Simple Summary
The location of the primary tumor in the right colon, left colon, or rectum affects the efficacy of biological drugs used in the treatment of metastatic colorectal cancer, but how? We examined how the primary tumor location affects disease characteristics, treatability, quality of life, and outcome in a real-life study population of 1080 Finnish patients in the RAXO study. The primary tumor location correlates with the location of metastases, the frequency of gene mutations, how often metastases can be operated upon, long-term survival after curative surgery or palliative chemotherapy, and the quality of life during the disease trajectory. The primary tumor location is a helpful surrogate for clinicians working with metastatic colorectal cancer patients in estimating the clinical course of the disease. This study cannot identify the reasons for the associations, i.e., whether it is the primary location per se, the different mutations, or other reasons.
Abstract
The primary tumor location (PTL) is associated with the phenotype, metastatic sites, mutations, and outcomes of metastatic colorectal cancer (mCRC) patients, but this has mostly been studied according to sidedness (right vs. left sided). We studied right colon vs. left colon vs. rectal PTL in a real-life study population (n = 1080). Health-related quality of life (HRQoL) was assessed multi-cross-sectionally with QLQ-C30, QLQ-CR29, EQ-5D, and 15D. A chi-square, Kaplan–Meier, and Cox regression were used to compare the groups. The PTL was in the right colon in 310 patients (29%), the left colon in 396 patients (37%), and the rectum in 375 patients (35%). The PTL was associated with distinct differences in metastatic sites during the disease trajectory. The resectability, conversion, and resection rates were lowest in the right colon, followed by the rectum, and were highest in the left colon. Overall survival was shortest for right colon compared with left colon or rectal PTL (median 21 vs. 35 vs. 36 months), with the same trends after metastasectomy or systemic therapy only. PTL also remained statistically significant in a multivariable model. The distribution of symptoms varied according to PTL, especially between the right colon (with general symptoms of metastases) and rectal PTL (with sexual- and bowel-related symptoms). mCRC, according to PTL, behaves differently regarding metastatic sites, resectability of the metastases, outcomes of treatment, and HRQoL.
1. Introduction
The primary tumor location (PTL) affects the phenotype, treatment alternatives, and prognosis of metastatic colorectal cancer (mCRC) [1]. The negative prognostic and predictive value of PTL for epidermal growth factor receptor (EGFR)-inhibitor treatment has led to renewed interest in the PTL [2,3]. In a large population-based SEER and national program of cancer registries material from the US, in patients with stage I–IV cancers, right colon primaries were present in 39%, left colon cancers in 24%, and rectal cancers in 30% [4].
The right side of the colon located proximal to the splenic flexure (caecum, ascending colon, and proximal two-thirds of the transverse colon), arises from the midgut during embryological development, while the left colon and rectum arise from the hindgut. Probably due to differences in the gut microbiota, the right side of the colon displays differences in its mucosal immunology [5]. Right-sided tumors are more frequently mucinous, are associated with an inflammatory response, and have a higher frequency of BRAF-V600E mutations (mt), deficient mismatch repair (dMMR)/microsatellite instability high (MSI-H), and hypermutated tumors [6,7]. Left-sided colon or rectal tumors more frequently have chromosomal alterations, amplification of EGFR and HER2 genes, and aberrant EGFR signaling. There are also variations between left colon and rectum, for example, regarding KRAS frequencies [6,7,8]. The location of metastases also differs notably, with right-sided cancers having more peritoneal carcinomatosis, left-sided colon cancers having more liver metastases, and rectal cancers having more lung metastases [9]. The surgical and radiation therapy approaches also differ for rectal and colon primaries. This means that right colon, left colon, and rectal PTLs can, at least in some respects, be considered as different diseases from a clinical perspective.
Patients with an unresectable right-sided mCRC have worse overall survival (OS) and progression-free survival (PFS) rates, independent of the treatment regimens studied, compared to those with left-sided primaries [3,10,11,12,13]. PTL also influences the prognosis after a liver resection, with metachronous left-sided mCRCs having the best survival, and right-sided the worst [14,15]. The first-line combination of chemotherapy with EGFR-inhibitors has clearly benefitted patients with left-sided tumors, whereas patients with right-sided tumors have derived limited benefits in most studies, but, on the contrary, seem to benefit from the addition of bevacizumab [2,3,10,16].
Health-related quality of life (HRQoL) and maintenance of functionality are highly important to patients [17,18]. Thus, in addition to classical clinical trial endpoints, such as survival, measuring functional, social, and emotional parameters of HRQoL is especially valuable in studies exploring treatment decisions, to maximize resectability, survival, and palliation [19]. In phase III studies of systemic treatments, a HRQoL measurement is recommended but far from always reported, and real-life data for HRQoL during and after systemic treatment are scarce [20,21,22]. Metastasectomies have been used increasingly and improve survival significantly and, thus, focus on long-term adverse events is of great importance. There are HRQoL data after a single organ metastasectomy, but very scarce data for multisite metastasectomies [21,23,24,25,26,27]. The HRQoL is also affected by the surgery for the primary tumor and the radiotherapy. To the best of our knowledge, there are no data on HRQoL in patients treated for multisite metastatic disease according to the PTL.
Our aim was to assess the impact of right colon, left colon, or rectal PTLs on the demographics, resectability, and outcomes after metastasectomy and/or local ablative therapy (LAT), systemic therapy, or best supportive care (BSC) in a real-life Finnish study population of treatable patients with comprehensive clinical data and molecular testing. A secondary aim was to study HRQoL divided by the PTL during different disease phases.
2. Materials and Methods
The RAXO study included 1086 mCRC patients between 2012 and 2018 [28]. Inclusion criteria were, in brief, patients eligible for first-line systemic therapy, an age of over 18 years, and a histologically confirmed CRC with distant metastases, or a locally advanced primary tumor not curatively treatable (but, in the end, no locally advanced patients were included, only metastatic). The resectability assessment, definitions of resectability, and data collection have been explained in a previous paper by Osterlund et al. [28].
The patients were treated according to local clinical guidelines, based on the ESMO and NCCN guidelines [29,30,31,32].
The molecular testing and testing for dMMR were described in an earlier study [33].
Clinical trial identification for the RAXO study is NCT01531595 https://classic.clinicaltrials.gov/ct2/show/NCT01531621, accessed on 26 February 2024 and EudraCT 2011-003137-33 https://eudract.ema.europa.eu/results-web/, accessed on 26 February 2024. Ethical permission for the study was obtained by the Ethical Board at the Helsinki University Hospital (number 242/13/03/02/2011 and HUS/1288/2016). The study was conducted in accordance with the Declaration of Helsinki. All patients gave their informed consent to participation to the prospective study and separately to the QoL study.
HRQoL was evaluated using four different HRQoL measures: the generic 15D [34] and EQ-5D-3L (index score and visual analogue scale [VAS]) [35], which produce both index and profile data, and the cancer-specific EORTC QLQ-C30 [36] and colorectal cancer-specific QLQ-CR29 [37], of which QLQ-C30 produces both an index (global health status—GHS) and profile measures, and QLQ-CR29 colorectal cancer-specific profile measures. The HRQoL assessments were previously described in detail [21]. The HRQoL data were collected multi-cross-sectionally (1–13 times) and analyzed according to disease phase (Figure 1). The questionnaires were given to the patients at the hospital or sent out by mail. The patients were instructed to fill out the questionnaires just before a response evaluation and/or a doctor’s appointment. The time points were, thus, not treatment-phase-dependent or scheduled to baseline, at certain timepoints during a treatment phase, or after progression. Four disease phases were used: post-resection, remission, systemic treatment, and BSC. The phases of curative treatment were defined as post-resection during the first 6 months after metastasectomy and/or LAT including any adjuvant therapy, and the remission phase started if the patient had been disease-free for more than 6 months from the last metastasectomy and/or LAT or had a complete response to the systemic therapy for more than 6 months. The systemic treatment phase included both non-curative systemic therapy in one or several lines that was given with the goal of life-prolongation and palliation but also neoadjuvant or conversion treatment that was given before metastasectomy and/or LAT. These two situations were combined to one group as it is not possible to know upfront if the treatment will result in a potentially curative metastasectomy/LAT or palliation [21]. The BSC phase was the time after ending active cancer treatment for mCRC (no patient was in the BSC only group since all patients should be treatable).
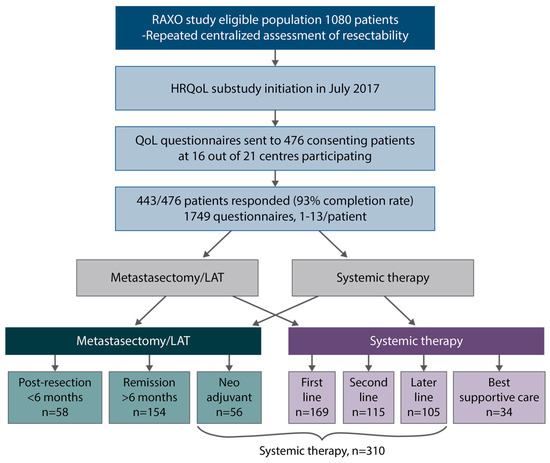
Figure 1.
