Long-Term Impact of Severe Postoperative Complications after Esophagectomy for Cancer: Individual Patient Data Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Selection Process
2.3. Data Extraction
2.4. Outcomes and Definitions
2.5. Quality Assessment and Assessment of Certainty of Evidence
2.6. Statistical Analysis
3. Results
3.1. Systematic Review
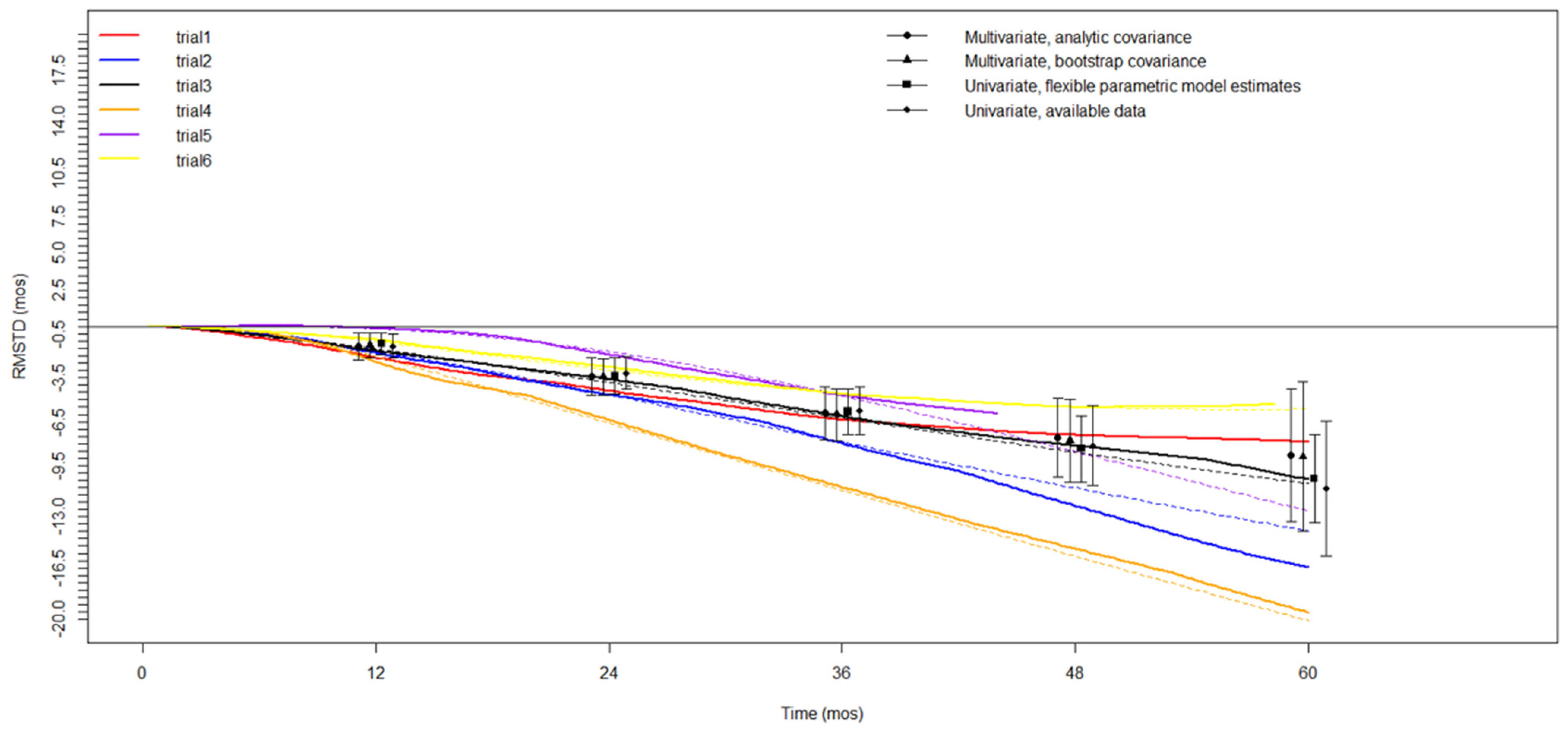
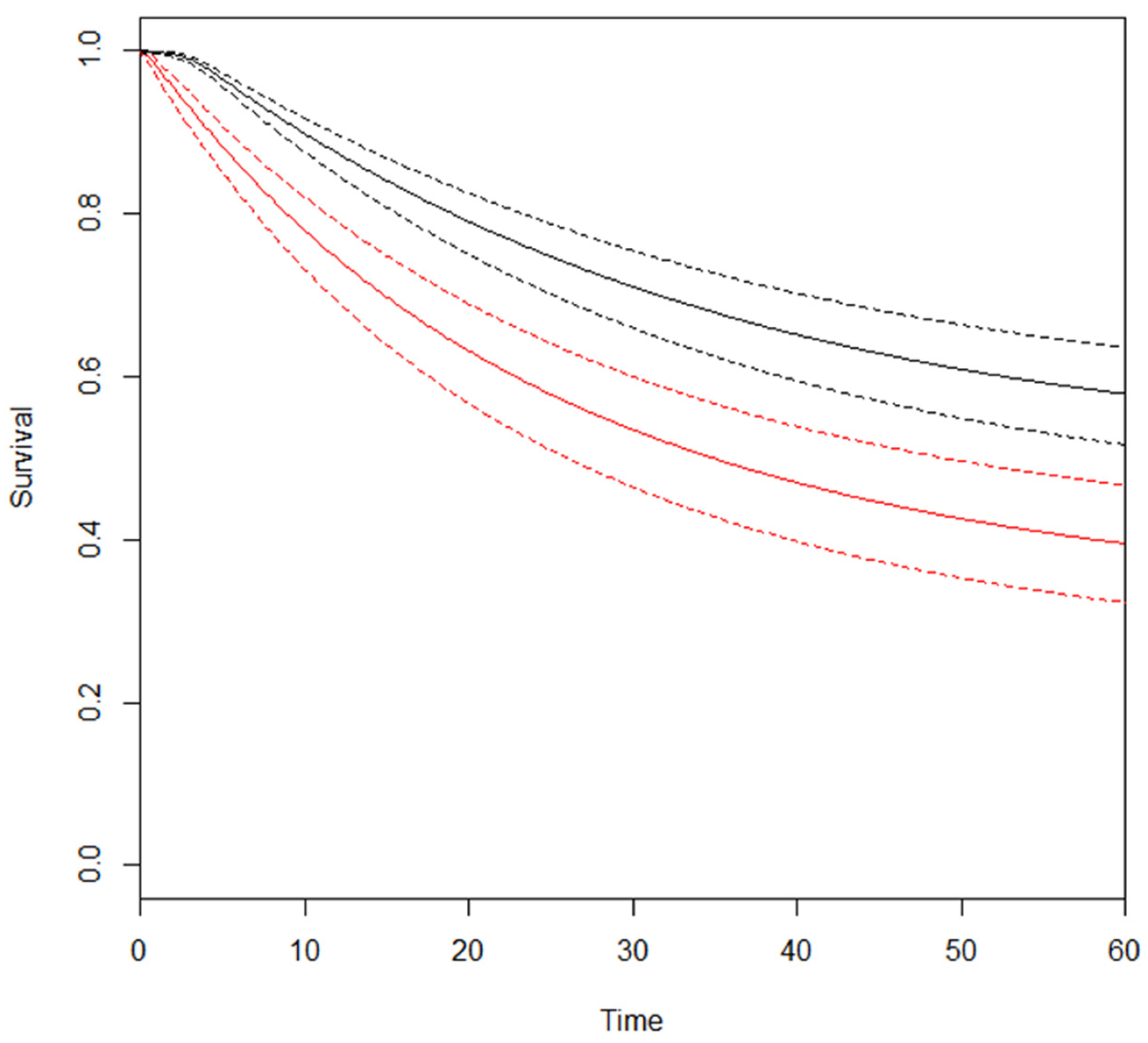
3.2. Meta-Analysis—Overall Survival (OS)
3.3. Secondary Outcomes—CSS/DFS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Q.; Ma, Y.L.; Qin, Q.; Wang, P.H.; Luo, Y.; Xu, P.F.; Cui, Y. Epidemiology of Esophageal Cancer in 2020 and Projections to 2030 and 2040. Thorac. Cancer 2023, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Donlon, N.E.; Moran, B.; Kamilli, A.; Davern, M.; Sheppard, A.; King, S.; Donohoe, C.L.; Lowery, M.; Cunningham, M.; Ravi, N.; et al. Cross versus Flot Regimens in Esophageal and Esophagogastric Junction Adenocarcinoma a Propensity-Matched Comparison. Ann. Surg. 2022, 276, 792–798. [Google Scholar] [CrossRef]
- Lordick, F.; Mariette, C.; Haustermans, K.; Obermannová, R.; Arnold, D.; on behalf of the ESMO Guidelines Committee. Oesophageal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2016, 27, v50–v57. [Google Scholar] [CrossRef] [PubMed]
- Bonavina, L.; Asti, E.; Sironi, A.; Bernardi, D.; Aiolfi, A. Hybrid and Total Minimally Invasive Esophagectomy: How I Do It. J. Thorac. Dis. 2017, 9, S761–S772. [Google Scholar] [CrossRef] [PubMed]
- van der Wilk, B.J.; Hagens, E.R.C.; Eyck, B.M.; Gisbertz, S.S.; van Hillegersberg, R.; Nafteux, P.; Schröder, W.; Nilsson, M.; Wijnhoven, B.P.L.; Lagarde, S.M.; et al. Outcomes after Totally Minimally Invasive versus Hybrid and Open Ivor Lewis Oesophagectomy: Results from the International Esodata Study Group. Br. J. Surg. 2022, 109, 283–290. [Google Scholar] [CrossRef]
- Viklund, P.; Lindblad, M.; Lu, M.; Ye, W.; Johansson, J.; Lagergren, J. Risk Factors for Complications After Esophageal Cancer Resection: A Prospective Population-Based Study in Sweden. Ann. Surg. 2006, 243, 204. [Google Scholar] [CrossRef] [PubMed]
- Markar, S.R.; Zaninotto, G.; Castoro, C.; Johar, A.; Lagergren, P.; Elliott, J.A.; Gisbertz, S.S.; Mariette, C.; Alfieri, R.; Huddy, J.; et al. Lasting Symptoms After Esophageal Resection (LASER): European Multicenter Cross-Sectional Study. Ann. Surg. 2022, 275, E392–E400. [Google Scholar] [CrossRef]
- Aiolfi, A.; Griffiths, E.A.; Sozzi, A.; Manara, M.; Bonitta, G.; Bonavina, L.; Bona, D. Effect of Anastomotic Leak on Long-Term Survival After Esophagectomy: Multivariate Meta-Analysis and Restricted Mean Survival Times Examination. Ann. Surg. Oncol. 2023, 30, 5564–5572. [Google Scholar] [CrossRef]
