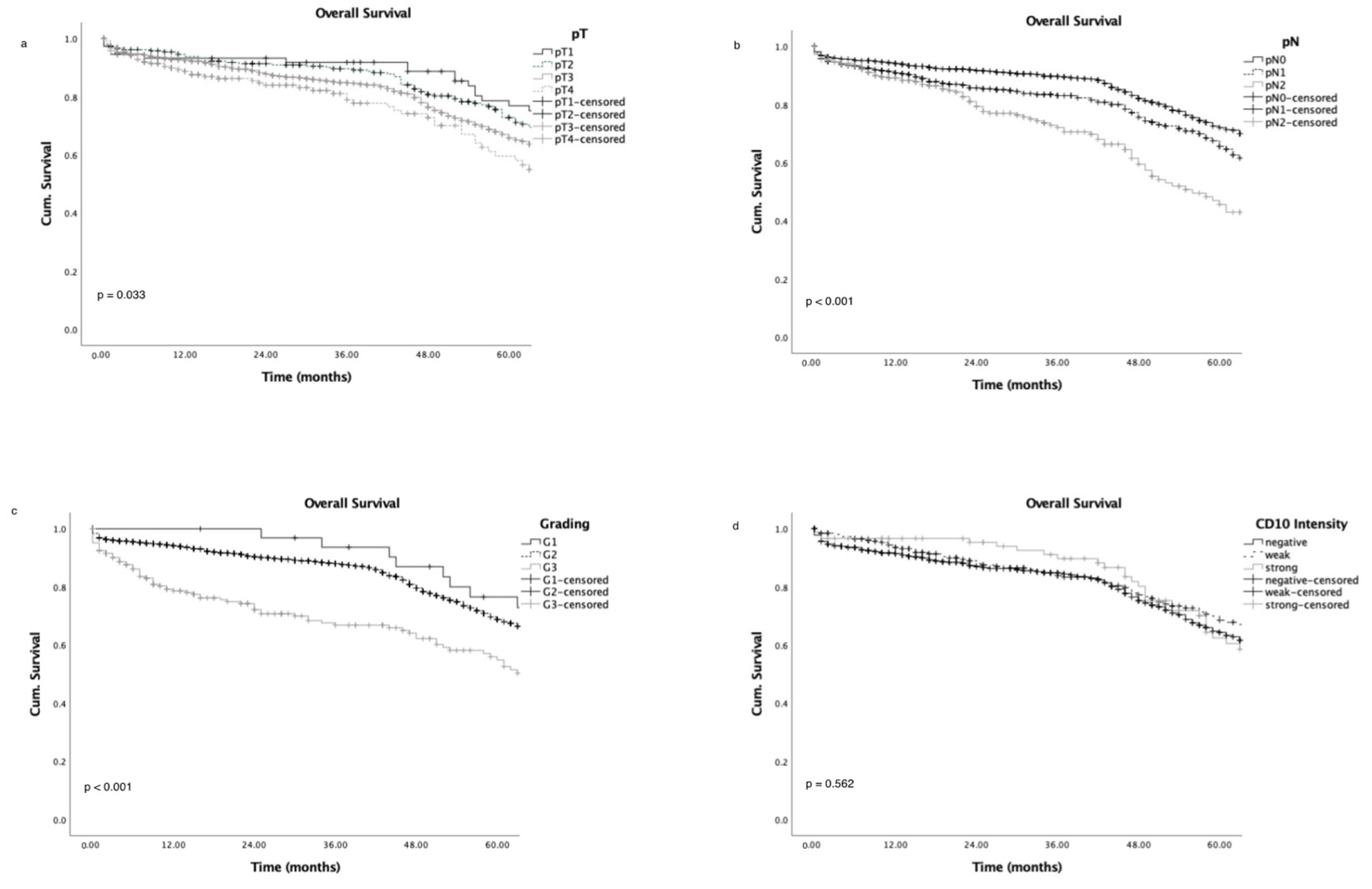
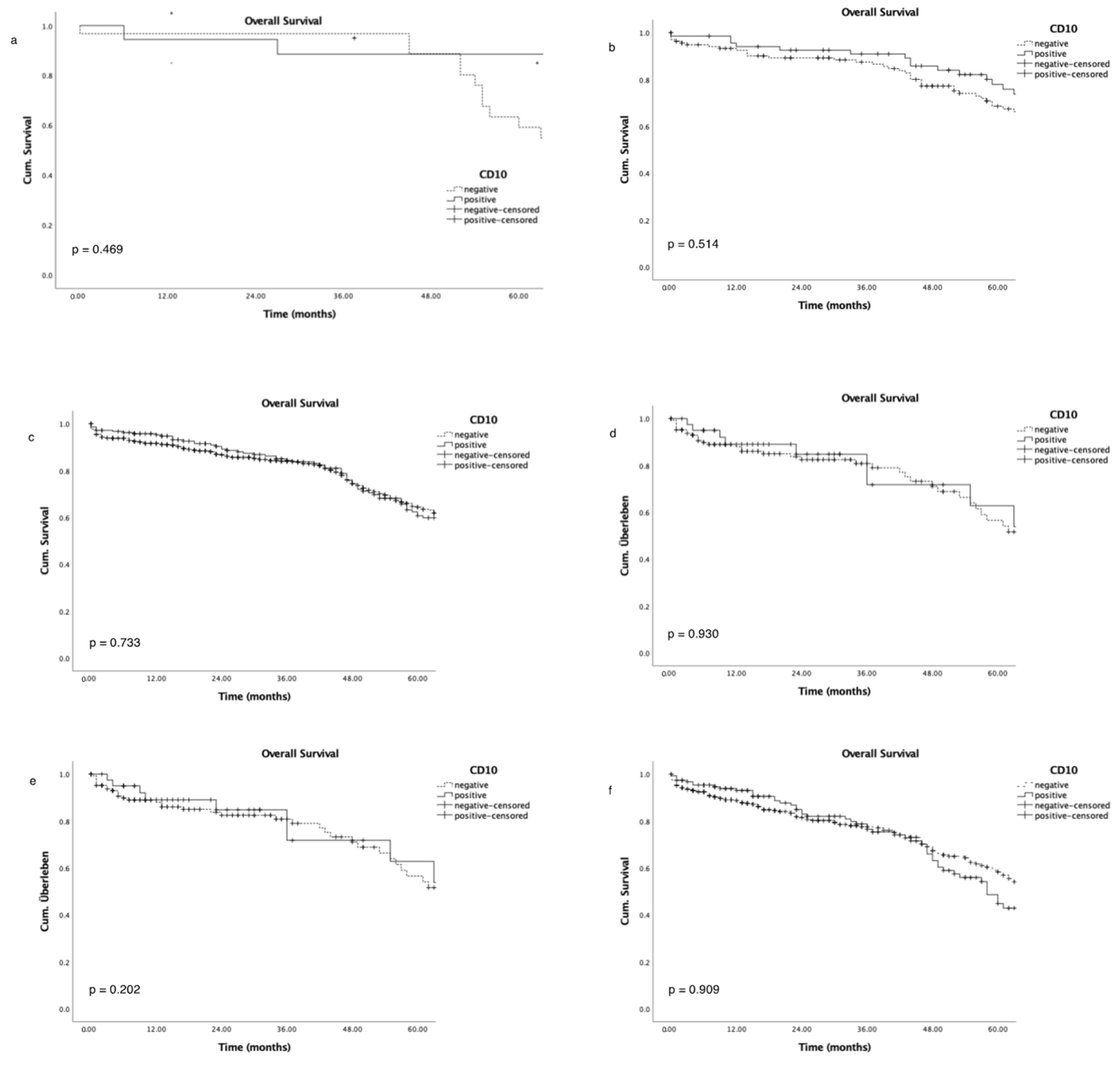
3.5. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itoh, F.; Hinoda, Y.; Ohe, Y.; Nakagawa, N.; Ueda, R.; Yachi, A.; Imai, K. Expression of CD10/neutral endopeptidase in normal and malignant tissues of the human stomach and colon. J. Gastroenterol. 1996, 31, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Ordi, J.; Romagosa, C.; Tavassoli, F.A.; Nogales, F.; Palacin, A.; Condom, E.; Torne, A.; Cardesa, A. CD10 expression in epithelial tissues and tumors of the gynecologic tract: A useful marker in the diagnosis of mesonephric, trophoblastic, and clear cell tumors. Am. J. Surg. Pathol. 2003, 27, 178–186. [Google Scholar] [CrossRef]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.F.; Hariri, G.; Newman, R.A.; Sutherland, D.R.; Ritter, M.A.; Ritz, J. Selective expression of the common acute lymphoblastic leukemia (gp 100) antigen on immature lymphoid cells and their malignant counterparts. Blood 1983, 61, 628–639. [Google Scholar] [CrossRef]
- McIntosh, G.G.; Lodge, A.J.; Watson, P.; Hall, A.G.; Wood, K.; Anderson, J.J.; Angus, B.; Horne, C.H.; Milton, I.D. NCL-CD10-270: A new monoclonal antibody recognizing CD10 in paraffin-embedded tissue. Am. J. Pathol. 1999, 154, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Louhichi, T.; Saad, H.; Dhiab, M.B.; Ziadi, S.; Trimeche, M. Stromal CD10 expression in breast cancer correlates with tumor invasion and cancer stem cell phenotype. BMC Cancer 2018, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Kuniyasu, H.; Luo, Y.; Fujii, K.; Sasahira, T.; Moriwaka, Y.; Tatsumoto, N.; Sasaki, T.; Yamashita, Y.; Ohmori, H. CD10 enhances metastasis of colorectal cancer by abrogating the anti-tumoural effect of methionine-enkephalin in the liver. Gut 2010, 59, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Khanh, D.T.; Mekata, E.; Mukaisho, K.; Sugihara, H.; Shimizu, T.; Shiomi, H.; Murata, S.; Naka, S.; Yamamoto, H.; Endo, Y.; et al. Prognostic role of CD10(+) myeloid cells in association with tumor budding at the invasion front of colorectal cancer. Cancer Sci. 2011, 102, 1724–1733. [Google Scholar] [CrossRef]