Study design, patient flow, health-related quality of life (HRQoL) questionnaires, post-resection (within 6 months after metastasectomy and/or local ablative therapy (LAT) including adjuvant-like treatment), remission (more than 6 months after metastasectomy and/or LAT), systemic therapy (mean of neoadjuvant/conversion, first-, second- and later-line), best supportive care (after ending active cancer treatment).
Results are presented as proportions, median with range or mean values with standard deviations or 95% confidence intervals (CI). Proportions between the three PTL groups and all demographic alternatives with percentage presented were compared using crosstabs. All comparisons were performed with the non-parametric Mann–Whitney or Kruskal–Wallis tests. Minimal clinically important difference (MID) was used with cut-offs as described in [21]. The reverse Kaplan–Meier method was used for estimation of median follow-up time. OS was estimated using Kaplan–Meier; it was calculated from time of mCRC diagnosis to death by any reason or censored if alive at last follow-up (7 October 2020). PFS was estimated from time of first-line systemic therapy initiation to progression or censored if no progression was noted at cut-off dates. Relapse-free survival (RFS) was calculated from first metastasectomy or LAT to relapse, death, or censored at last date of follow-up, and non-radical resection or second organ not resected denoted 0 months. OS and PFS were compared using Cox regression with 95% CI. A multivariable cox regression model adjusting for clinically meaningful variables was also fitted. Two-sided p-values < 0.05 and 95% CIs not crossing 1.00 were considered statistically significant. All analyses were performed using SPSS statistics version 28 or 29, IBM corporation, Armonk, NY, USA.
3. Results
3.1. Baseline Demographics
The total number of patients was 1080, as multiple colorectal primaries were present in 6 cases omitted from further analysis in this sub-study. The PTL was right colon in 310 (29%), left colon in 396 (37%), and rectum in 374 (35%) (Table 1).

Table 1.
Baseline demographics by primary tumor location.
Patients with right colon tumors were more often over 70-years-old and females than patients with left colon or rectal PTLs. Right colon tumors more often had mucinous or signet cell histology and high-grade tumors than left colon or rectal primaries. Anemia was more common among right colon patients compared with left colon and rectum, but no other laboratory parameters, including CEA, differed according to PTL (Table S1).
Of the 1080 patients, 833 (77%) had their primary tumor resected. Surgical procedures for primary tumors are presented in Table 1. Primary tumors in the right or left colon were resected more often than the rectal tumors.
Patients with left colon tumors more often had liver metastases, both at baseline and during trajectory, than patients with right colon or rectal primaries (Figure 2). Rectal PTL was associated with a higher prevalence of lung metastases, almost doubling during trajectory, compared with patients with right colon and left colon primaries. Right colon PTL was associated with a higher prevalence of peritoneal, distant lymph node, and ovarian metastases compared to patients with left-sided colon and rectal PTLs. Liver metastases were mostly already present at baseline whereas lung, distant lymph node, and peritoneal metastases became more common over time, as did rarer metastatic sites such as bone, adrenal, or brain.
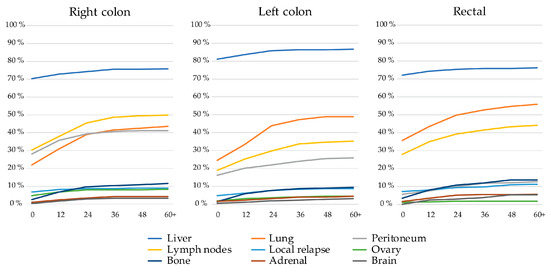
Figure 2.
Frequency of metastatic sites of the nine most common metastatic sites at baseline and during disease trajectory (presented to 60+ months) divided by primary tumor location.
Of the included patients, 93% were adequately tested for RAS and BRAF-V600E mutations in clinical routine. BRAF-V600E and/or NRAS were missing in 59 patients (denoted (K)RAS) before the ESMO recommendation of extended testing in 2016 (Table 1). RAS & BRAF wild type (wt) status was more uncommon among the right colon PTL than in left colon or rectal PTLs. RAS mt were more common in rectal and right colon PTLs than in left colon PTL. Right colon PTL was associated with a higher proportion of BRAF-V600E mt compared with left colon or rectal PTLs.
MMR testing was performed in 39% of the patients (Table 1). dMMR was more common in right colon PTL compared with left colon, with none identified in rectal PTL.
3.2. Resectability, Resections, and LAT
Technical resectability of metastases was centrally assessed for all patients (Figure 3). For patients with upfront borderline and non-resectable metastases, re-assessment was performed after 2–3 months and after 4–5 months of systemic therapy.
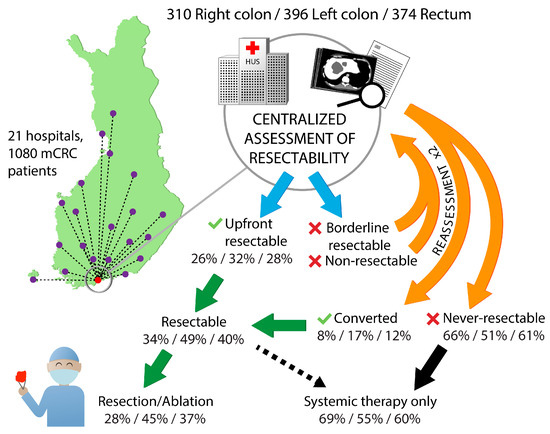
Figure 3.
Technical resectability assessment at the tertiary center in Helsinki and the upfront resectable and converted proportions, proportions that underwent resection or ablation according to primary tumor location.
Patients with right colon or rectal primaries had non-resectable metastases upfront more often compared with left colon primaries (60% vs. 58% vs. 48%), and after conversion therapy they remained never-resectable more often (Table 2).

Table 2.
Resectability and resections and/or local ablative therapy (LAT) according to PTL.
Multisite and multiple metastasectomies were performed in 37% of the patients, with single site metastatic disease having a metastasectomy and/or LAT in 53% of patients and multiple metastatic site patients in 18%, respectively (Table 2). Mean number of metastasectomies/LAT per patient with a procedure was 1.6 in right colon PTL, 1.5 in left colon PTL, and 1.7 in rectal PTL. Resections and/or LAT were less frequently performed in patients with right colon PTL compared with left colon and rectum (28% vs. 45% vs. 37%), and, as a consequence they received ‘systemic therapy only’ more often (69% vs. 55% vs. 60%).
Liver resections were performed most often in left colon PTL (Table 2). Of patients with baseline liver metastases or liver-only disease, metastasectomy and/or LAT was performed in 46% and 50%, respectively, compared with 26% and 24% in right colon PTL, and 39% and 36% of rectal PTL patients, respectively (p < 0.001).
Lung resections were most often performed in rectal PTL (Table 2), with lung resections or LAT performed in 17% of the patients with baseline lung metastases and in 38% of patients with lung-only disease, compared with 9% and 40% in right colon PTL, and 12% and 50% in left colon PTL, respectively.
Cytoreductive surgery, distant lymphadenectomy, gynecologic resection, or subcutaneous resections were performed more often in right colon PTL.
3.3. Treatments
Systemic therapy was given to 97% (1052/1080) of the patients, either as neoadjuvant/conversion/adjuvant or as non-curative treatment. The maximum number of treatment lines in a patient was seven.
In first-line treatment, patients with right colon PTL were more often treated with bevacizumab-containing regimens compared to patients with left colon or rectal PTLs (66% vs. 54% vs. 56%, p = 0.005; Table 3), and on the contrary, less often with EGFR-inhibitors (5% vs. 18% vs. 17%, p < 0.001). EGFR-inhibitors were also less common in the RAS & BRAF wt subgroup (n = 354) in patients with right colon PTL (13% vs. 39% vs. 40%, p < 0.001).

Table 3.
Treatment and response to treatment according to primary tumor location.
During all lines of systemic therapy, patients with right colon PTL received VEGF-inhibitors (bevacizumab or aflibercept) as often as left colon or rectal PTL patients (76% vs. 70% vs. 71%, p = 0.176), whereas EFGR-inhibitors were given less often (15% vs. 38% vs. 33%, p < 0.001, in all patients; and 46% vs. 77% vs. 72%, p < 0.001, in the RAS & BRAF wt subgroup).
Good treatment response (i.e., partial or complete response, and no evidence of disease with metastasectomy and/or LAT) to first-line treatment was seen less often in right colon PTL compared with left colon and rectal PTLs (54% vs. 67% vs. 64%, p < 0.001; Table 3), and progressive disease as best response more often (16% vs. 6% vs. 8%, p < 0.001).
3.4. Overall Survival and Progression-Free Survival
Median follow-up time was 57 months (95% CI 54–60). OS from diagnosis of mCRC irrespective of treatment given was worse in right colon PTL compared with left colon and rectal PTLs (median OS 21 vs. 35 vs. 35 months, Figure 4). OS stratified by treatment group showed similar associations with PTL for ‘systemic therapy only’ (median OS 18 vs. 22 vs. 23 months), and ‘metastasectomy and/or LAT’ (median OS 69 vs. 72 vs. 73 months), but not for the ‘best supportive care only’ group (median 2 vs. 2. vs. 3 months, respectively).