- Booka, E.; Takeuchi, H.; Suda, K.; Fukuda, K.; Nakamura, R.; Wada, N.; Kawakubo, H.; Kitagawa, Y. Meta-Analysis of the Impact of Postoperative Complications on Survival after Oesophagectomy for Cancer. BJS Open 2018, 2, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Nuytens, F.; Dabakuyo-Yonli, T.S.; Meunier, B.; Gagnière, J.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; Mabrut, J.Y.; et al. Five-Year Survival Outcomes of Hybrid Minimally Invasive Esophagectomy in Esophageal Cancer: Results of the MIRO Randomized Clinical Trial. JAMA Surg. 2021, 156, 323. [Google Scholar] [CrossRef] [PubMed]
- Manara, M.; Bona, D.; Bonavina, L.; Aiolfi, A. Impact of Pulmonary Complications Following Esophagectomy on Long-Term Survival: Multivariate Meta-Analysis and Restricted Mean Survival Time Assessment. Updates Surg. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Aiolfi, A.; Asti, E.; Rausa, E.; Bonavina, G.; Bonitta, G.; Bonavina, L. Use of C-Reactive Protein for the Early Prediction of Anastomotic Leak after Esophagectomy: Systematic Review and Bayesian Meta-Analysis. PLoS ONE 2018, 13, e0209272. [Google Scholar] [CrossRef] [PubMed]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’Journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking Complications Associated with Esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Bundred, J.R.; Hollis, A.C.; Evans, R.; Hodson, J.; Whiting, J.L.; Griffiths, E.A. Impact of Postoperative Complications on Survival after Oesophagectomy for Oesophageal Cancer. BJS Open 2020, 4, 405. [Google Scholar] [CrossRef]
- D’Annoville, T.; D’Journo, X.B.; Trousse, D.; Brioude, G.; Dahan, L.; Seitz, J.F.; Doddoli, C.; Thomas, P.A. Respiratory Complications after Oesophagectomy for Cancer Do Not Affect Disease-Free Survival. Eur. J. Cardiothorac. Surg. 2012, 41, e66–e73. [Google Scholar] [CrossRef] [PubMed]
- Lerut, T.; Moons, J.; Coosemans, W.; Van Raemdonck, D.; De Leyn, P.; Decaluwé, H.; Decker, G.; Nafteux, P. Postoperative Complications after Transthoracic Esophagectomy for Cancer of the Esophagus and Gastroesophageal Junction Are Correlated with Early Cancer Recurrence: Role of Systematic Grading of Complications Using the Modified Clavien Classification. Ann. Surg. 2009, 250, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Wang, Y.J.; Liu, X.H.; Tan, Q.Y.; Jiang, Y.G.; Guo, W. The Effect of Postoperative Complications on Survival of Patients after Minimally Invasive Esophagectomy for Esophageal Cancer. Surg. Endosc. 2017, 31, 3475–3482. [Google Scholar] [CrossRef]
- Xia, B.T.; Rosato, E.L.; Chojnacki, K.A.; Crawford, A.G.; Weksler, B.; Berger, A.C. Major Perioperative Morbidity Does Not Affect Long-Term Survival in Patients Undergoing Esophagectomy for Cancer of the Esophagus or Gastroesophageal Junction. World J. Surg. 2013, 37, 408–415. [Google Scholar] [CrossRef]
