The Gut Connection: Exploring the Possibility of Implementing Gut Microbial Metabolites in Lymphoma Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Lymphoma
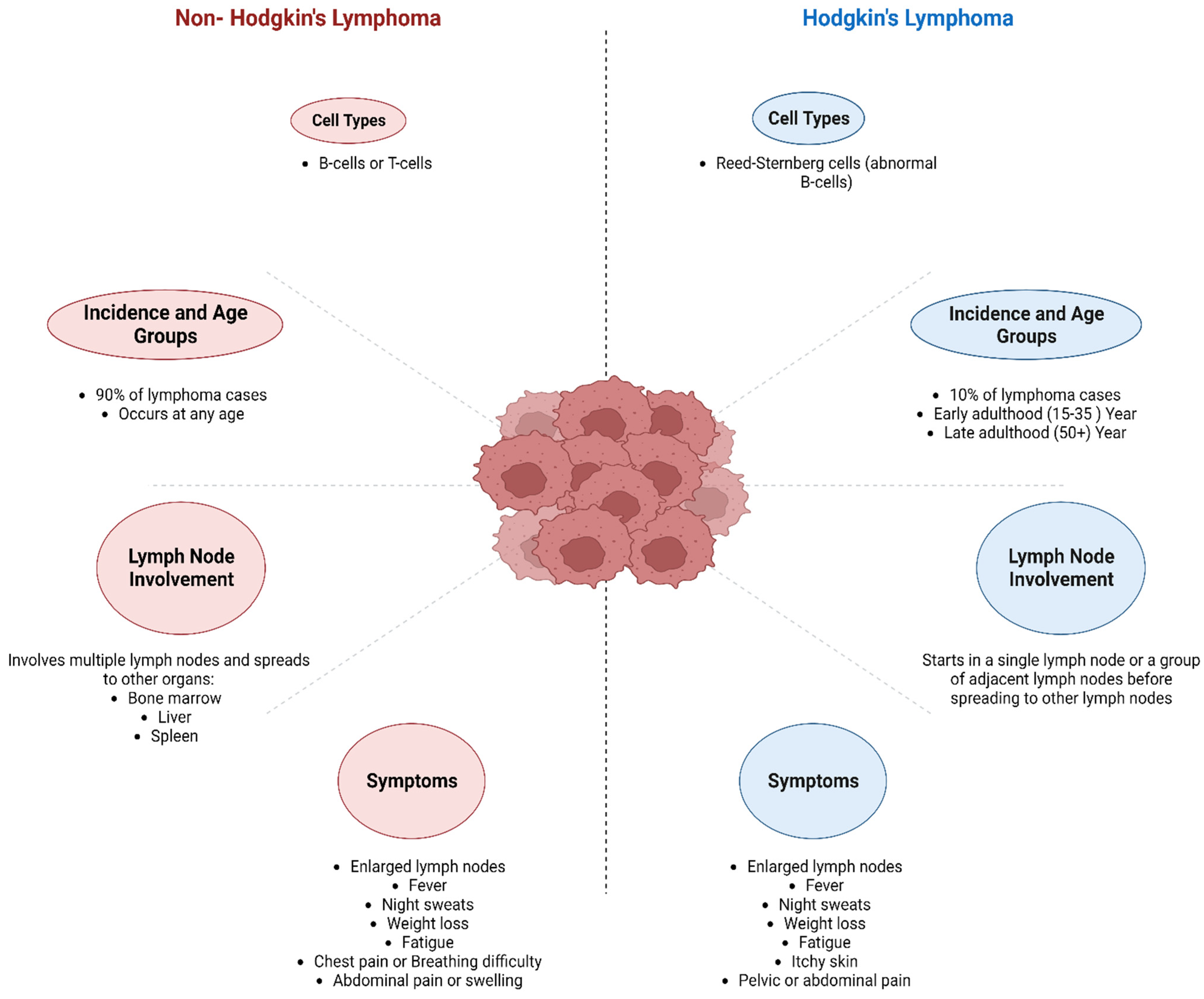
3. Classification of Lymphoma
3.1. Hodgkin’s Lymphoma (HL)
3.2. Non-Hodgkin Lymphoma (NHL)
Burkitt Lymphoma (BL)
4. Treatment and Side Effects
4.1. HL
4.2. NHL
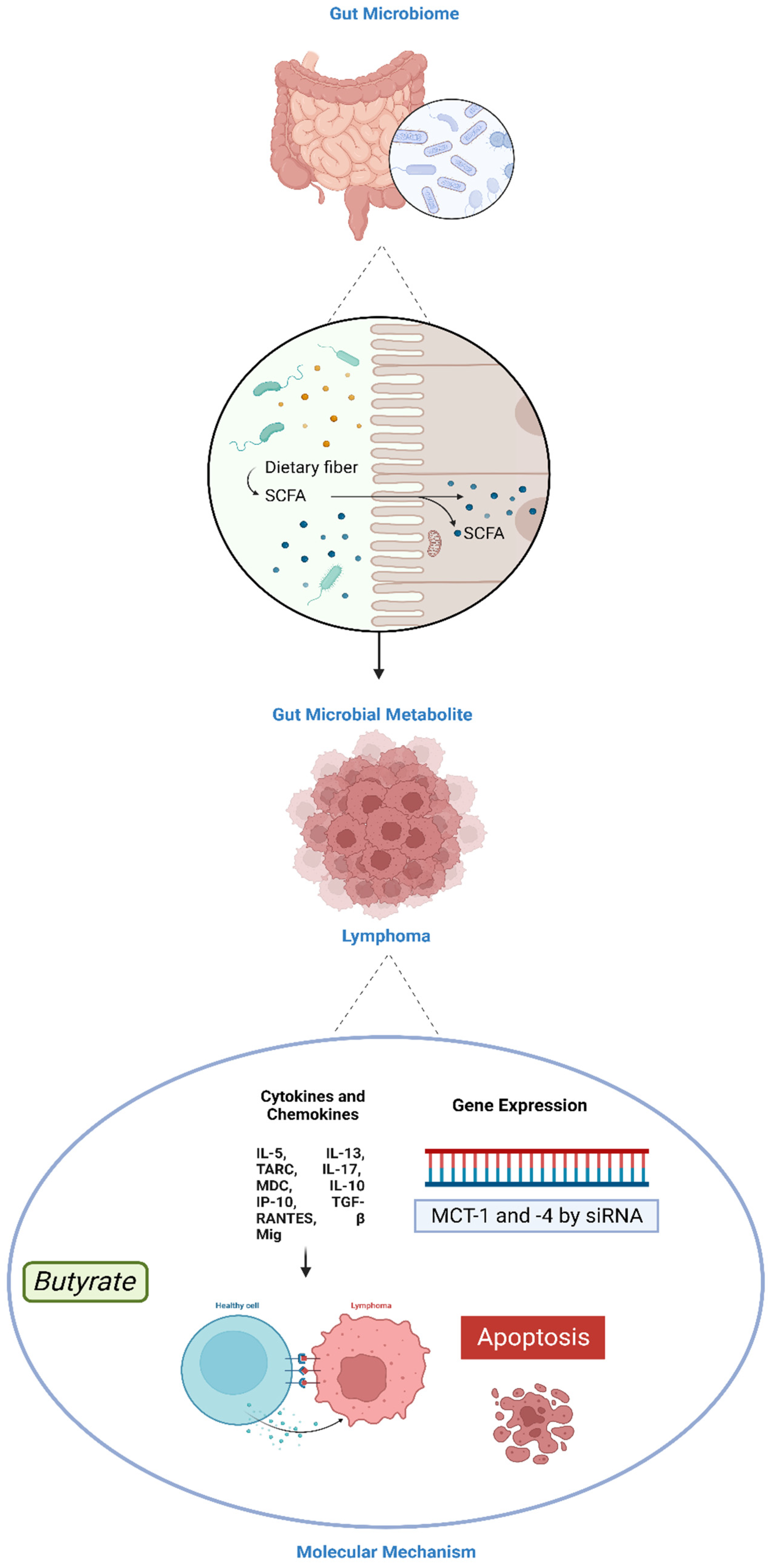
5. Role of Gut Microbiota in Lymphoma
5.1. Prebiotics
5.2. Probiotics
5.3. Postbiotics
5.3.1. SCFAs
5.3.2. Bacteriocins
5.3.3. Inosine
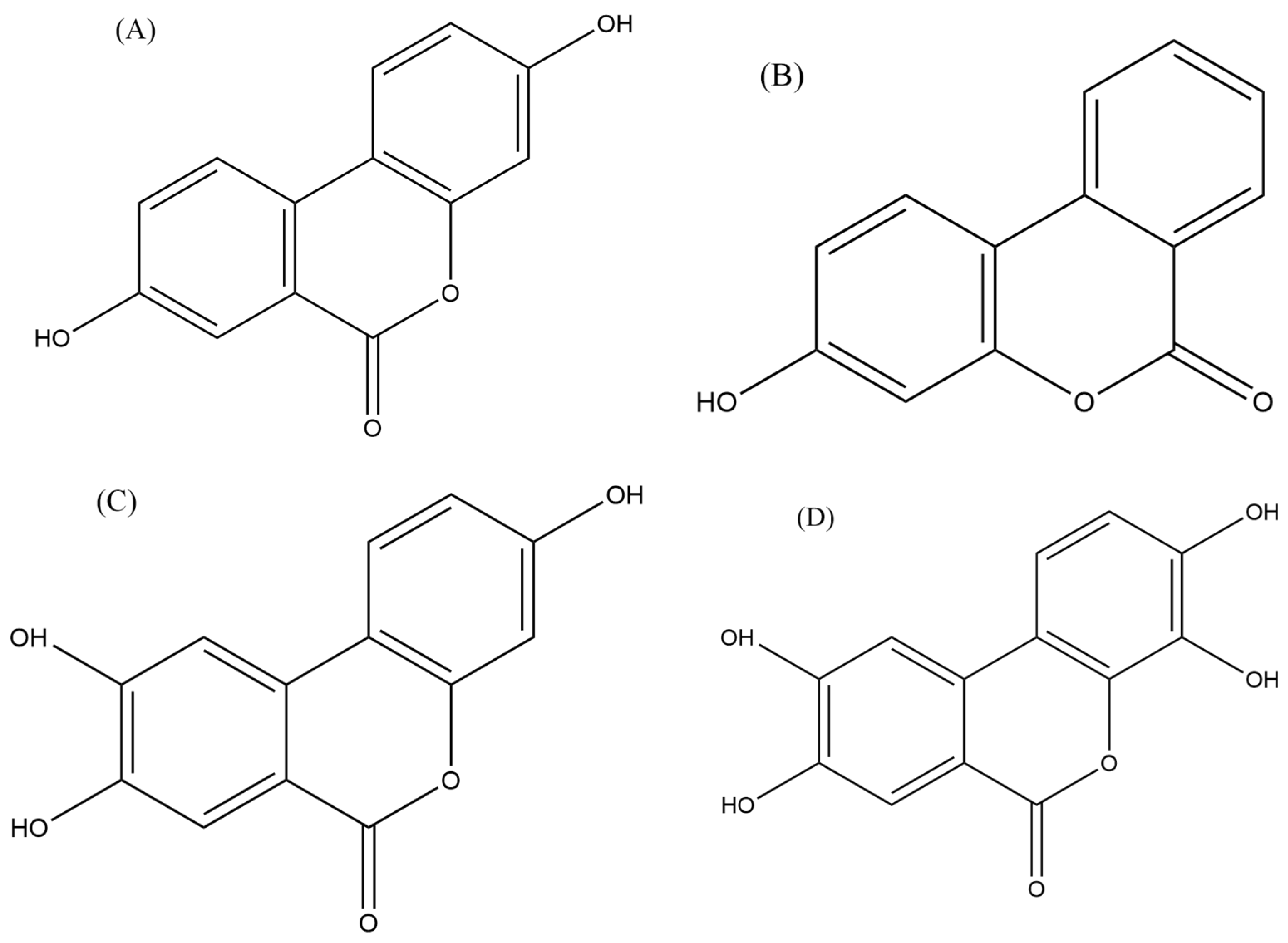
5.3.4. Urolithins
6. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, E.N.; Ghosh, N. Lymphoma. Prim. Care Clin. Off. Pract. 2016, 43, 661–675. [Google Scholar] [CrossRef]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Westin, J.R.; Kersten, M.J.; Salles, G.; Abramson, J.S.; Schuster, S.J.; Locke, F.L.; Andreadis, C. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: Observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am. J. Hematol. 2021, 96, 1295–1312. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Wu, J.; Ye, B.-C.; Qi, N. Gut microbiota-derived metabolites in the development of diseases. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6658674. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut microbiota and immune system interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-A.; Lee, S.; Choi, J.K.; Kang, B.-C.; Kim, M.-J.; Dhakal, H.; Kwon, T.K.; Khang, D.; Kim, S.-H. The suppressive effect of dabrafenib, a therapeutic agent for metastatic melanoma, in IgE-mediated allergic inflammation. Int. Immunopharmacol. 2020, 83, 106398. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Wang, X.; Waschke, B.C.; Woolaver, R.A.; Chen, S.M.Y.; Chen, Z.; Wang, J.H. HDAC inhibitors overcome immunotherapy resistance in B-cell lymphoma. Protein Cell 2020, 11, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Cure Cancer Australia. Lymphoma 2022. Available online: https://www.curecancer.com.au/cancer/blood?gclid=CjwKCAjwitShBhA6EiwAq3RqAxkSFH_j88v6iU1QXyT5O2Bd7yaC39GQhXFNvPFpP_A_jEv-Ot5rhRoCklAQAvD_BwE (accessed on 7 June 2023).
- Yung, L.; Linch, D. Hodgkin’s lymphoma. Lancet 2003, 361, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R.; Engert, A.; Hansmann, M.-L. Hodgkin lymphoma. J. Clin. Investig. 2012, 122, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Cellular and Molecular Immunology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Bowzyk Al-Naeeb, A.; Ajithkumar, T.; Behan, S.; Hodson, D.J. Non-Hodgkin lymphoma. BMJ 2018, 362, k3204. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Crouch, S.; Lax, S.; Li, J.; Painter, D.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Lymphoma incidence, survival and prevalence 2004–2014: Sub-type analyses from the UK’s Haematological Malignancy Research Network. Br. J. Cancer 2015, 112, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Shankland, K.R.; Armitage, J.O.; Hancock, B.W. Non-hodgkin lymphoma. Lancet 2012, 380, 848–857. [Google Scholar] [CrossRef]
- Parkin, D. 11. Cancers attributable to infection in the UK in 2010. Br. J. Cancer 2011, 105, S49–S56. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Slager, S.L.; Cerhan, J.R.; Wang, S.S.; Vajdic, C.M.; Skibola, C.F.; Bracci, P.M.; de Sanjosé, S.; Smedby, K.E.; Chiu, B.C. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: The InterLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.F.; Rumgay, H.; Dunlop, C.; Ryan, M.; Quartly, F.; Cox, A.; Deas, A.; Elliss-Brookes, L.; Gavin, A.; Hounsome, L. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br. J. Cancer 2018, 118, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Vardiman, J.W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; International Agency for research on Cancer: Lyon, France, 2008; Volume 2.