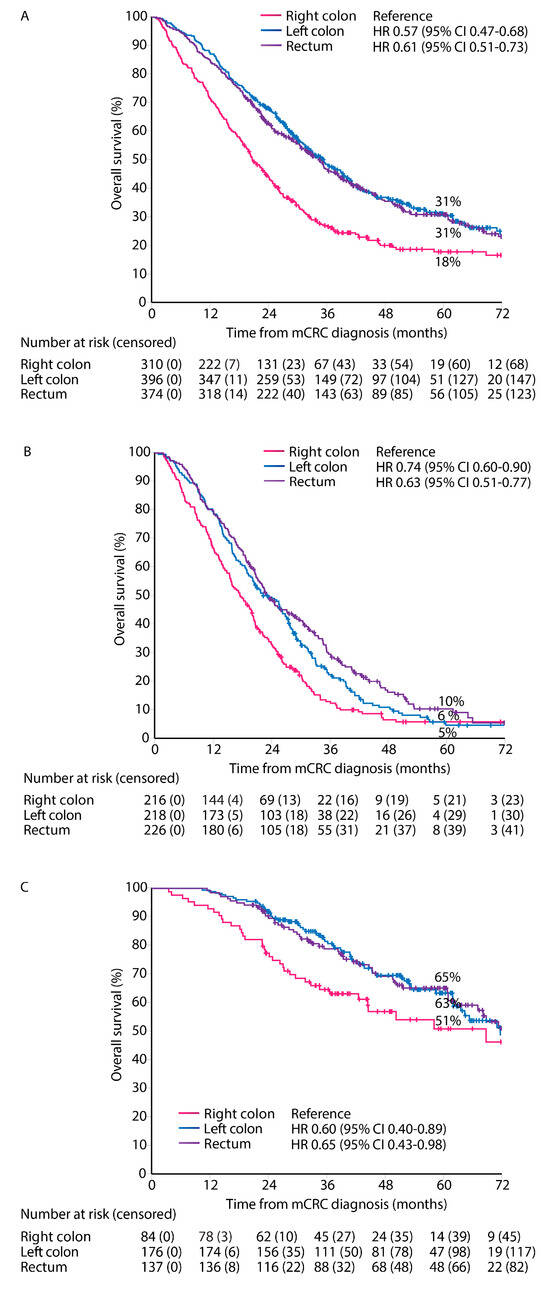
Figure 4.
Overall survival according to primary tumor location. All patients (A), systemic therapy only (B), and metastasectomy and/or LAT (C).
PFS for first-line treatment for ‘systemic therapy only’ patients showed a worse PFS for right and left colon PTLs compared with rectal PTL (median PFS 7.0 vs. 7.6 vs. 8.3 months, Figure 5A).
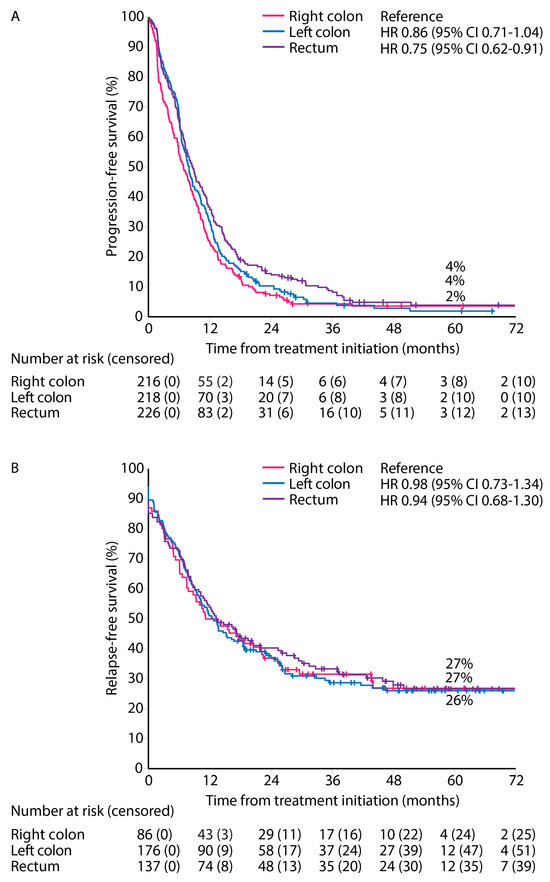
Figure 5.
Progression-free survival for ‘systemic therapy only’ (A), and relapse-free survival after first metastasectomy and/or local ablative therapy according to primary tumor location (B).
RFS from first metastasectomy and/or LAT was similar for all PTLs (median RFS 11.2 vs. 12.6 vs. 13.4 months) and 5-year RFS rates (27% vs. 27% vs. 26%) for right colon, left colon, and rectal PTLs, respectively (Figure 5B).
3.5. Multivariable Model for OS
OS was also impaired in right colon PTL compared with left colon and rectal PTLs in a multivariable model adjusted for age, number of metastatic sites, ECOG performance status, treatment modalities, and molecular pathology (HR reference, 0.77 [95% CI 0.63–0.93], 0.64 [0.53–0.78], Table 4).

Table 4.
Univariable and multivariable Cox regression model for overall survival.
Since treatment modality is not a “true” baseline factor, a second model using baseline resectability was constructed (Table S2). The results did not change substantially and the PTL was still statistically significant.
3.6. HRQoL and PTL in Different Treatment Phases
HRQoL questionnaires were answered by 443 patients (1–13 questionnaires per patient), with 1749 questionnaires in total. In the post-resection phase, i.e., within 6 months from resection including adjuvant therapy, 58 patients responded, and during the remission phase, without relapse more than 6 months after metastasectomy/LAT, 154 patients responded. In the systemic treatment phase, where half of the patients were treated with curative neoadjuvant/conversion intent and half with non-curative intent, 310 patients answered and in the best supportive care phase, after stopping cancer treatment, 34 patients answered (Figure 1).
Mean and SD values for the QoL indexes are presented in Table S3. The index scores for EQ-5D according to the three PTLs were 0.76–0.90 in the post-resection phase and 0.87–0.88 in the remission phase.
When comparing right colon to left colon, an MID for worse index measures as 15D, EQ-5D, and GHS (the higher the better) was noted for the BSC phase, but no other MIDs or statistically significant differences were noted for the post-resection, remission, or systemic treatment phases (Table 5).

Table 5.
Comparisons of index measures between primary tumor location in different treatment phases.
In a comparison between the right colon vs. rectal PTL, MIDs for 15D, EQ-5D, VAS, and GHS in the post-resection phase were noted; additionally, for 15D in the remission phase, and for GHS in the BSC phase (Table 5). Only the difference in VAS was statistically significant in the post-resection phase.
There were some minimal clinically important differences (Δ |0.05| or more) in 15D dimensions between the PTLs (Figure S1).
In the post-resection phase, patients with right colon PTL scored better than those with left colon or rectal PTLs for excretion, discomfort, and symptoms, and worse for regarding sleeping, breathing, distress, and mental function.
In the remission phase, patients with right colon PTL showed better MID compared with the left colon or rectal PTLs for sexual activity and excretion. For systemic treatment, those with right colon PTL showed better MIDs for sexual activity and worse for sleeping. In the BSC phase, right colon patients compared with rectal PTL scored better for breathing, excretion, usual activities, and mental function.
3.7. QLQ-C30 and QLQ-CR29 Symptom Scales
Symptom burden (the lower the better, range 0–2600, MID ≥ |26 × 5 = 130|) is the individual sum of the means for the 26 different symptom scales of the QLQ-C30 and QLQ-CR29 questionnaires. The sum of 10 functioning scales (the higher the better, range 0–1000, MID ≥ |10 × 5 = 50|) from QLQ-C30 and QLQ-CR29 were also calculated.
In the post-resection phase, patients with right colon PTL had a higher symptom burden and lower functioning scale sum, compared with those with left colon and rectal PTLs with a difference of 142 and −104, respectively, reaching the MID level but not statistical significance (Table 5 and Figure 6). In the post-resection phase, fatigue, dyspnea, insomnia, diarrhea, appetite loss, hair loss, sore skin, and stool frequency were clinically significantly (MID > 5) worse in patients with right colon PTL than in left colon or rectal PTLs. Symptoms more common in rectal PTL than in right colon and/or left colon PTLs were flatulence, embarrassment, and dyspareunia.
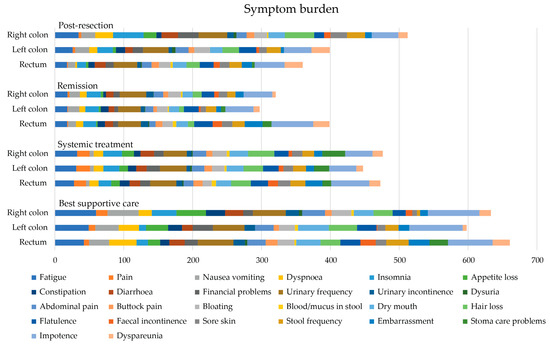
Figure 6.
Symptom burden as sum of 26 symptom scales (0 no symptoms to 100 most symptoms, theoretical maximum 2600) measured with the QLQ-C30 and QLQ-CR29 during different treatment phases according to primary tumor location.