- Yamamoto, M.; Shimokawa, M.; Yoshida, D.; Yamaguchi, S.; Ohta, M.; Egashira, A.; Ikebe, M.; Morita, M.; Toh, Y. The Survival Impact of Postoperative Complications after Curative Resection in Patients with Esophageal Squamous Cell Carcinoma: Propensity Score-Matching Analysis. J. Cancer Res. Clin. Oncol. 2020, 146, 1351–1360. [Google Scholar] [CrossRef]
- Luc, G.; Durand, M.; Chiche, L.; Collet, D. Major Post-Operative Complications Predict Long-Term Survival after Esophagectomy in Patients with Adenocarcinoma of the Esophagus. World J. Surg. 2015, 39, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Hamai, Y.; Emi, M.; Ibuki, Y.; Yoshikawa, T.; Ohsawa, M.; Hirohata, R.; Okada, M. Risk Factors for Recurrence in Esophageal Squamous Cell Carcinoma Without Pathological Complete Response After Trimodal Therapy. Anticancer Res. 2020, 40, 4387–4394. [Google Scholar] [CrossRef] [PubMed]
- Kiyozumi, Y.; Yoshida, N.; Ishimoto, T.; Yagi, T.; Koga, Y.; Uchihara, T.; Sawayama, H.; Hiyoshi, Y.; Iwatsuki, M.; Baba, Y.; et al. Prognostic Factors of Salvage Esophagectomy for Residual or Recurrent Esophageal Squamous Cell Carcinoma After Definitive Chemoradiotherapy. World J. Surg. 2018, 42, 2887–2893. [Google Scholar] [CrossRef] [PubMed]
- Aahlin, E.K.; Olsen, F.; Uleberg, B.; Jacobsen, B.K.; Lassen, K. Major Postoperative Complications Are Associated with Impaired Long-Term Survival after Gastro-Esophageal and Pancreatic Cancer Surgery: A Complete National Cohort Study. BMC Surg. 2016, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Makino, T.; Miyata, H.; Miyazaki, Y.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Takiguchi, S.; Mori, M.; et al. Postoperative Infectious Complications Are Associated with Adverse Oncologic Outcomes in Esophageal Cancer Patients Undergoing Preoperative Chemotherapy. Ann. Surg. Oncol. 2016, 23, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Goossen, K.; Tenckhoff, S.; Probst, P.; Grummich, K.; Mihaljevic, A.L.; Büchler, M.W.; Diener, M.K. Optimal Literature Search for Systematic Reviews in Surgery. Langenbecks Arch. Surg. 2018, 403, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Royston, P.; Parmar, M.K.B. Restricted Mean Survival Time: An Alternative to the Hazard Ratio for the Design and Analysis of Randomized Trials with a Time-to-Event Outcome. BMC Med. Res. Methodol. 2013, 13, 152. [Google Scholar] [CrossRef]
- Wei, Y.; Royston, P.; Tierney, J.F.; Parmar, M.K.B. Meta-Analysis of Time-to-Event Outcomes from Randomized Trials Using Restricted Mean Survival Time: Application to Individual Participant Data. Stat. Med. 2015, 34, 2881–2898. [Google Scholar] [CrossRef]
- Jackson, D.; White, I.R.; Riley, R.D. A Matrix-Based Method of Moments for Fitting the Multivariate Random Effects Model for Meta-Analysis and Meta-Regression. Biom. J. 2013, 55, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.N.M.; Welton, N.J. Enhanced Secondary Analysis of Survival Data: Reconstructing the Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 2 March 2023).