- Dall’Era, M.A.; True, L.D.; Siegel, A.F.; Porter, M.P.; Sherertz, T.M.; Liu, A.Y. Differential expression of CD10 in prostate cancer and its clinical implication. BMC Urol. 2007, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, A.; Rocha, C.; Saxer-Sekulic, N.; Zlobec, I.; Sauter, G.; Thalmann, G.N. High CD10 expression in lymph node metastases from surgically treated prostate cancer independently predicts early death. Virchows Arch. 2011, 458, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: Multifunctional contributors to tumor progression. Mol. Med. Today 2000, 6, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Raposo, T.P.; Comes, M.S.; Idowu, A.; Agit, B.; Hassall, J.; Fadhil, W.; Nica, R.; Ecker, R.; Yao, T.; Ilyas, M. CD10 inhibits cell motility but expression is associated with advanced stage disease in colorectal cancer. Exp. Mol. Pathol. 2018, 104, 190–198. [Google Scholar] [CrossRef]
- Yao, T.; Tsutsumi, S.; Akaiwa, Y.; Takata, M.; Nishiyama, K.; Kabashima, A.; Tsuneyoshi, M. Phenotypic expression of colorectal adenocarcinomas with reference to tumor development and biological behavior. Jpn. J. Cancer Res. 2001, 92, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Nakanishi, Y.; Sekine, S.; Yoshimura, K.; Akasu, T.; Moriya, Y.; Shimoda, T. CD10 expression in colorectal carcinoma correlates with liver metastasis. Dis. Colon Rectum 2005, 48, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Taniguchi, H.; Yao, T.; Shimoda, T.; Ueno, H.; Hirai, T.; Ohue, M. Multi-institutional study of risk factors of liver metastasis from colorectal cancer: Correlation with CD10 expression. Int. J. Color. Dis. 2010, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Ohji, Y.; Yao, T.; Eguchi, T.; Yamada, T.; Hirahashi, M.; Iida, M.; Tsuneyoshi, M. Evaluation of risk of liver metastasis in colorectal adenocarcinoma based