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Sbih-Lammali, F.; Djennaoui, D.; Belaoui, H.; Bouguermouh, A.; Decaussin, G.; Ooka, T. Transcriptional expression of Epstein-Barr virus genes and proto-oncogenes in north African nasopharyngeal carcinoma. J. Med. Virol. 1996, 49, 7–14. [Google Scholar] [CrossRef]
- Moore, P.S.; Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat. Rev. Cancer 2010, 10, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Fatima, I.; Kanwal, S.; Mahmood, T. Natural Products Mediated Targeting of Virally Infected Cancer. Dose Response 2019, 17, 1559325818813227. [Google Scholar] [CrossRef] [PubMed]
- zur Hausen, H.; de Villiers, E.M. Cancer “causation” by infections--individual contributions and synergistic networks. Semin. Oncol. 2014, 41, 860–875. [Google Scholar] [CrossRef] [PubMed]
- Eladwy, R.A.; Vu, H.T.; Shah, R.; Li, C.G.; Chang, D.; Bhuyan, D.J. The Fight against the Carcinogenic Epstein-Barr Virus: Gut Microbiota, Natural Medicines, and Beyond. Int. J. Mol. Sci. 2023, 24, 1716. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Trompet, E.; Snoeck, R. Novel therapeutics for Epstein–Barr virus. Molecules 2019, 24, 997. [Google Scholar] [CrossRef] [PubMed]
- Pitot, H.C. The molecular biology of carcinogenesis. Cancer 1993, 72 (Suppl. S3), 962–970. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, A.; Fuentes-Pananá, E.M. Human viruses and cancer. Viruses 2014, 6, 4047–4079. [Google Scholar] [CrossRef]
- Khidr, L.; Chen, P.L. RB, the conductor that orchestrates life, death and differentiation. Oncogene 2006, 25, 5210–5219. [Google Scholar] [CrossRef] [PubMed]
- Murata, T. Regulation of Epstein–Barr virus reactivation from latency. Microbiol. Immunol. 2014, 58, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Pagano, J.S.; Whitehurst, C.B.; Andrei, G. Antiviral drugs for EBV. Cancers 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Pinnix, C.C.; Wirth, A.; Milgrom, S.A.; Andraos, T.Y.; Aristophanous, M.; Pham, M.; Hancock, D.; Ludmir, E.B.; Gunther, J.R.; Fanale, M.A.; et al. Omitting cardiophrenic lymph nodes in the treatment of patients with Hodgkin lymphoma via modified involved-site radiation therapy. Leuk. Lymphoma 2018, 59, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Adult Hodgkin Lymphoma Treatment (PDQ®): Patient Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Mondello, P.; Musolino, C.; Dogliotti, I.; Bohn, J.P.; Cavallo, F.; Ferrero, S.; Botto, B.; Cerchione, C.; Nappi, D.; De Lorenzo, S.; et al. ABVD vs BEACOPP escalated in advanced-stage Hodgkin’s lymphoma: Results from a multicenter European study. Am. J. Hematol. 2020, 95, 1030–1037. [Google Scholar] [CrossRef]
- Engert, A.; Plütschow, A.; Eich, H.T.; Lohri, A.; Dörken, B.; Borchmann, P.; Berger, B.; Greil, R.; Willborn, K.C.; Wilhelm, M. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N. Engl. J. Med. 2010, 363, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Johnson, S.B.; Yuan, G.; Arriba, A.K.; Zubizarreta, M.E.; Chatterjee, S.; Nagarkatti, M.; Nagarkatti, P.; Xiao, S. Doxorubicin obliterates mouse ovarian reserve through both primordial follicle atresia and overactivation. Toxicol. Appl. Pharmacol. 2019, 381, 114714. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Ewer, S.M. Cardiotoxicity of anticancer treatments. Nat. Rev. Cardiol. 2015, 12, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Munoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Vaklavas, C.; Forero-Torres, A. Safety and efficacy of brentuximab vedotin in patients with Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Ther. Adv. Hematol. 2012, 3, 209–225. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Long, G.V.; Scolyer, R.A.; Teng, M.W.L.; Smyth, M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017, 52, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Imber, B.S.; Yahalom, J. Radiotherapy for Non-Hodgkin Lymphomas. Cancer J. 2020, 26, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Oki, Y.; Ewer, M.S.; Lenihan, D.J.; Fisch, M.J.; Hagemeister, F.B.; Fanale, M.; Romaguera, J.; Pro, B.; Fowler, N.; Younes, A. Pegylated liposomal doxorubicin replacing conventional doxorubicin in standard R-CHOP chemotherapy for elderly patients with diffuse large B-cell lymphoma: An open label, single arm, phase II trial. Clin. Lymphoma Myeloma Leuk. 2015, 15, 152–158. [Google Scholar] [CrossRef]
- Sitzia, J.; North, C.; Stanley, J.; Winterberg, N. Side effects of CHOP in the treatment of non-Hodgkin’s lymphoma. Cancer Nurs. 1997, 20, 430–439. [Google Scholar] [CrossRef]
- Ansell, S.M.; Armitage, J. Non-Hodgkin lymphoma: Diagnosis and treatment. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Kuruvilla, J.; Armand, P.; Hamadani, M.; Kline, J.; Moskowitz, C.H.; Avigan, D.; Brody, J.D.; Ribrag, V.; Herrera, A.F.; Morschhauser, F. Pembrolizumab for patients with non-Hodgkin lymphoma: Phase 1b KEYNOTE-013 study. Leuk. Lymphoma 2023, 64, 130–139. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Guevara-Ramírez, P.; Cadena-Ullauri, S.; Paz-Cruz, E.; Tamayo-Trujillo, R.; Ruiz-Pozo, V.A.; Zambrano, A.K. Role of the gut microbiota in hematologic cancer. Front. Microbiol. 2023, 14, 1185787. [Google Scholar] [CrossRef]
- Lin, Z.; Mao, D.; Jin, C.; Wang, J.; Lai, Y.; Zhang, Y.; Zhou, M.; Ge, Q.; Zhang, P.; Sun, Y.; et al. The gut microbiota correlate with the disease characteristics and immune status of patients with untreated diffuse large B-cell lymphoma. Front. Immunol. 2023, 14, 1105293. [Google Scholar] [CrossRef]
- Upadhyay Banskota, S.; Skupa, S.A.; El-Gamal, D.; D’Angelo, C.R. Defining the Role of the Gut Microbiome in the Pathogenesis and Treatment of Lymphoid Malignancies. Int. J. Mol. Sci. 2023, 24, 2309. [Google Scholar] [CrossRef]
- Hussein, N.; Rajasuriar, R.; Khan, A.M.; Lim, Y.A.-L.; Gan, G.G. The Role of the Gut Microbiome in Hematological Cancers. Mol. Cancer Res. 2024, 22, 7–20. [Google Scholar] [CrossRef]
- Xu, Z.-F.; Yuan, L.; Zhang, Y.; Zhang, W.; Wei, C.; Wang, W.; Zhao, D.; Zhou, D.; Li, J. The Gut Microbiome Correlated to Chemotherapy Efficacy in Diffuse Large B-Cell Lymphoma Patients. Hematol. Rep. 2024, 16, 63–75. [Google Scholar] [CrossRef]
- Xu, Z.-F.; Zhao, D.; Wei, C.; Wang, W.; Zhang, Y.; Zhang, W.; Zhou, D. Characteristics and prognostic value of gut microbiota in follicular lymphoma. Oncol. Lett. 2024, 27, 207. [Google Scholar] [CrossRef]
- Mamgain, G.; Patra, P.; Naithani, M.; Nath, U.K. The Role of Microbiota in the Development of Cancer Tumour Cells and Lymphoma of B and T Cells. Cureus 2021, 13, e19047. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, W.; Zhang, W.; Zhang, Y.; Wei, C.; Li, J.; Zhou, D. Gut Microbiota in Untreated Diffuse Large B Cell Lymphoma Patients. Front. Microbiol. 2021, 12, 646361. [Google Scholar] [CrossRef] [PubMed]
- Meyn, M.S. Ataxia-telangiectasia, cancer and the pathobiology of the ATM gene. Clin. Genet. 1999, 55, 289–304. [Google Scholar] [CrossRef] [PubMed]
- MW, B.A. Treatment of lymphoid malignancies in patients with ataxia-telangiectasia. Med. Pediatr. Oncol. 1999, 32, 479–480. [Google Scholar]
- Taylor, A.; Metcalfe, J.; Thick, J.; Mak, Y. Leukemia and lymphoma in ataxia telangiectasia. Blood 1996, 87, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.; Funkhouser, J.; Tuck-Muller, C.; Gatti, R. Cancer susceptibility in ataxia-telangiectasia. Leukemia 1992, 6, 8–13. [Google Scholar]