- Charvat, H.; Belot, A. Mexhaz: An R Package for Fitting Flexible Hazard-Based Regression Models for Overall and Excess Mortality with a Random Effect. J. Stat. Softw. 2021, 98, 1–36. [Google Scholar] [CrossRef]
- Schuring, N.; Jezerskyte, E.; van Berge Henegouwen, M.I.; Sprangers, M.A.G.; Lagergren, P.; Johar, A.; Markar, S.R.; Gisbertz, S.S.; Markar, S.R.; Zaninotto, G.; et al. Influence of Postoperative Complications Following Esophagectomy for Cancer on Quality of Life: A European Multicenter Study. Eur. J. Surg. Oncol. 2023, 49, 97–105. [Google Scholar] [CrossRef]
- Kalata, S.; Singh, B.; Graham, N.; Fan, Z.; Chang, A.C.; Lynch, W.R.; Lagisetty, K.H.; Lin, J.; Yeung, J.; Reddy, R.M.; et al. Epidemiology of Postoperative Complications After Esophagectomy: Implications for Management. Ann. Thorac. Surg. 2023, 116, 1168–1175. [Google Scholar] [CrossRef]
- Bailey, S.H.; Bull, D.A.; Harpole, D.H.; Rentz, J.J.; Neumayer, L.A.; Pappas, T.N.; Daley, J.; Henderson, W.G.; Krasnicka, B.; Khuri, S.F.; et al. Outcomes after Esophagectomy: A Ten-Year Prospective Cohort. Ann. Thorac. Surg. 2003, 75, 217–222. [Google Scholar] [CrossRef]
- Liou, D.Z.; Serna-Gallegos, D.; Mirocha, J.; Bairamian, V.; Alban, R.F.; Soukiasian, H.J. Predictors of Failure to Rescue after Esophagectomy. Ann. Thorac. Surg. 2018, 105, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, Z.M.; Habermann, E.; Borah, B.J.; Moriarty, J.P.; Rojas, R.L.; Blackmon, S.H. Understanding Failure to Rescue After Esophagectomy in the United States. Ann. Thorac. Surg. 2020, 109, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Fabbi, M.; Hagens, E.R.C.; Van Berge Henegouwen, M.I.; Gisbertz, S.S. Anastomotic Leakage after Esophagectomy for Esophageal Cancer: Definitions, Diagnostics, and Treatment. Dis. Esophagus 2021, 34, doaa039. [Google Scholar] [CrossRef]
- Jezerskyte, E.; van Berge Henegouwen, M.I.; van Laarhoven, H.W.M.; van Kleef, J.J.; Eshuis, W.J.; Heisterkamp, J.; Hartgrink, H.H.; Rosman, C.; van Hillegersberg, R.; Hulshof, M.C.C.M.; et al. Postoperative Complications and Long-Term Quality of Life After Multimodality Treatment for Esophageal Cancer: An Analysis of the Prospective Observational Cohort Study of Esophageal-Gastric Cancer Patients (POCOP). Ann. Surg. Oncol. 2021, 28, 7259–7276. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.D.; Zheng, H.; Gaissert, H.; Mathisen, D.; Muniappan, A.; Wright, C.; Lanuti, M. Relative Incremental Cost of Postoperative Complications of Esophagectomy. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, A.; Åkesson, O.; Johansson, J.; Persson, J. Hospital Costs and Health-Related Quality of Life from Complications after Esophagectomy. Eur. J. Surg. Oncol. 2021, 47, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli Romario, U.; de Pascale, S.; Manara, M.; Colombo, S.; Attanasio, A.; Sabbatini, A.; Sandrin, F. Esophagectomy-Prevention of Complications-Tips and Tricks for the Preoperative, Intraoperative and Postoperative Stage. Updates Surg. 2023, 75, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Fransen, L.F.C.; Berkelmans, G.H.K.; Asti, E.; van Berge Henegouwen, M.I.; Berlth, F.; Bonavina, L.; Brown, A.; Bruns, C.; van Daele, E.; Gisbertz, S.S.; et al. The Effect of Postoperative Complications after Minimally Invasive Esophagectomy on Long-Term Survival an International Multicenter Cohort Study. Ann. Surg. 2021, 274, E1129–E1137. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.; Sepesi, B.; Hofstetter, W.L.; Correa, A.M.; Bhutani, M.S.; Watson, T.J.; Swisher, S.G.; Ajani, J.A.; Erasmus, J.; Komaki, R.; et al. Endoscopic Esophageal Tumor Length: A Prognostic Factor for Patients with Esophageal Cancer. Cancer 2011, 117, 63–69. [Google Scholar] [CrossRef] [PubMed]