on the combination of risk factors including CD10 expression: Multivariate analysis of clinicopathological and immunohistochemical factors. Oncol. Rep. 2007, 17, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Zurawski, J.; Talarska, P.; de Mezer, M.; Kaszkowiak, K.; Chalcarz, M.; Iwanik, K.; Karon, J.; Krokowicz, P. Evaluation of CD10 expression as a diagnostic marker for colorectal cancer. Gastroenterol. Hepatol. Bed Bench 2022, 15, 24–31. [Google Scholar] [PubMed]
- Nishida, T.; Egashira, Y.; Akutagawa, H.; Fujii, M.; Uchiyama, K.; Shibayama, Y.; Hirose, Y. Predictors of lymph node metastasis in T1 colorectal carcinoma: An immunophenotypic analysis of 265 patients. Dis. Colon Rectum 2014, 57, 905–915. [Google Scholar] [CrossRef]
- Jang, T.J.; Park, J.B.; Lee, J.I. The Expression of CD10 and CD15 Is Progressively Increased during Colorectal Cancer Development. Korean J. Pathol. 2013, 47, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Nimura, S.; Mizoguchi, M.; Hamada, Y.; Yamashita, Y.; Iwasaki, H. Early colorectal carcinomas: CD10 expression, mucin phenotype and submucosal invasion. Pathol. Int. 2012, 62, 600–611. [Google Scholar] [CrossRef]
- Del Rio, P.; Crafa, P.; Papadia, C.; Benecchi, L.; Campanini, N.; Sianesi, N.; Montana, C.M.; Sianesi, M. Is CD10 a reliable marker of invasive colorectal cancer? Ann. Ital. Chir. 2011, 82, 279–282. [Google Scholar] [PubMed]
- Iwase, T.; Kushima, R.; Mukaisho, K.; Mitsufuji, S.; Okanoue, T.; Hattori, T. Overexpression of CD10 and reduced MUC2 expression correlate with the development and progression of colorectal neoplasms. Pathol. Res. Pract. 2005, 201, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Kononen, J.; Bubendorf, L.; Kallioniemi, A.; Barlund, M.; Schraml, P.; Leighton, S.; Torhorst, J.; Mihatsch, M.J.; Sauter, G.; Kallioniemi, O.P. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 1998, 4, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Mirlacher, M.; Sauter, G. Tissue microarrays. Biotechniques 2004, 36, 98–105. [Google Scholar] [CrossRef]