In the remission phase, symptom burden was numerically higher in patients with a rectal primary tumor compared to the right colon or left colon (Table 5). The symptoms that affected patient’s HRQoL after metastasectomy the most, regardless of PTL, were principally bowel- and sexuality-related (Figure 7). More symptoms in patients with rectal PTL compared with right and/or left colon were seen for constipation, flatulence, fecal incontinence, stool frequency, embarrassment, stoma care problems, impotence, and dyspareunia.
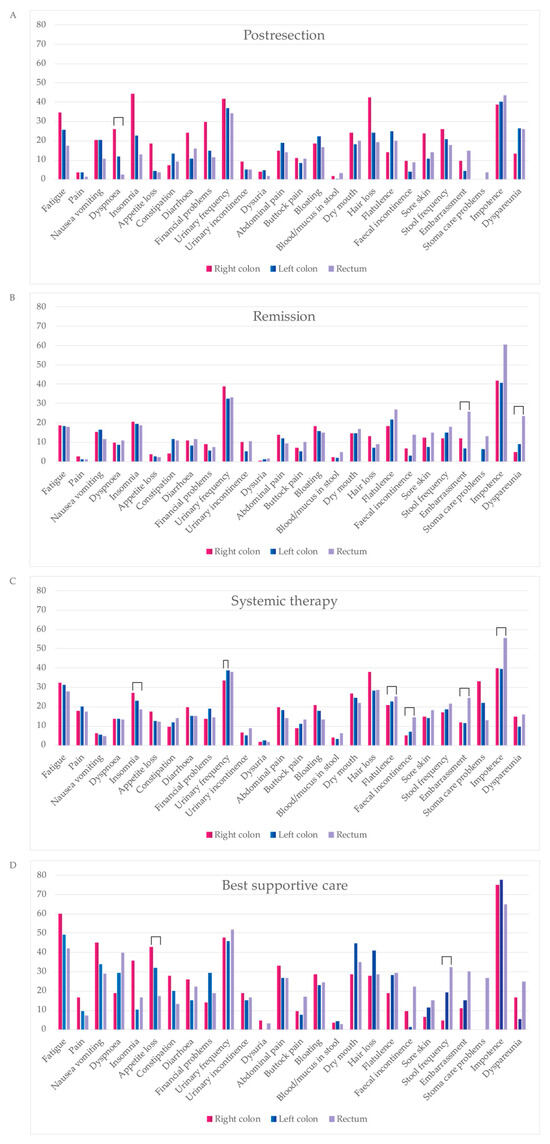
Figure 7.
Comparison of symptom scales from QLQC30 during four treatment phases: post-resection ((A), within 6 months after metastasectomy and/or local ablative therapy (LAT) including adjuvant-like treatment), remission ((B), more than 6 months after metastasectomy and/or LAT), systemic therapy ((C), mean of neoadjuvant/conversion, first-, second- and later-line), best supportive care ((D), after ending active cancer treatment). Statistically significant differences between primary tumor locations are marked with brackets.
In the systemic treatment phase, the primary tumor location did not statistically or clinically significantly affect the symptom burden (Table 5). During systemic treatment, stoma care problems were more common in patients with primaries in the right colon compared to the left and rectal PTLs (Figure 7). Instead, impotence and embarrassment were more common in patients with rectal PTL compared with the right or left colon PTLs.
For the BSC phase, no statistically significant differences between different PTLs for symptom burden were noted. The functioning scale sum was lower for rectal PTL compared with right and left colon PTLs (Table 5). In BSC, patients with a right colon PTLhad more fatigue, pain, insomnia, and appetite loss compared to left and rectal primary tumors (Figure 7). Patients with a left colon PTL had more dry mouth and hair loss compared with a right colon and rectal PTLs. Patients with a rectal PTL complained more often about dyspnea, stool frequency, fecal incontinence, embarrassment, stoma care problems, and impotence compared to right or left colon PTLs.
4. Discussion
We have previously reported that by maximizing metastasectomies, it is possible to achieve excellent survival with maintained HRQoL in mCRC patients [21,28]. We now add that this is seen in all three PTLs. Patients with right colon PTL, associated with several negative predictive and prognostic factors, tended to do worse regarding resectability, conversions, resections, systemic treatment, and outcomes than those with the left colon PTL and more in line with the rectal PTL. There were also clinically important differences between left colon and rectal PTLs, and it may not always be accurate to lump them together as life-sided. Distribution of symptoms rendered typical for CRC and mCRC, nonetheless, varied according to the three PTLs, which has rarely been reported in the literature. HRQoL indexes were worse for right colon vs. left colon or rectal PTLs in post-resection and best supportive care phases, which has never been reported before.
4.1. Resectability and Resections
To the best of our knowledge, resectability of mCRC metastases according to three PTL groups has not been reported before. Upfront resectability was slightly higher for left colon PTL compared with right colon and rectal PTLs (32% vs. 26% vs. 28%). However, a major difference is seen in the proportion of unconvertable and never-resectable, which was 66% among the right colon PTL compared with 51% for left colon and in between, 61%, for the rectal PTL. Thus, mCRC patients with a right colon PTL are also less often treated with curative intent metastasectomy and/or LAT (27%), compared with a left colon (44%) or rectal (37%) PTL. This is in line with differences noted in a population-based cohort of synchronous mCRC cases (11% vs. 13% vs. 16%) [9], or in the Cairo5 study with borderline or non-resectable liver metastases with resection rates of 58% in left-sided RAS & BRAF wt and 37% and 51% for right-sided and/or RAS/BRAF mt [38].
Right colon PTLs were associated with peritoneal, distant lymph node, and ovarian metastases, in line with previous findings [9]. Since these metastatic sites, as opposed to the liver and lungs, are rarely resectable, this can at least partly explain the differences in resectability and resection rates. Cytoreductive surgery was for this reason also performed more often in a right colon PTL. Liver metastases and liver-only were most common in left colon PTLs, in line with the findings in a Dutch study [9]. When liver metastases are present, they are more often synchronous, multiple, and affect more liver segments in a right colon PTL, compared with left colon or rectal PTLs [39], reflected in our liver-only patients who were resected in 24% of right colon PTL patients, compared with 50% of left colon, and 36% of rectal PTL patients. Further, a right colon PTL is more often BRAF mt and MSI-H, two groups associated with a more rapid clinical course, poorer performance, being another reason for fewer metastasectomies [40,41]. Patients with a rectal PTL had more lung metastases than right or left colon PTLs, in line with previously published reports [42], further adding to the difference in resectability and resection rates (9% in patients with baseline lung metastases and a right colon PTL vs. 12% in a left colon PTL vs. 17% in a rectal PTL). Metastases of right colon PTLs are thus more often unresectable at baseline and they also stay never-resectable more often than metastases in patients with left colon or rectal PTLs. Further, they more often have an unfavorable biology being less responsive to conventional chemotherapy. Treatments, thus, more often become non-curative systemic therapy. The lower conversion rate probably mimics the lower response rates seen in right colon PTL receiving systemic therapy whether with or without EGFR- or VEGF-inhibitors [2]; lower response rates were also seen here. The increasing incidence of lung, peritoneal, distant lymph node, and brain metastases during trajectory highlights a need for re-resections and multisite resections. An ambition of the RAXO study was to maximize resection rates and multiple resections also were more common (mean 1.5 resections per metastasectomized in right colon, 1.6 in left colon, and 1.7 in rectal PTL patients) in this study than in other studies.
Right colon PTL was, as discussed above, associated with a higher incidence of BRAF mt and dMMR, and also high RAS mt rates, which were associated with lower metastasectomy rates [7,43,44]. This is not unexpected, since BRAF mt patients often have metastases in organs considered unresectable, such as the peritoneum and distant lymph nodes, while RAS mt patients have metastases in lungs and brain. It has been suggested that BRAF mt could be the genetic alteration that is responsible for differences in metastatic sites between the right colon and left colon PTLs [7].
The noted differences in patient demographics, such as female sex, older age, and high grade tumors, for a right colon PTL in this study are in line with previously published studies [45], and may also explain the lower metastasectomy rates.
4.2. Survival, Prognosis, and Predictive Factors
Due to poor prognostic features such as BRAF mt, dMMR, adverse metastatic sites, mucinous and signet cell tumor histology, high grade, female sex and older age, comorbidities, etc., associated with a right colon PTL, patients with a right colon PTL have a worse outcome as compared to left colon and/or rectal PTLs [9,45,46], in line with our findings. Earlier disease stages show similar differences in demographics between right colon and left colon PTLs as in mCRC, but there are no clinically meaningful differences in survival or recurrence [45,47,48]. This implies that the factors that drive CRC recurrence are, at least in part, distinct from those influencing survival in mCRC.
OS and PFS for all patients, regardless of treatment and in systemic therapy, are generally inferior for right-sided compared with left-sided colorectal cancer [2,3,9,10,46], as also shown in our study. Previous studies have reported that a right colon PTL is associated with a lack of benefit when treated with EGFR-inhibitors; additionally, when the tumor is RAS wt [2,3,10] or RAS & BRAF wt and pMMR [49]. On the contrary, numerical PFS and OS benefit from the addition of bevacizumab is seen in a right colon PTL [3,10,50]. An OS benefit from the addition of an EGFR-inhibitor in left-sided RAS and/or BRAF wt patients has been seen in most studies, apart from Cairo5 [2,3,38,50]. Also, there are studies showing worse survival for rectal PTLs compared to left colon PTLs when treated with an EGRF-inhibitor [51,52].