- Hecht, F.; Hecht, B.K. Cancer in ataxia-telangiectasia patients. Cancer Genet. Cytogenet. 1990, 46, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Morrell, D.; Cromartie, E.; Swift, M. Mortality and cancer incidence in 263 patients with ataxia-telangiectasia. J. Natl. Cancer Inst. 1986, 77, 89–92. [Google Scholar] [PubMed]
- Cheema, A.K.; Maier, I.; Dowdy, T.; Wang, Y.; Singh, R.; Ruegger, P.M.; Borneman, J.; Fornace, A.J., Jr.; Schiestl, R.H. Chemopreventive Metabolites Are Correlated with a Change in Intestinal Microbiota Measured in A-T Mice and Decreased Carcinogenesis. PLoS ONE 2016, 11, e0151190. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.L.; Maier, I.; Dang, A.T.; Berry, D.; Liu, J.; Ruegger, P.M.; Yang, J.-I.; Soto, P.A.; Presley, L.L.; Reliene, R.; et al. Intestinal Bacteria Modify Lymphoma Incidence and Latency by Affecting Systemic Inflammatory State, Oxidative Stress, and Leukocyte Genotoxicity. Cancer Res. 2013, 73, 4222–4232. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.L.; Schiestl, R.H. Lymphoma caused by intestinal microbiota. Int. J. Environ. Res. Public Health 2014, 11, 9038–9049. [Google Scholar] [CrossRef] [PubMed]
- Jaye, K.; Li, C.G.; Chang, D.; Bhuyan, D.J. The role of key gut microbial metabolites in the development and treatment of cancer. Gut Microbes 2022, 14, 2038865. [Google Scholar] [CrossRef] [PubMed]
- Sliva, J.; Pantzartzi, C.N.; Votava, M. Inosine Pranobex: A Key Player in the Game Against a Wide Range of Viral Infections and Non-Infectious Diseases. Adv. Ther. 2019, 36, 1878–1905. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.; Varannes, S.B.D.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetière, M.; Batard, E. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef]
- Pope, J.L.; Tomkovich, S.; Yang, Y.; Jobin, C. Microbiota as a mediator of cancer progression and therapy. Transl. Res. 2017, 179, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, M.; Ghidini, A.; Perego, G.; Cabiddu, M.; Khakoo, S.; Oggionni, E.; Abeni, C.; Hahne, J.C.; Tomasello, G. Use of antibiotics and risk of cancer: A systematic review and meta-analysis of observational studies. Cancers 2019, 11, 1174. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Wang, B.-Y.; Zhang, W.-N.; Huang, J.-Y.; Li, B.-S.; Zhang, M.; Jiang, L.; Li, J.-F.; Wang, M.-J.; Dai, Y.-J. Genomic profiling of adult and pediatric B-cell acute lymphoblastic leukemia. EBioMedicine 2016, 8, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Cani, P.D. Interaction between obesity and the gut microbiota: Relevance in nutrition. Annu. Rev. Nutr. 2011, 31, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Uccello, M.; Malaguarnera, G.; Basile, F.; D’agata, V.; Malaguarnera, M.; Bertino, G.; Vacante, M.; Drago, F.; Biondi, A. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 2012, 12, S35. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, K.M.; Conterno, L.; Gasperotti, M.; Viola, R. Up-regulating the human intestinal microbiome using whole plant foods, polyphenols, and/or fiber. J. Agric. Food Chem. 2012, 60, 8776–8782. [Google Scholar] [CrossRef]
- Rattanathammethee, T.; Tuitemwong, P.; Thiennimitr, P.; Sarichai, P.; Pombejra, S.N.; Piriyakhuntorn, P.; Hantrakool, S.; Chai-Adisaksopha, C.; Rattarittamrong, E.; Tantiworawit, A. Gut microbiota profiles of treatment-naïve adult acute myeloid leukemia patients with neutropenic fever during intensive chemotherapy. PLoS ONE 2020, 15, e0236460. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Resta-Lenert, S.; Barrett, K.E. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut. 2003, 52, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Clement, C.; Pogue, A.I.; Bhattacharjee, S.; Zhao, Y.; Lukiw, W.J. Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD). Front. Aging Neurosci. 2014, 6, 97417. [Google Scholar]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [PubMed]