- Marx, A.; Simon, P.; Simon, R.; Mirlacher, M.; Izbicki, J.R.; Yekebas, E.; Kaifi, J.T.; Terracciano, L.; Sauter, G. AMACR expression in colorectal cancer is associated with left-sided tumor localization. Virchows Arch. 2008, 453, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Bernescu, I.; Reichstein, A.C.; Luchtefeld, M.; Ogilvie, J.W. Does CD10 Expression Predict Lymph Node Metastasis in Colorectal Cancer? Dis. Colon Rectum 2016, 59, 22–27. [Google Scholar] [CrossRef]
- Kim, K.M.; Ahn, A.R.; Park, H.S.; Jang, K.Y.; Moon, W.S.; Kang, M.J.; Ha, G.W.; Lee, M.R.; Chung, M.J. Clinical significance of p53 protein expression and TP53 variation status in colorectal cancer. BMC Cancer 2022, 22, 940. [Google Scholar] [CrossRef] [PubMed]
- Liebl, M.C.; Hofmann, T.G. The Role of p53 Signaling in Colorectal Cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, M. Molecular Network of Colorectal Cancer and Current Therapeutic Options. Front. Oncol. 2022, 12, 852927. [Google Scholar] [CrossRef] [PubMed]
- Matly, A.; Quinn, J.A.; McMillan, D.C.; Park, J.H.; Edwards, J. The relationship between beta-catenin and patient survival in colorectal cancer systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103337. [Google Scholar] [CrossRef] [PubMed]
- Maguer-Satta, V.; Besancon, R.; Bachelard-Cascales, E. Concise review: Neutral endopeptidase (CD10): A multifaceted environment actor in stem cells, physiological mechanisms, and cancer. Stem Cells 2011, 29, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M.; Lopez-Otin, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087.e3. [Google Scholar] [CrossRef] [PubMed]
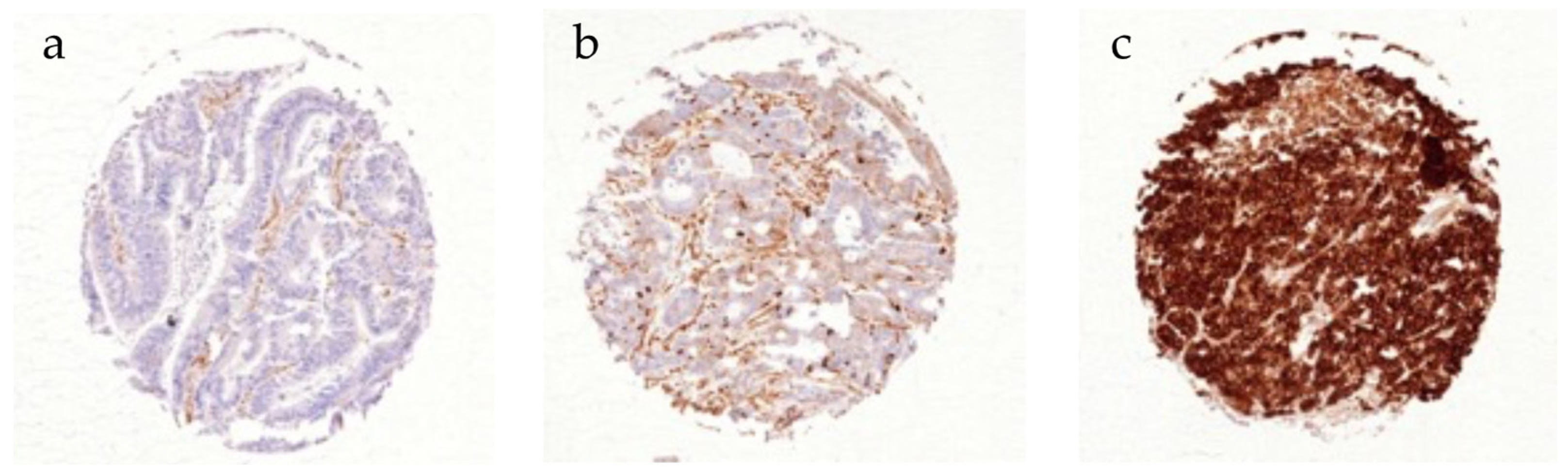

| Parameter | n (%) | |
|---|---|---|
| n = 1711 | ||
| Gender | female | 858 (50.1) |
| male | 853 (49.9) | |
| Age | (years) | 68.8 (11.9) |
| Tumour grade | G1 | 29 (1.7) |
| G2 | 1505 (88.0) | |
| G3 | 177 (10.3) | |
| Tumour stage | pT1 | 75 (4.4) |
| pT2 | 270 (15.8) | |
| pT3 | 1104 (64.5) | |
| pT4 | 262 (15.3) | |