A Dutch study showed impaired OS for patients with any metastasectomy or systemic therapy only in the right colon PTL subgroup compared with left colon and rectal PTLs [9]. This survival difference is also shown in the liver resection subgroup of that study (no OS-rates given) [9] and in an Austrian patient series (5-year OS rates approximately 30% for right-sided vs. 42% for left-sided) [15]. The Cairo5 study showed approximately 30+% 5-year OS rates in the right-sided or RAS/BRAF mt group and 40+% in the left-sided RAS & BRAF wt group [38]. This is in line with our findings of 5-year OS rates 51% vs. 63% vs. 65%, respectively, after any metastasectomy for a right colon PTL compared with left colon and rectal PTLs, with the caveat that survival after relapse is shorter in the right colon PTL.
4.3. Health-Related Quality of Life
HRQoL after metastasectomy in mCRC has rarely been reported, and mostly for single-site metastasectomies [24,25,26,27], with data for multisite and multiple CRC metastasectomies available only from this study and a Canadian study [21,23]. To our knowledge, there are no previous data presented in this patient population divided according to PTL.
As shown earlier, index measures, expressing the QoL with one number, without any profile or symptom measures, report preserved global HRQoL despite receiving more intense treatment, generally meaning more adverse events [53,54,55]. In line with this, this study shows more pronounced differences in symptom scores than in index scores. However, index scores (15D, EQ-5D, and GHS) showed worse HRQoL in patients with right colon PTL after metastasectomy compared to rectal PTL and worse HRQoL in BSC phase compared to left colon PTL. Patients with metastatic spread in the liver mostly recover quickly after modern liver surgery [56,57]. The fact that patients with right colon PTL have proportionally fewer liver and lung metastasectomies and more cytoreductive surgery could contribute to this HRQoL difference seen after metastasectomy.
The impact of the PTL on HRQoL in mCRC from a surgical point of view focuses on the question whether and when the primary tumor should be resected or not. Surgery may improve QoL, especially with symptomatic primaries, but without apparent survival benefit in asymptomatic primaries [58,59]. Studies unquestionably show that surgery of stage I-III rectal tumors is an important driver of poor HRQoL in CRC survivors [60,61,62,63].
In this study, symptoms impairing HRQoL of metastatic rectal cancer patients were related to bowel function or sexuality. Symptoms as embarrassment, dyspareunia, impotence, low sexual interest, stoma care problems, fecal incontinence, stool frequency, constipation, flatulence, etc., bothered the rectal PTL patients during all treatment phases. All these symptoms have been generally described in mCRC patients, but not confined to rectal PTL [64,65,66].
Patients with a right colon PTL, compared with left colon and/or rectal PTLs, more often had symptoms like dyspnea, insomnia, hair loss, or loss of appetite. These, along with fatigue and insomnia, have been the most common complaints previously published but again without differentiation according to PTL [67,68,69,70]. These symptoms of a right colon PTL may reflect the metastatic spread in the peritoneal cavity and lymph nodes and may, especially in the postresection phase, be caused by extensive cytoreductive surgery.
During the whole treatment trajectory, patients with a left colon PTL seemed to report less severe symptoms than those with right colon or rectal PTLs.
As observed previously, the most symptoms were reported during the BSC phase but no studies have reported symptoms according to PTL [66,71,72].
The symptom burden is caused by the cancer itself and by the treatments. Awareness of long-term adverse events with surgical, radiotherapy, and systemic treatment need to be minimized with individualized treatment planning in multi-disciplinary teams, and patient preferences kept in mind with shared decision making. To capture all symptoms causing shame, like sexual complaints, urinary frequency, bowel function, and stoma care problems, these themes need to be actively discussed with patients, with a special emphasis on rectal PTL patients. Meanwhile, patients with a right colon PTL need support especially after metastasectomy and during systemic treatment. This emphasizes the need for survivorship programs [73,74].
4.4. Strengths and Limitations
The strengths of this study include its prospective setting with a large national real-life population, extensive and complete patient-level details, high rate of RAS and BRAF testing, and inclusion of patients with multiple and multisite metastases. An additional strength of the study is the use of multiple HRQoL questionnaires and high questionnaire completion rate of 93%, in comparison with 73–91% for CRC patients in other cross-sectional studies [75,76]. We also captured HRQoL data throughout the disease trajectory.
An obvious limitation of this study is the observational design without randomization. Nevertheless, the long-term observational nature with a high number of patients has allowed us to describe the clinical behavior, treatments, and outcomes in detail. Another limitation is that MMR testing was not performed in the early days of inclusion. Central assessment without full knowledge of the patients’ condition may be criticized but provided a good estimate of technical resectability. A major limitation is that HRQoL questionnaires were recorded multi-cross-sectionally and not longitudinally at prespecified timepoints. The HRQoL sub-study started in 2017 when the RAXO study per se had been ongoing since 2012. This led to a risk of guarantee-time bias. The BSC phase describes the HRQoL after failing intensive treatment and not those receiving ‘BSC only’.
5. Conclusions
mCRC behaves differently according to the primary tumor location regarding metastatic sites, resectability of metastases, outcomes of treatment, and quality of life. Patients with right colon primaries have more cytoreductive surgery and less liver and lung resections than left colon or rectal cancers, and have worse survival after metastasectomy or systemic treatment. Left colon cancers have most liver resections and rectal cancers most lung resections, both with excellent outcomes. Rectal cancer patients seem to suffer from symptoms impairing quality of life caused by the primary tumor itself or the local treatments, while patients with right colon primaries suffer from symptoms caused by the metastatic spread. Thus, right colon, left colon, and rectal cancers are separate disease entities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16051052/s1, Table S1. Laboratory findings in right colon, left colon, and rectal primary tumor location; Table S2. Univariable and multivariable Cox regression model for overall survival using only baseline factors; Table S3. Health-related quality of life indexes in different treatment phases according to primary tumor location; Figure S1. The mean 15D dimensions in different treatment phases for right colon, left colon, and rectal primary tumor location.
Author Contributions
Conceptualization, S.A., E.O., H.S., A.U., B.G., H.I., K.L. and P.O.; Data curation, S.A., E.O., A.U., H.I., K.L. and P.O.; formal analysis, S.A., E.O., K.L. and P.O.; funding acquisition, A.R., J.S., M.J.M., T.K., A.Å., R.R., E.H., R.K., P.H., A.N., T.S., H.S., A.L., H.I., K.L. and P.O.; investigation, S.A., E.O., H.I., K.L. and P.O.; methodology, S.A., E.O., H.S., A.U., B.G., H.I., K.L. and P.O.; project administration, S.A., E.O., A.Å., R.R., R.K., P.H., L.-M.S., T.S., A.L., H.I. and P.O.; resources, S.A., E.O., A.R., J.S., M.J.M., T.K., S.K., A.Å., R.R., R.K., P.H., A.N., T.S., A.L., J.K., H.I., K.L. and P.O.; software, S.A., E.O., K.L. and P.O.; supervision, B.G., H.I., J.T.L., K.L. and P.O.; validation, S.A., E.O., A.U., B.G., H.I., J.T.L., K.L. and P.O.; visualization, S.A., E.O., B.G., J.T.L., K.L. and P.O.; writing—original draft, S.A., E.O., B.G., H.I., J.T.L., K.L. and P.O.; writing—review and editing, S.A., E.O., A.R., L.N., J.S., M.J.M., T.K., S.K., A.Å., R.R., E.H., R.K., P.H., L.-M.S., A.N., A.U., T.S., H.S., A.L., T.M., J.K., B.G., H.I., J.T.L., K.L. and P.O. All authors have read and agreed to the published version of the manuscript.
Funding
This investigator-initiated study was supported by Finska Läkaresällskapet (2016, 2018, 2019, 2020, 2021, 2022, 2023), The Finnish Cancer Foundation (2019–2020, 2021, 2022–2023), Relander’s foundation (2020–2022) the Competitive State Research Financing of the Expert Responsibility Area of Tampere, Helsinki, Turku, Kuopio, Oulu, and Satakunta Hospitals (2012, 2016, 2017, 2018, 2019, 2020, 2021, 2022, 2023), Tampere University Hospital Fund (Tukisäätiö 2019, 2020, 2023 and OOO-project 2020), Helsinki University Hospital research fund (2019, 2020, 2021, 2022, 2023), and the infrastructure with the database and study nurses partly supported by pharmaceutical companies: Amgen—unrestricted grant (2012–2020, 2023), Eli Lilly and Company (2012–2017), Merck KGaA (2012–2020), Roche Oy (2012–2020), Sanofi (2012–2017), and Servier—unrestricted grant (2016–2023). Open access funding provided by University of Helsinki. The funders had no role in the study design, analysis, interpretation of the data or decision to publish.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Regional Scientific Ethical Board at Helsinki University Hospital (number 242/13/03/02/2011 and HUS/1288/2016).
Informed Consent Statement
An informed consent statement was obtained from all subjects involved in the study.