- McCain, R.S.; McManus, D.T.; McQuaid, S.; James, J.A.; Salto-Tellez, M.; Reid, N.B.; Craig, S.; Chisambo, C.; Bingham, V.; McCarron, E.; et al. Alcohol Intake, Tobacco Smoking, and Esophageal Adenocarcinoma Survival: A Molecular Pathology Epidemiology Cohort Study. Cancer Causes Control 2020, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abou Chaar, M.K.; Godin, A.; Harmsen, W.S.; Wzientek, C.; Saddoughi, S.A.; Hallemeier, C.L.; Cassivi, S.D.; Nichols, F.C.; Reisenauer, J.S.; Shen, K.R.; et al. Determinants of Long-Term Survival Decades After Esophagectomy for Esophageal Cancer. Ann. Thorac. Surg. 2023, 116, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Boshier, P.R.; Ziff, C.; Adam, M.E.; Fehervari, M.; Markar, S.R.; Hanna, G.B. Effect of Perioperative Blood Transfusion on the Long-Term Survival of Patients Undergoing Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-Analysis. Dis. Esophagus 2018, 31, dox134. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, M.; Ghidini, A.; Sgroi, G.; Vavassori, I.; Petrò, D.; Cabiddu, M.; Aiolfi, A.; Bonitta, G.; Zaniboni, A.; et al. Red Blood Cell Transfusions and the Survival in Patients with Cancer Undergoing Curative Surgery: A Systematic Review and Meta-Analysis. Surg. Today 2021, 51, 1535–1557. [Google Scholar] [CrossRef]
- Markar, S.; Gronnier, C.; Duhamel, A.; Bigourdan, J.M.; Badic, B.; du Rieu, M.C.; Lefevre, J.H.; Turner, K.; Luc, G.; Mariette, C. Pattern of Postoperative Mortality After Esophageal Cancer Resection According to Center Volume: Results from a Large European Multicenter Study. Ann. Surg. Oncol. 2015, 22, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Bona, D.; Lombardo, F.; Matsushima, K.; Cavalli, M.; Lastraioli, C.; Bonitta, G.; Cirri, S.; Danelli, P.; Aiolfi, A. Three-Field versus Two-Field Lymphadenectomy for Esophageal Squamous Cell Carcinoma: A Long-Term Survival Meta-Analysis. Surgery 2022, 171, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Markar, S.R.; Lagergren, J. Surgical and Surgeon-Related Factors Related to Long-Term Survival in Esophageal Cancer: A Review. Ann. Surg. Oncol. 2020, 27, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Khoushhal, Z.; Canner, J.; Schneider, E.; Stem, M.; Haut, E.; Mungo, B.; Lidor, A.; Molena, D. Influence of Specialty Training and Trainee Involvement on Perioperative Outcomes of Esophagectomy. Ann. Thorac. Surg. 2016, 102, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal Cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Dubecz, A.; Gall, I.; Solymosi, N.; Schweigert, M.; Peters, J.H.; Feith, M.; Stein, H.J. Temporal Trends in Long-Term Survival and Cure Rates in Esophageal Cancer: A SEER Database Analysis. J. Thorac. Oncol. 2012, 7, 443–447. [Google Scholar] [CrossRef]
- Griffin, S.M.; Jones, R.; Kamarajah, S.K.; Navidi, M.; Wahed, S.; Immanuel, A.; Hayes, N.; Phillips, A.W. Evolution of Esophagectomy for Cancer Over 30 Years: Changes in Presentation, Management and Outcomes. Ann. Surg. Oncol. 2021, 28, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Van Hagen, P.; Hulshof, M.C.C.M.; Van Lanschot, J.J.B.; Steyerberg, E.W.; Van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; Richel, D.J.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant Chemoradiotherapy plus Surgery versus Surgery Alone for Oesophageal or Junctional Cancer (CROSS): Long-Term Results of a Randomised Controlled Trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Steber, C.; Hughes, R.T.; McTyre, E.R.; Soike, M.; Farris, M.; Levine, B.J.; Pasche, B.; Levine, E.; Blackstock, A.W. Cisplatin/5-Fluorouracil (5-FU) Versus Carboplatin/Paclitaxel Chemoradiotherapy as Definitive or Pre-Operative Treatment of Esophageal Cancer. Cureus 2021, 13, e12574. [Google Scholar] [CrossRef]