| Nodal status | pN0 | 889 (52.0) |
| pN1 | 458 (26.8) | |
| pN2 | 364 (21.3) | |
| Tumour type | tubular carcinoma | 1644 (96.0) |
| mucinous carcinoma | 59 (3.4) | |
| others | 8 (0.5) | |
| Tumour localization | right colon | 414 (24.2) |
| transverse colon | 149 (8.7) | |
| left colon | 514 (30.0) | |
| rectum | 637 (37.2) | |
| CD10 | |||||
|---|---|---|---|---|---|
| Parameter | n | Negative (%) | Weak (%) | Strong (%) | p Value |
| All Cancers | 1469 | 72.6 | 20.1 | 7.4 | |
| Tumour Stage | |||||
| pT1 | 53 | 62.3 | 32.1 | 5.7 | 0.017 |
| pT2 | 221 | 66.5 | 22.1 | 11.3 | |
| pT3 | 850 | 73.3 | 20.4 | 6.4 | |
| pT4 | 198 | 78.5 | 16.1 | 5.6 | |
| Lymph Node Metastasis | 0.711 | ||||
| pN0 | 698 | 71.6 | 21.2 | 7.2 | |
| pN1 | 329 | 75.7 | 17.4 | 7 | |
| pN2 | 277 | 69.7 | 22.7 | 7.6 | |
| pN3 | 1 | 100 | 0 | 0 | |
| Grading | 0.397 | ||||
| G1 | 21 | 81 | 14.3 | 4.8 | |
| G2 | 1139 | 71.5 | 21.3 | 7.2 | |
| G3 | 157 | 78.3 | 16 | 5.7 | |
| Tumour Localization | <0.001 | ||||
| Right colon | 258 | 81.4 | 12.8 | 5.8 | |
| Transverse colon | 88 | 84.1 | 11.4 | 4.5 | |
| Left colon | 250 | 66 | 26.4 | 7.6 | |
| Rectum | 416 | 71.9 | 22.6 | 5.5 | |
| Histological Subtype | 0.048 | ||||
| Adenocarcinoma | 938 | 72.8 | 20.8 | 6.4 | |
| Mucinous | 67 | 91 | 6 | 3 | |
| Others | 9 | 66.7 | 33.3 | 0 | |
| Peritumoural Lymphocytes | 0.871 | ||||
| Absent | 567 | 74.3 | 19.4 | 6.3 | |
| Present | 438 | 74 | 20.3 | 5.7 | |
| Vascular Invasion | 0.376 | ||||
| No | 565 | 74.7 | 18.6 | 6.7 | |
| Yes | 439 | 73.3 | 21.5 | 5.2 |
| CD10 | |||||
|---|---|---|---|---|---|
| Parameter | n Evaluable | Negative (%) | Weak (%) | Strong (%) | p Value |
| ß-catenin membranous | 0.070 | ||||
| negative | 110 | 80.0 | 12.7 | 7.3 | |
| weak | 328 | 77.7 | 12.8 | 9.5 | |
| moderate | 93 | 65.6 | 17.2 | 17.2 | |
| strong | 329 | 69.9 | 17.0 | 13.1 | |
| ß-catenin nuclear | <0.001 | ||||
| negative | 444 | 80.4 | 11.5 | 8.1 | |
| weak | 157 | 68.8 | 20.4 | 10.8 | |
| moderate | 140 | 70.7 | 13.6 | 15.7 | |