Data Availability Statement
The data collected for this study can be made available to others in a de-identified form after all primary and secondary endpoints have been published, in the presence of a data transfer agreement, and if the purpose of use complies with Finnish legislation. Requests for data sharing can be made to the corresponding author, including a proposal that must be approved by the steering committee.
Acknowledgments
We thank the patients and their families, the investigators, study personnel, and the hospitals that have participated in this study. We thank Celina Österlund for the preparation of the figures.
Conflicts of Interest
All authors report institutional research funding from Eli Lilly, Merck KGaA, Roche Finland, Sanofi, and unrestricted grants from Amgen and Servier, during the conduct of the study. S.A., E.O., A.R., L.N., J.S., M.J.M., T.K., S.K., A.Å., R.R., E.H., R.K., P.H., L.-M.S., A.N., A.U., T.S., H.S., A.L., T.M., J.K., B.G., H.I., K.L. and P.O. report grants, personal fees, or non-financial support from AbbVie, Amgen, Agenus, Astellas, Astra- Zeneca, Baxalta/Shire, Bayer, BMS, Celgene, Eisai/Ewopharma, Eli Lilly, Erythech Pharma, Fresenius, Incyte, Jansen-Cilag, Medicom, Merck, MSD, Nordic Drugs/Pharma, Novartis, Nutricia/Danone, Pierre-Fabre, Roche, Sanofi, Servier, Sobi, Takeda, and/or Varian.
References
- Iacopetta, B. Are There Two Sides to Colorectal Cancer? Int. J. Cancer 2002, 101, 403–408. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and Predictive Value of Primary Tumour Side in Patients with RAS Wild-Type Metastatic Colorectal Cancer Treated with Chemotherapy and EGFR Directed Antibodies in Six Randomized Trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef] [PubMed]
- Venook, A.P.; Niedzwiecki, D.; Innocenti, F.; Fruth, B.; Greene, C.; O’Neil, B.H.; Shaw, J.E.; Atkins, J.N.; Horvath, L.E.; Polite, B.N.; et al. Impact of Primary (1°) Tumor Location on Overall Survival (OS) and Progression-Free Survival (PFS) in Patients (Pts) with Metastatic Colorectal Cancer (MCRC): Analysis of CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2016, 34, 3504. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Flemer, B.; Lynch, D.B.; Brown, J.M.R.; Jeffery, I.B.; Ryan, F.J.; Claesson, M.J.; O’Riordain, M.; Shanahan, F.; O’Toole, P.W. Tumour-Associated and Non-Tumour-Associated Microbiota in Colorectal Cancer. Gut 2017, 66, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Dienstmann, R.; Vermeulen, L.; Guinney, J.; Kopetz, S.; Tejpar, S.; Tabernero, J. Consensus Molecular Subtypes and the Evolution of Precision Medicine in Colorectal Cancer. Nat. Rev. Cancer 2017, 17, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.-Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF Mutation and Microsatellite Instability on the Pattern of Metastatic Spread and Prognosis in Metastatic Colorectal Cancer. Cancer 2011, 117, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, R.; Chatila, W.K.; Lipsyc, M.D.; Hechtman, J.F.; Cercek, A.; Sanchez-Vega, F.; Jayakumaran, G.; Middha, S.; Zehir, A.; Donoghue, M.T.A.; et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell 2018, 33, 125–136.e3. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.P.M.; Van Der Kruijssen, D.E.W.; Hugen, N.; De Hingh, I.H.J.T.; Nagtegaal, I.D.; Verhoeven, R.H.A.; Koopman, M.; De Wilt, J.H.W. The Impact of Primary Tumor Location in Synchronous Metastatic Colorectal Cancer: Differences in Metastatic Sites and Survival. Ann. Surg. Oncol. 2019, 27, 1580–1588. [Google Scholar] [CrossRef]
- Tejpar, S.; Stintzing, S.; Ciardiello, F.; Tabernero, J.; Van Cutsem, E.; Beier, F.; Esser, R.; Lenz, H.J.; Heinemann, V. Prognostic and Predictive Relevance of Primary Tumor Location in Patients with Ras Wild-Type Metastatic Colorectal Cancer Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017, 3, 194–201. [Google Scholar] [CrossRef]
- Loupakis, F.; Yang, D.; Yau, L.; Feng, S.; Cremolini, C.; Zhang, W.; Maus, M.K.H.; Antoniotti, C.; Langer, C.; Scherer, S.J.; et al. Primary Tumor Location as a Prognostic Factor in Metastatic Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2015, 107, 427. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Taieb, J.; Kuboki, Y.; Pfeiffer, P.; Kumar, A.; Hochster, H.S. Trifluridine/Tipiracil with or without Bevacizumab in Metastatic Colorectal Cancer: Results of a Systematic Review and Meta-Analysis. Ther. Adv. Med. Oncol. 2023, 15, 17588359221146137. [Google Scholar] [CrossRef] [PubMed]
- Boeckx, N.; Koukakis, R.; Op De Beeck, K.; Rolfo, C.; Van Camp, G.; Siena, S.; Tabernero, J.; Douillard, J.-Y.; André, T.; Peeters, M. Primary Tumor Sidedness Has an Impact on Prognosis and Treatment Outcome in Metastatic Colorectal Cancer: Results from Two Randomized First-Line Panitumumab Studies. Ann. Oncol. 2017, 28, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; Amodio, P.M.; Chilelli, M.G.; Santoro, R.; Schirripa, M.; Ranalli, T.V.; Pessina, G.; Giron Berrios, J.R.; Natoni, F.; Virtuoso, A.; et al. Real-Life Experience of the Prognostic Significance of the Primary Tumor Location on the Timing of Colorectal Liver Metastases: A Retrospective Analysis. Cureus 2022, 14, e30607. [Google Scholar] [CrossRef] [PubMed]
- Gasser, E.; Braunwarth, E.; Riedmann, M.; Cardini, B.; Fadinger, N.; Presl, J.; Klieser, E.; Ellmerer, P.; Dupré, A.; Imai, K.; et al. Primary Tumour Location Affects Survival after Resection of Colorectal Liver Metastases: A Two-Institutional Cohort Study with International Validation, Systematic Meta-Analysis and a Clinical Risk Score. PLoS ONE 2019, 14, e0217411. [Google Scholar] [CrossRef]
- Loupakis, F.; Hurwitz, H.I.; Saltz, L.; Arnold, D.; Grothey, A.; Nguyen, Q.L.; Osborne, S.; Talbot, J.; Srock, S.; Lenz, H.J. Impact of Primary Tumour Location on Efficacy of Bevacizumab plus Chemotherapy in Metastatic Colorectal Cancer. Br. J. Cancer 2018, 119, 1451–1455. [Google Scholar] [CrossRef]
- Lapinsky, E.; Man, L.C.; MacKenzie, A.R. Health-Related Quality of Life in Older Adults with Colorectal Cancer. Curr. Oncol. Rep. 2019, 21, 81. [Google Scholar] [CrossRef]
- Wedding, U.; Ködding, D.; Pientka, L.; Steinmetz, H.T.; Schmitz, S. Physicians’ Judgement and Comprehensive Geriatric Assessment (CGA) Select Different Patients as Fit for Chemotherapy. Crit. Rev. Oncol. Hematol. 2007, 64, 1–9. [Google Scholar] [CrossRef]
- Bonnetain, F.; Borg, C.; Adams, R.R.; Ajani, J.A.; Benson, A.; Bleiberg, H.; Chibaudel, B.; Diaz-Rubio, E.; Douillard, J.Y.; Fuchs, C.S.; et al. How Health-Related Quality of Life Assessment Should Be Used in Advanced Colorectal Cancer Clinical Trials. Ann. Oncol. 2017, 28, 2077–2085. [Google Scholar] [CrossRef]
- Hamers, P.A.H.; Vink, G.R.; Elferink, M.A.G.; Stellato, R.K.; Dijksterhuis, W.P.M.; Punt, C.J.A.; Koopman, M.; May, A.M.; Beerepoot, L.V.; Creemers, G.-J.; et al. Quality of Life and Survival of Metastatic Colorectal Cancer Patients Treated with Trifluridine-Tipiracil (QUALITAS). Clin. Color. Cancer 2022, 21, 154–166. [Google Scholar] [CrossRef]
- Lehtomäki, K.; Stedt, H.P.; Osterlund, E.; Muhonen, T.; Soveri, L.-M.; Halonen, P.; Salminen, T.K.; Kononen, J.; Kallio, R.; Ålgars, A.; et al. Health-Related Quality of Life in Metastatic Colorectal Cancer Patients Treated with Curative Resection and/or Local Ablative Therapy or Systemic Therapy in the Finnish RAXO-Study. Cancers 2022, 14, 1713. [Google Scholar] [CrossRef]