- Prins, M.J.D.; Ruurda, J.P.; van Diest, P.J.; van Hillegersberg, R.; ten Kate, F.J.W. The Significance of the HER-2 Status in Esophageal Adenocarcinoma for Survival: An Immunohistochemical and an in Situ Hybridization Study. Ann. Oncol. 2013, 24, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- van Putten, M.; de Vos-Geelen, J.; Nieuwenhuijzen, G.A.P.; Siersema, P.D.; Lemmens, V.E.P.P.; Rosman, C.; van der Sangen, M.J.C.; Verhoeven, R.H.A. Long-Term Survival Improvement in Oesophageal Cancer in the Netherlands. Eur. J. Cancer 2018, 94, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, A.; Rahman, S.; Grace, B.; Walker, R.; Noble, F.; Kelly, J.; Byrne, J.; Underwood, T. The Effect of Surgical Complications on Long-Term Prognosis Following Oesophagectomy. Eur. J. Surg. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.P.; Bach, P.B.; Schrag, D.; Bains, M.S.; Turnbull, A.D.; Karpeh, M.; Brennan, M.F.; Rusch, V.W. The Impact of Complications on Outcomes after Resection for Esophageal and Gastroesophageal Junction Carcinoma. J. Am. Coll. Surg. 2004, 198, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, S.M.; Reitsma, J.B.; Maris, A.K.D.; van Berge Henegouwen, M.I.; Busch, O.R.C.; Obertop, H.; Zwinderman, A.H.; van Lanschot, J.J.B. Preoperative Prediction of the Occurrence and Severity of Complications after Esophagectomy for Cancer with Use of a Nomogram. Ann. Thorac. Surg. 2008, 85, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Ancona, E.; Cagol, M.; Epifani, M.; Cavallin, F.; Zaninotto, G.; Castoro, C.; Alfieri, R.; Ruol, A. Surgical Complications Do Not Affect Longterm Survival after Esophagectomy for Carcinoma of the Thoracic Esophagus and Cardia. J. Am. Coll. Surg. 2006, 203, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ferri, L.E.; Law, S.; Wong, K.H.; Kwok, K.F.; Wong, J. The Influence of Technical Complications on Postoperative Outcome and Survival after Esophagectomy. Ann. Surg. Oncol. 2006, 13, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Saunders, J.H.; Yanni, F.; Dorrington, M.S.; Bowman, C.R.; Vohra, R.S.; Parsons, S.L. Impact of Postoperative Complications on Disease Recurrence and Long-Term Survival Following Oesophagogastric Cancer Resection. Br. J. Surg. 2020, 107, 103–112. [Google Scholar] [CrossRef]
- Rutegård, M.; Lagergren, P.; Rouvelas, I.; Mason, R.; Lagergren, J. Surgical Complications and Long-Term Survival after Esophagectomy for Cancer in a Nationwide Swedish Cohort Study. Eur. J. Surg. Oncol. 2012, 38, 555–561. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-Related Inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Okamura, A.; Takeuchi, H.; Matsuda, S.; Ogura, M.; Miyasho, T.; Nakamura, R.; Takahashi, T.; Wada, N.; Kawakubo, H.; Saikawa, Y.; et al. Factors Affecting Cytokine Change After Esophagectomy for Esophageal Cancer. Ann. Surg. Oncol. 2015, 22, 3130–3135. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving Postoperative Immune Status and Resistance to Cancer Metastasis: A Combined Perioperative Approach of Immunostimulation and Prevention of Excessive Surgical Stress Responses. Ann. Surg. 2011, 253, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Song, Q.; Jia, Y.; Chen, X.; Wang, C.; Chen, P.; Min, R.; Cheng, Y. The Clinical Significance of Systemic Inflammation Score in Esophageal Squamous Cell Carcinoma. Tumor Biol. 2016, 37, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Takeuchi, H.; Kawakubo, H.; Nishi, T.; Fukuda, K.; Nakamura, R.; Takahashi, T.; Wada, N.; Saikawa, Y.; Omori, T.; et al. Clinical Significance of CXCL-8/CXCR-2 Network in Esophageal Squamous Cell Carcinoma. Surgery 2013, 154, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Spicer, J.D.; McDonald, B.; Cools-Lartigue, J.J.; Chow, S.C.; Giannias, B.; Kubes, P.; Ferri, L.E. Neutrophils Promote Liver Metastasis via Mac-1-Mediated Interactions with Circulating Tumor Cells. Cancer Res. 2012, 72, 3919–3927. [Google Scholar] [CrossRef] [PubMed]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells and Promote Metastasis. J. Clin. Investig. 