| Strong | 130 | 58.5 | 21.5 | 20.0 | |
| Ki67 | 0.085 | ||||
| <10% | 501 | 75.8 | 13.8 | 10.4 | |
| ≥10% | 776 | 70.2 | 17.4 | 12.4 | |
| p53 | 0.013 | ||||
| negative | 374 | 77.5 | 13.9 | 8.6 | |
| positive | 270 | 68.5 | 15.9 | 15.6 | |
| MSH2 | 0.212 | ||||
| negative | 190 | 78.4 | 12.1 | 9.5 | |
| positive | 454 | 71.8 | 15.9 | 12.3 | |
| MLH1 | <0.001 | ||||
| negative | 128 | 87.5 | 5.5 | 7.0 | |
| positive | 515 | 70.3 | 17.1 | 12.6 | |
| BRAF | 0.001 | ||||
| negative | 1141 | 69.1 | 17.2 | 13.7 | |
| positive | 100 | 84.0 | 13.0 | 3.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grass, J.-K.; Grupp, K.; Kluth, M.; Hube-Magg, C.; Simon, R.; Kemper, M.; Izbicki, J.R.; Sauter, G.; Melling, N. CD10 Expression Correlates with Earlier Tumour Stages and Left-Sided Tumour Location in Colorectal Cancer but Has No Prognostic Impact in a European Cohort. Cancers 2024, 16, 1473. https://doi.org/10.3390/cancers16081473
Grass J-K, Grupp K, Kluth M, Hube-Magg C, Simon R, Kemper M, Izbicki JR, Sauter G, Melling N. CD10 Expression Correlates with Earlier Tumour Stages and Left-Sided Tumour Location in Colorectal Cancer but Has No Prognostic Impact in a European Cohort. Cancers. 2024; 16(8):1473. https://doi.org/10.3390/cancers16081473
Chicago/Turabian StyleGrass, Julia-Kristin, Katharina Grupp, Martina Kluth, Claudia Hube-Magg, Ronald Simon, Marius Kemper, Jakob R. Izbicki, Guido Sauter, and Nathaniel Melling. 2024. "CD10 Expression Correlates with Earlier Tumour Stages and Left-Sided Tumour Location in Colorectal Cancer but Has No Prognostic Impact in a European Cohort" Cancers 16, no. 8: 1473. https://doi.org/10.3390/cancers16081473
APA StyleGrass, J.-K., Grupp, K., Kluth, M., Hube-Magg, C., Simon, R., Kemper, M., Izbicki, J. R., Sauter, G., & Melling, N. (2024). CD10 Expression Correlates with Earlier Tumour Stages and Left-Sided Tumour Location in Colorectal Cancer but Has No Prognostic Impact in a European Cohort. Cancers, 16(8), 1473. https://doi.org/10.3390/cancers16081473