- Lehtomäki, K.; Soveri, L.M.; Osterlund, E.; Lamminmäki, A.; Uutela, A.; Heervä, E.; Halonen, P.; Stedt, H.; Aho, S.; Muhonen, T.; et al. Resectability, Resections, Survival Outcomes, and Quality of Life in Older Adult Patients with Metastatic Colorectal Cancer (the RAXO-Study). J. Clin. Med. 2023, 12, 3541. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.C.; Coburn, N.G.; Devitt, K.S.; Serrano, P.E.; Moulton, C.-A.; Cleary, S.P.; Law, C.; Moore, M.J.; Gallinger, S. Survival Following Resection of Intra- and Extra-Hepatic Metastases from Colorectal Cancer: A Phase II Trial. Ann. Surg. Oncol. 2016, 23, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Wiering, B.; Oyen, W.J.G.; Adang, E.M.M.; van der Sijp, J.R.M.; Roumen, R.M.; de Jong, K.P.; Ruers, T.J.M.; Krabbe, P.F.M. Long-Term Global Quality of Life in Patients Treated for Colorectal Liver Metastases. Br. J. Surg. 2011, 98, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Langenhoff, B.S.; Krabbe, P.F.M.; Peerenboom, L.; Wobbes, T.; Ruers, T.J.M. Quality of Life after Surgical Treatment of Colorectal Liver Metastases. Br. J. Surg. 2006, 93, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Studer, P.; Horn, T.; Haynes, A.; Candinas, D.; Banz, V.M. Quality of Life after Hepatic Resection. Br. J. Surg. 2018, 105, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Cashin, P.; Mahteme, H.; Syk, I.; Frödin, J.; Glimelius, B.; Graf, W. Quality of Life and Cost Effectiveness in a Randomized Trial of Patients with Colorectal Cancer and Peritoneal Metastases. Eur. J. Surg. Oncol. 2018, 44, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, P.; Salminen, T.; Soveri, L.M.; Kallio, R.; Kellokumpu, I.; Lamminmäki, A.; Halonen, P.; Ristamäki, R.; Lantto, E.; Uutela, A.; et al. Repeated Centralized Multidisciplinary Team Assessment of Resectability, Clinical Behavior, and Outcomes in 1086 Finnish Metastatic Colorectal Cancer Patients (RAXO): A Nationwide Prospective Intervention Study. Lancet Reg. Health Eur. 2021, 3, 100049. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; Van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO Consensus Guidelines for Management of Patients with Colon and Rectal Cancer. A Personalized Approach to Clinical Decision Making. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 1139–1167. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Osterlund, E.; Ristimäki, A.; Kytölä, S.; Kuopio, T.; Heervä, E.; Muhonen, T.; Halonen, P.; Kallio, R.; Soveri, L.M.; Sundström, J.; et al. KRAS-G12C Mutation in One Real-Life and Three Population-Based Nordic Cohorts of Metastatic Colorectal Cancer. Front. Oncol. 2022, 12, 826073. [Google Scholar] [CrossRef] [PubMed]
- Sintonen, H. The 15D Instrument of Health-Related Quality of Life: Properties and Applications. In Proceedings of the Annals of Medicine; Royal Society of Medicine Press Ltd.: London, UK, 2001; Volume 33, pp. 328–336. [Google Scholar]
- Brooks, R. EuroQol: The Current State of Play. Health Policy N. Y. 1996, 37, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Gujral, S.; Conroy, T.; Fleissner, C.; Sezer, O.; King, P.M.; Avery, K.N.L.; Sylvester, P.; Koller, M.; Sprangers, M.A.G.; Blazeby, J.M. Assessing Quality of Life in Patients with Colorectal Cancer: An Update of the EORTC Quality of Life Questionnaire. Eur. J. Cancer 2007, 43, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Punt, C.J.A.; Bond, M.J.G.; Bolhuis, K.; Loosveld, O.J.L.; de Groot, J.W.; Droogendijk, H.; Helgason, H.H.; Hendriks, M.P.; Klaase, J.M.; Kazemier, G.; et al. LBA27 First-Line Systemic Treatment in Patients with Initially Unresectable Colorectal Cancer Liver Metastases (CRLM): Overall Survival of the Phase III CAIRO5 Study of the Dutch Colorectal Cancer Group. Ann. Oncol. 2023, 34, S1268. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Strömberg, C.; Jonas, E.; Freedman, J. Colorectal Cancer Liver Metastases—A Population-Based Study on Incidence, Management and Survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Dragomir, A.; Sundström, M.; Pfeiffer, P.; Thunberg, U.; Bergfors, M.; Aasebø, K.; Eide, G.E.; Ponten, F.; Qvortrup, C.; et al. High BRAF Mutation Frequency and Marked Survival Differences in Subgroups According to KRAS/BRAF Mutation Status and Tumor Tissue Availability in a Prospective Population-Based Metastatic Colorectal Cancer Cohort. PLoS ONE 2015, 10, e0131046. [Google Scholar] [CrossRef]
- Nunes, L.; Aasebø, K.; Mathot, L.; Ljungström, V.; Edqvist, P.H.; Sundström, M.; Dragomir, A.; Pfeiffer, P.; Ameur, A.; Ponten, F.; et al. Molecular Characterization of a Large Unselected Cohort of Metastatic Colorectal Cancers in Relation to Primary Tumor Location, Rare Metastatic Sites and Prognosis. Acta Oncol. 2020, 59, 417–426. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of Metastasis in Colon and Rectal Cancer OPEN. Nat. Publ. Gr. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Uutela, A.; Osterlund, E.; Halonen, P.; Kallio, R.; Ålgars, A.; Salminen, T.; Lamminmäki, A.; Soveri, L.M.; Ristamäki, R.; Lehtomäki, K.; et al. Resectability, Conversion, Metastasectomy and Outcome According to RAS and BRAF Status for Metastatic Colorectal Cancer in the Prospective RAXO Study. Br. J. Cancer 2022, 127, 686. [Google Scholar] [CrossRef] [PubMed]
- Uutela, A.; Nordin, A.; Osterlund, E.; Halonen, P.; Kallio, R.; Soveri, L.M.; Salminen, T.; Ålgars, A.; Ristimäki, A.; Ovissi, A.; et al. Resectability and Resection Rates of Colorectal Liver Metastases According to RAS and BRAF Mutational Status: Prospective Study. Br. J. Surg. 2023, 110, 931. [Google Scholar] [CrossRef] [PubMed]
- Benedix, F.; Kube, R.; Meyer, F.; Schmidt, U.; Gastinger, I.; Lippert, H. Comparison of 17,641 Patients with Right- and Left-Sided Colon Cancer: Differences in Epidemiology, Perioperative Course, Histology, and Survival. Dis. Colon Rectum 2010, 53, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Tomasello, G.; Borgonovo, K.; Ghidini, M.; Turati, L.; Dallera, P.; Passalacqua, R.; Sgroi, G.; Barni, S. Prognostic Survival Associated with Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-Analysis. JAMA Oncol. 2017, 3, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; MacKinlay, A.; Semira, C.; Schieber, C.; Jimeno Yepes, A.J.; Lee, B.; Wong, R.; Hettiarachchige, C.K.H.; Gunn, N.; Tie, J.; et al. Stage-Based Variation in the Effect of Primary Tumor Side on All Stages of Colorectal Cancer Recurrence and Survival. Clin. Color. Cancer 2018, 17, e569–e577. [Google Scholar] [CrossRef] [PubMed]
- Hamfjord, J.; Myklebust, T.Å.; Larsen, I.K.; Kure, E.H.; Glimelius, B.; Guren, T.K.; Tveit, K.M.; Guren, M.G. Survival Trends of Right- and Left-Sided Colon Cancer across Four Decades: A Norwegian Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 342. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Muro, K.; Watanabe, J.; Shitara, K.; Ohori, H.; Shiozawa, M.; Yasui, H.; Oki, E.; Sato, T.; Naito, T.; et al. Efficacy of Panitumumab in Patients with Left-Sided Disease, MSS/MSI-L, and RAS/BRAF WT: A Biomarker Study of the Phase III PARADIGM Trial. J. Clin. Oncol. 2023, 41, 3508. [Google Scholar] [CrossRef]
- Watanabe, J.; Muro, K.; Shitara, K.; Yamazaki, K.; Shiozawa, M.; Ohori, H.; Takashima, A.; Yokota, M.; Makiyama, A.; Akazawa, N.; et al. Panitumumab vs Bevacizumab Added to Standard First-Line Chemotherapy and Overall Survival Among Patients with RAS Wild-Type, Left-Sided Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2023, 329, 1271–1282. [Google Scholar] [CrossRef]
- Lee, K.-H.; Chen, W.-S.; Jiang, J.-K.; Yang, S.-H.; Wang, H.-S.; Chang, S.-C.; Lan, Y.-T.; Lin, C.-C.; Lin, H.-H.; Huang, S.-C.; et al. The Efficacy of Anti-EGFR Therapy in Treating Metastatic Colorectal Cancer Differs between the Middle/Low Rectum and the Left-Sided Colon. Br. J. Cancer 2021, 125, 816–825. [Google Scholar] [CrossRef]