2013, 123, 3446–3458. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, S.; Tachibana, M.; Yoshimura, H.; Ueda, S.; Fujii, T.; Dhar, D.K.; Nakamoto, T.; Nagasue, N. Postoperative Pulmonary Complications Are Associated with Worse Short- and Long-Term Outcomes after Extended Esophagectomy. J. Surg. Oncol. 2004, 88, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Booka, E.; Takeuchi, H.; Nishi, T.; Matsuda, S.; Kaburagi, T.; Fukuda, K.; Nakamura, R.; Takahashi, T.; Wada, N.; Kawakubo, H.; et al. The Impact of Postoperative Complications on Survivals After Esophagectomy for Esophageal Cancer. Medicine 2015, 94, e1369. [Google Scholar] [CrossRef]
- Baba, Y.; Yoshida, N.; Shigaki, H.; Iwatsuki, M.; Miyamoto, Y.; Sakamoto, Y.; Watanabe, M.; Baba, H. Prognostic Impact of Postoperative Complications in 502 Patients with Surgically Resected Esophageal Squamous Cell Carcinoma: A Retrospective Single-Institution Study. Ann. Surg. 2016, 264, 305–311. [Google Scholar] [CrossRef]
- Kataoka, K.; Takeuchi, H.; Mizusawa, J.; Igaki, H.; Ozawa, S.; Abe, T.; Nakamura, K.; Kato, K.; Ando, N.; Kitagawa, Y. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann. Surg. 2017, 265, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Nevins, E.J.; Chmelo, J.; Prasad, P.; Brown, J.; Phillips, A.W. Long-Term Survival Is Not Affected by Severity of Complications Following Esophagectomy. Eur. J. Surg. Oncol. 2024, 50, 108232. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.B.; Tian, Z.B.; Ding, X.L.; Guo, Y.J.; Mao, T.; Yu, Y.N.; Wang, K.X.; Jing, X. The Impact of Preoperative Sarcopenia on Survival Prognosis in Patients Receiving Neoadjuvant Therapy for Esophageal Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 619592. [Google Scholar] [CrossRef] [PubMed]
- Coupland, V.H.; Lagergren, J.; Lüchtenborg, M.; Jack, R.H.; Allum, W.; Holmberg, L.; Hanna, G.B.; Pearce, N.; Møller, H. Hospital Volume, Proportion Resected and Mortality from Oesophageal and Gastric Cancer: A Population-Based Study in England, 2004–2008. Gut 2013, 62, 961–966. [Google Scholar] [CrossRef]
- Patel, D.C.; Jeffrey Yang, C.F.; He, H.; Liou, D.Z.; Backhus, L.M.; Lui, N.S.; Shrager, J.B.; Berry, M.F. Influence of Facility Volume on Long-Term Survival of Patients Undergoing Esophagectomy for Esophageal Cancer. J. Thorac. Cardiovasc. Surg. 2022, 163, 1536–1546.e3. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative Chemotherapy with Fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bowlby, R.; Mungall, A.J.; Robertson, A.G.; Odze, R.D.; Cherniack, A.D.; Shih, J.; Pedamallu, C.S.; Cibulskis, C.; Dunford, A.; et al. Integrated Genomic Characterization of Oesophageal Carcinoma. Nature 2017, 541, 169. [Google Scholar] [CrossRef]
- Aiolfi, A.; Sozzi, A.; Bonitta, G.; Lombardo, F.; Cavalli, M.; Cirri, S.; Campanelli, G.; Danelli, P.; Bona, D. Linear- versus Circular-Stapled Esophagogastric Anastomosis during Esophagectomy: Systematic Review and Meta-Analysis. Langenbecks Arch. Surg. 2022, 407, 3297–3309. [Google Scholar] [CrossRef]
- Szakó, L.; Németh, D.; Farkas, N.; Kiss, S.; Dömötör, R.Z.; Engh, M.A.; Hegyi, P.; Eross, B.; Papp, A. Network Meta-Analysis of Randomized Controlled Trials on Esophagectomies in Esophageal Cancer: The Superiority of Minimally Invasive Surgery. World J. Gastroenterol. 2022, 28, 4019–4234. [Google Scholar] [CrossRef]
- Low, D.E.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.E.; D’Journo, X.B.; Griffin, S.M.; Hölscher, A.H.; Hofstetter, W.L.; Jobe, B.A.; et al. International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann. Surg. 2015, 262, 286–294. [Google Scholar] [CrossRef]
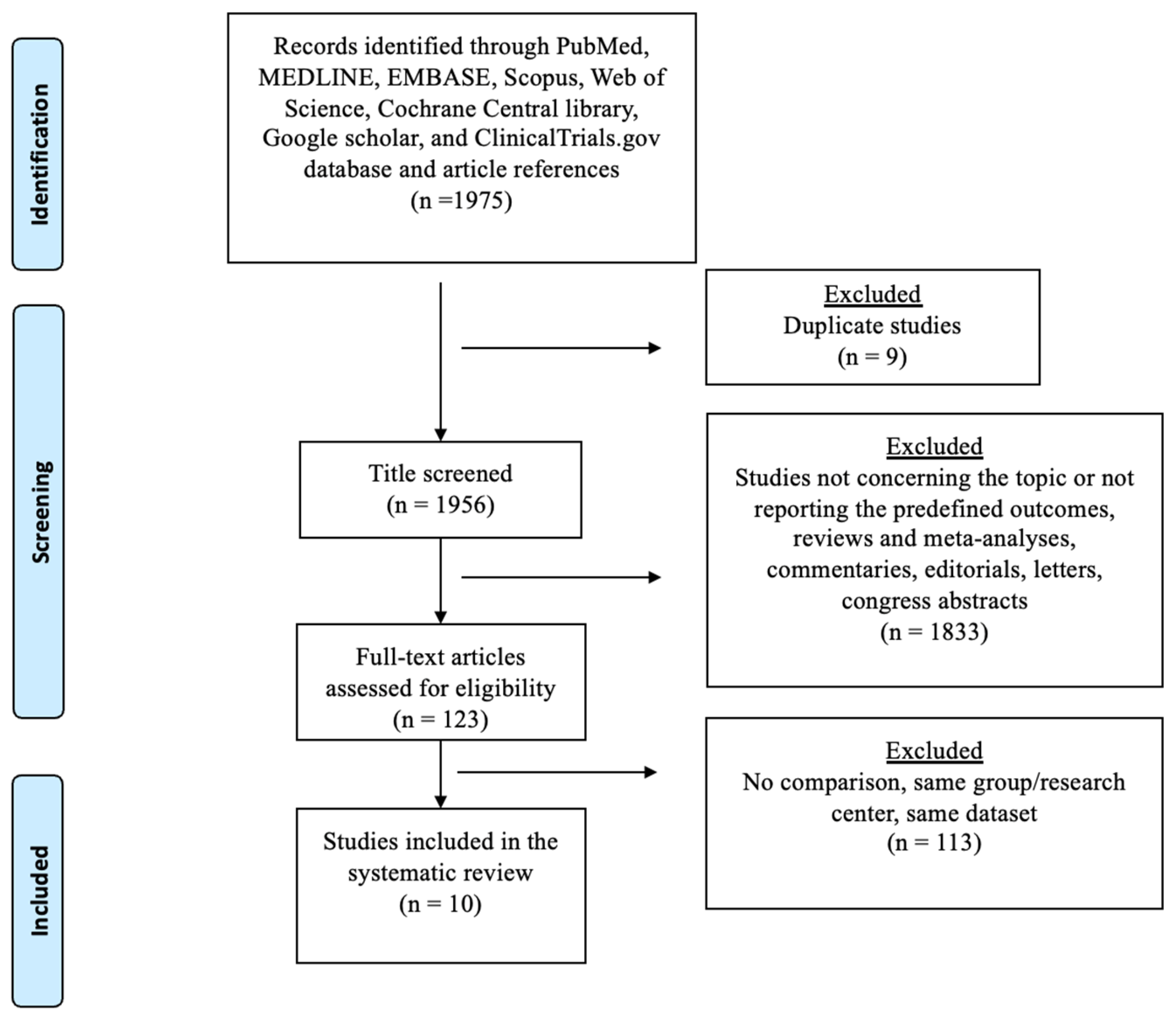
| Author, Year | Country | Study Design | No. Pts | Sex M | Age (yrs) | Tumor Histology (SCC-ADK-Other) | Location (U-M-L) | Neoadjuvant Treatment | pStage 0–I | pStage II | pStage III | pStage IV | Surgical Approach |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D’annoville et al., 2012 [17] | France | Ret | 341 | 286 | 60.1 ± 10 | 127-214-0 | 14-77-250 | 179 | nr | nr | nr | nr | Open |
| Xia et al., 2013 [20] | USA | Ret | 237 | 195 | 62 (32–86) | 36-201-0 | 5-17-215 | 155 | 90 | 84 | 52 | 11 | Open/MIE |
| Luc et al., 2015 [22] | France | Ret | 116 | 106 | 64.6 (40–79) | 0-106-0 | 0-0-116 | 106 | nr | nr | nr | nr | Open |
| Yamashita et al., 2016 [26] | Japan | Ret | 255 | 220 | 65 (35–85) | 255-0-0 | nr | 255 | 49 | 76 | 120 | 10 | Open/Hybrid |
| Aahlin et al., 2016 [25] | Norway | Ret | 331 | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr |
| Li et al., 2017 [19] | China | Ret | 214 | 170 | 60.2 ± 8.1 | 214-0-0 | 41-152-21 | nr | nr | nr | nr | nr | Hybrid/MIE |
| Kiyozumi et al., 2018 [24] | Japan | Ret | 50 | 46 | nr | 50-0-0 | 8-40-2 | 50 | nr | nr | nr | nr | Open |
| Bundred et al., 2020 [16] | UK | Ret | 430 | 342 | 64.9 ± 9.4 | 70-337-23 | 0-24-368 | nr | 89 | 95 | 232 | 9 | Open/Hybrid/MIE |
| Yamamoto et al., 2020 [21] | Japan | Ret | 102 | 92 | nr | 102-0-0 | nr | 42 | 28 | 30 | 31 | 13 | Open/Hybrid |
| Kurokawa et al., 2020 [23] | Japan | Ret | 105 | 88 | 63.6 ± 7.8 | 105-0-0 | 20-52-33 | 105 | 20 | 39 | 27 | 5 | Open/Hybrid |
| Time Horizon | No. Trials | RMSTD (mos) | SE | 95% CIs | p Value |
|---|---|---|---|---|---|
| 12-month | 6 | −1.4 | 0.5 | −2.4, −0.4 | 0.008 |
| 24-month | 6 | −3.4 | 0.6 | −4.6, −2.1 | <0.001 |
| 36-month | 5 | −5.8 | 0.9 | −7.7, −3.9 | <0.001 |
| 48-month | 5 | −7.4 | 1.4 | −10.1, −4.7 | <0.001 |
| 60-month | 3 | −8.6 | 1.9 | −12.5, −4.7 | <0.001 |
| Time Horizon | No. Trials | RMSTD (mos) | SE | 95% CIs | p Value |
|---|---|---|---|---|---|
| 12-month | 3 | −0.7 | 0.3 | −1.2, −0.2 | 0.009 |
| 24-month | 3 | −1.9 | 1.1 | −4.0, 0.3 | 0.09 |
| 36-month | 3 | −3.3 | 1.9 | −7.0, 0.4 | 0.08 |
| 48-month | 3 | −3.9 | 3.1 | −10.1, 2.3 | 0.22 |
| 60-month | 2 | −6.8 | 2.6 | −11.9, 1.7 | 0.21 |
| Time Horizon | No. Trials | RMSTD (mos) | SE | 95% CIs | p Value |
|---|---|---|---|---|---|
| 12-month | 6 | −0.6 | 0.4 | −1.4, 0.2 | 0.13 |
| 24-month | 6 | −2.3 | 0.9 | −4.2, −0.5 | 0.01 |
| 36-month | 6 | −3.7 | 1.6 | −6.8, −0.5 | 0.02 |
| 48-month | 6 | −4.4 | 2.4 | −9.2, 0.3 | 0.06 |
| 60-month | 3 | −4.6 | 3.3 | −11.1, 1.9 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bona, D.; Manara, M.; Bonitta, G.; Guerrazzi, G.; Guraj, J.; Lombardo, F.; Biondi, A.; Cavalli, M.; Bruni, P.G.; Campanelli, G.; et al. Long-Term Impact of Severe Postoperative Complications after Esophagectomy for Cancer: Individual Patient Data Meta-Analysis. Cancers 2024, 16, 1468. https://doi.org/10.3390/cancers16081468
Bona D, Manara M, Bonitta G, Guerrazzi G, Guraj J, Lombardo F, Biondi A, Cavalli M, Bruni PG, Campanelli G, et al. Long-Term Impact of Severe Postoperative Complications after Esophagectomy for Cancer: Individual Patient Data Meta-Analysis. Cancers. 2024; 16(8):1468. https://doi.org/10.3390/cancers16081468
Chicago/Turabian StyleBona, Davide, Michele Manara, Gianluca Bonitta, Guglielmo Guerrazzi, Juxhin Guraj, Francesca Lombardo, Antonio Biondi, Marta Cavalli, Piero Giovanni Bruni, Giampiero Campanelli, and et al. 2024. "Long-Term Impact of Severe Postoperative Complications after Esophagectomy for Cancer: Individual Patient Data Meta-Analysis" Cancers 16, no. 8: 1468. https://doi.org/10.3390/cancers16081468
APA StyleBona, D., Manara, M., Bonitta, G., Guerrazzi, G., Guraj, J., Lombardo, F., Biondi, A., Cavalli, M., Bruni, P. G., Campanelli, G., Bonavina, L., & Aiolfi, A. (2024). Long-Term Impact of Severe Postoperative Complications after Esophagectomy for Cancer: Individual Patient Data Meta-Analysis. Cancers, 16(8), 1468. https://doi.org/10.3390/cancers16081468