- Kodama, H.; Masuishi, T.; Wakabayashi, M.; Nakata, A.; Kumanishi, R.; Nakazawa, T.; Ogata, T.; Matsubara, Y.; Honda, K.; Narita, Y.; et al. Differential Efficacy of Targeted Monoclonal Antibodies in Left-Sided Colon and Rectal Metastatic Cancers. Clin. Color. Cancer 2023, 22, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Schuurhuizen, C.S.E.W.; Braamse, A.M.J.; Konings, I.R.H.M.; Sprangers, M.A.G.; Ket, J.C.F.; Dekker, J.; Verheul, H.M.W. Does Severe Toxicity Affect Global Quality of Life in Patients with Metastatic Colorectal Cancer during Palliative Systemic Treatment? A Systematic Review. Ann. Oncol. 2017, 28, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Bakkerus, L.; Buffart, L.M.; Buffart, T.E.; Meyer, Y.M.; Zonderhuis, B.M.; Haasbeek, C.J.A.; Versteeg, K.S.; Loosveld, O.J.L.; de Groot, J.W.B.; Hendriks, M.P.; et al. Health-Related Quality of Life in Patients with Metastatic Colorectal Cancer Undergoing Systemic Therapy with or Without Maximal Tumor Debulking. J. Natl. Compr. Cancer Netw. 2023, 21, 1059–1066.e5. [Google Scholar] [CrossRef] [PubMed]
- Hinz, A.; Mehnert, A.; Dégi, C.; Reissmann, D.R.; Schotte, D.; Schulte, T. The Relationship between Global and Specific Components of Quality of Life, Assessed with the EORTC QLQ-C30 in a Sample of 2019 Cancer Patients. Eur. J. Cancer Care 2017, 26, e12416. [Google Scholar] [CrossRef]
- Savikko, J.; Vikatmaa, L.; Hiltunen, A.M.; Mallat, N.; Tukiainen, E.; Salonen, S.M.; Nordin, A. Enhanced Recovery Protocol in Laparoscopic Liver Surgery. Surg. Endosc. 2021, 35, 1058. [Google Scholar] [CrossRef]
- Savikko, J.; Ilmakunnas, M.; Mäkisalo, H.; Nordin, A.; Isoniemi, H. Enhanced Recovery Protocol after Liver Resection. Br. J. Surg. 2015, 102, 1526–1532. [Google Scholar] [CrossRef]
- Bai, J.; Yang, M.; Liu, Z.; Efetov, S.; Kayaalp, C.; Dulskas, A.; Shaw, D.; Wang, X. Primary Tumor Resection in Colorectal Cancer Patients with Unresectable Distant Metastases: A Minireview. Front. Oncol. 2023, 13, 1138407. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, Y.; Shitara, K.; Mizusawa, J.; Hamaguchi, T.; Shida, D.; Komori, K.; Ikeda, S.; Ojima, H.; Ike, H.; Shiomi, A.; et al. Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients with Asymptomatic, Synchronous Unresectable Metastases (JCOG1007, IPACS): A Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 1098. [Google Scholar] [CrossRef]
- Świątkowski, F.; Górnicki, T.; Bułdyś, K.; Chabowski, M. The Quality of Life of Patients with Surgically Treated Colorectal Cancer: A Narrative Review. J. Clin. Med. 2022, 11, 6211. [Google Scholar] [CrossRef]
- Ratjen, I.; Schafmayer, C.; Enderle, J.; di Giuseppe, R.; Waniek, S.; Koch, M.; Burmeister, G.; Nöthlings, U.; Hampe, J.; Schlesinger, S.; et al. Health-Related Quality of Life in Long-Term Survivors of Colorectal Cancer and Its Association with All-Cause Mortality: A German Cohort Study. BMC Cancer 2018, 18, 1156. [Google Scholar] [CrossRef]
- Qaderi, S.M.; van der Heijden, J.A.G.; Verhoeven, R.H.A.; de Wilt, J.H.W.; Custers, J.A.E.; Beets, G.L.; Belt, E.J.T.; Berbée, M.; Beverdam, F.H.; Blankenburgh, R.; et al. Trajectories of Health-Related Quality of Life and Psychological Distress in Patients with Colorectal Cancer: A Population-Based Study. Eur. J. Cancer 2021, 158, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Valeikaite-Tauginiene, G.; Kraujelyte, A.; Poskus, E.; Jotautas, V.; Saladzinskas, Z.; Tamelis, A.; Lizdenis, P.; Dulskas, A.; Samalavicius, N.E.; Strupas, K.; et al. Predictors of Quality of Life Six Years after Curative Colorectal Cancer Surgery: Results of the Prospective Multicenter Study. Medicina 2022, 58, 482. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R.; van der Kaaij, M.; van Dorst, E.; Creutzberg, C.; Huyghe, E.; Kiserud, C.E. Sexual Dysfunction and Infertility as Late Effects of Cancer Treatment. Eur. J. Cancer Suppl. 2014, 12, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Sadovsky, R.; Basson, R.; Krychman, M.; Morales, A.M.; Schover, L.; Wang, R.; Incrocci, L. Cancer and Sexual Problems. J. Sex. Med. 2010, 7, 349–373. [Google Scholar] [CrossRef] [PubMed]
- Färkkilä, N.; Sintonen, H.; Saarto, T.; Järvinen, H.; Hänninen, J.; Taari, K.; Roine, R.P. Health-Related Quality of Life in Colorectal Cancer. Color. Dis. 2013, 15, e215–e222. [Google Scholar] [CrossRef] [PubMed]
- Hamidou, Z.; Chibaudel, B.; Hebbar, M.; Hug de Larauze, M.; André, T.; Louvet, C.; Brusquant, D.; Garcia-Larnicol, M.-L.; de Gramont, A.; Bonnetain, F. Time to Definitive Health-Related Quality of Life Score Deterioration in Patients with Resectable Metastatic Colorectal Cancer Treated with FOLFOX4 versus Sequential Dose-Dense FOLFOX7 Followed by FOLFIRI: The MIROX Randomized Phase III Trial. PLoS ONE 2016, 11, e0157067. [Google Scholar] [CrossRef] [PubMed]
- Láng, I.; Köhne, C.-H.; Folprecht, G.; Rougier, P.; Curran, D.; Hitre, E.; Sartorius, U.; Griebsch, I.; Van Cutsem, E. Quality of Life Analysis in Patients with KRAS Wild-Type Metastatic Colorectal Cancer Treated First-Line with Cetuximab plus Irinotecan, Fluorouracil and Leucovorin. Eur. J. Cancer 2013, 49, 439–448. [Google Scholar] [CrossRef]
- Quidde, J.; Hegewisch-Becker, S.; Graeven, U.; Lerchenmüller, C.A.; Killing, B.; Depenbusch, R.; Steffens, C.-C.; Lange, T.; Dietrich, G.; Stoehlmacher, J.; et al. Quality of Life Assessment in Patients with Metastatic Colorectal Cancer Receiving Maintenance Therapy after First-Line Induction Treatment: A Preplanned Analysis of the Phase III AIO KRK 0207 Trial. Ann. Oncol. 2016, 27, 2203–2210. [Google Scholar] [CrossRef]
- Bertaut, A.; Touchefeu, Y.; Blanc, J.; Bouché, O.; François, E.; Conroy, T.; Artru, P.; Adenis, A.; Gobbo, J.; Borg, C.; et al. Health-Related Quality of Life Analysis in Metastatic Colorectal Cancer Patients Treated by Second-Line Chemotherapy, Associated with Either Cetuximab or Bevacizumab: The PRODIGE 18 Randomized Phase II Study. Clin. Color. Cancer 2022, 21, e49–e61. [Google Scholar] [CrossRef]
- Flyum, I.R.; Mahic, S.; Grov, E.K.; Joranger, P. Health-Related Quality of Life in Patients with Colorectal Cancer in the Palliative Phase: A Systematic Review and Meta-Analysis. BMC Palliat. Care 2021, 20, 144. [Google Scholar] [CrossRef]
- Färkkilä, N.; Torvinen, S.; Roine, R.P.; Sintonen, H.; Hänninen, J.; Taari, K.; Saarto, T. Health-Related Quality of Life among Breast, Prostate, and Colorectal Cancer Patients with End-Stage Disease. Qual. Life Res. 2014, 23, 1387–1394. [Google Scholar] [CrossRef]
- Luo, X.; Li, J.; Chen, M.; Gong, J.; Xu, Y.; Li, Q. A Literature Review of Post-treatment Survivorship Interventions for Colorectal Cancer Survivors and/or Their Caregivers. Psychooncology 2021, 30, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Jefford, M.; Emery, J.D.; James Martin, A.; De Abreu Lourenco, R.; Lisy, K.; Grunfeld, E.; Mohamed, M.A.; King, D.; Tebbutt, N.C.; Lee, M.; et al. SCORE: A Randomised Controlled Trial Evaluating Shared Care (General Practitioner and Oncologist) Follow-up Compared to Usual Oncologist Follow-up for Survivors of Colorectal Cancer. eClinicalMedicine 2023, 66, 102346. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; Oerlemans, S.; Mols, F.; Aaronson, N.K.; van der Graaf, W.T.A.; van de Poll-Franse, L.V. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the “Real-World”: Results from the Population-Based PROFILES Registry. Oncologist 2020, 25, e722. [Google Scholar] [CrossRef] [PubMed]
- Pate, A.; Lowery, J.; Kilbourn, K.; Blatchford, P.J.; McNulty, M.; Risendal, B. Quality of Life and the Negative Impact of Comorbidities in Long-Term Colorectal Cancer Survivors: A Population-Based Comparison. J. Cancer Surviv. 2020, 14, 653–659. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).