- Delia, P.; Sansotta, G.; Donato, V.; Frosina, P.; Messina, G.; De Renzis, C.; Famularo, G. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. WJG 2007, 13, 912. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Off. J. Am. Coll. Gastroenterol.|ACG 2006, 101, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre-and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Díaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Peluzio, M.d.C.G.; Martinez, J.A.; Milagro, F.I. Postbiotics: Metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci. Technol. 2021, 108, 11–26. [Google Scholar] [CrossRef]
- Inamura, K. Gut microbiota contributes towards immunomodulation against cancer: New frontiers in precision cancer therapeutics. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Gill, P.; Van Zelm, M.; Muir, J.; Gibson, P. Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Astakhova, L.; Ngara, M.; Babich, O.; Prosekov, A.; Asyakina, L.; Dyshlyuk, L.; Midtvedt, T.; Zhou, X.; Ernberg, I.; Matskova, L. Short Chain Fatty Acids (SCFA) Reprogram Gene Expression in Human Malignant Epithelial and Lymphoid Cells. PLoS ONE 2016, 11, e0154102. [Google Scholar] [CrossRef] [PubMed]
- Mukovozov, I.; Huang, Y.-W.; Zhang, Q.; Liu, G.Y.; Siu, A.; Sokolskyy, Y.; Patel, S.; Hyduk, S.J.; Kutryk, M.J.; Cybulsky, M.I. The neurorepellent Slit2 inhibits postadhesion stabilization of monocytes tethered to vascular endothelial cells. J. Immunol. 2015, 195, 3334–3344. [Google Scholar] [CrossRef]
- Janíčková, O.; Ančicová, L.; Briestenska, K.; Mistrikova, J. The effect of Isoprinosine treatment on persistent infection of Balb/c mice infected with murine gammaherpesvirus 68. Acta Virol. 2017, 61, 32–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaur, S.; Kaur, S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Korpole, S.; Grover, V. Bacteriocins: Perspective for the development of novel anticancer drugs. Appl. Microbiol. Biotechnol. 2018, 102, 10393–10408. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T. The Potential as New Treatment Agent of Urolithin-A Metabolized from Ellagic Acid by Gut Microbiota in Cancer. Juntendo Med. J. 2021, 67, 131–139. [Google Scholar] [CrossRef]
- Lv, M.; Shi, C.; Pan, F.; Shao, J.; Feng, L.; Chen, G.; Ou, C.; Zhang, J.; Fu, W. Urolithin B suppresses tumor growth in hepatocellular carcinoma through inducing the inactivation of Wnt/β-catenin signaling. J. Cell. Biochem. 2019, 120, 17273–17282. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, J.; Yang, G.; Yu, N.; Huang, Z.; Xu, H.; Li, J.; Qiu, J.; Zeng, X.; Chen, S. Posttranscriptional regulation of Galectin-3 by miR-128 contributes to colorectal cancer progression. Oncotarget 2017, 8, 15242. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Scholte, J.; Scheurink, A.J.; van den Berg, M.; Bruggeman, G.; Bruininx, E.; de Vos, P.; Schols, H.A.; Gruppen, H. Effect of oat and soybean rich in distinct non-starch polysaccharides on fermentation, appetite regulation and fat accumulation in rat. Int. J. Biol. Macromol. 2019, 140, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Pomare, E.; Branch, W.; Naylor, C.; MacFarlane, G. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short-and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Zaky, A.; Glastras, S.J.; Wong, M.Y.; Pollock, C.A.; Saad, S. The role of the gut microbiome in diabetes and obesity-related kidney disease. Int. J. Mol. Sci. 2021, 22, 9641. [Google Scholar] [CrossRef] [PubMed]
- Poll, B.G.; Cheema, M.U.; Pluznick, J.L. Gut microbial metabolites and blood pressure regulation: Focus on SCFAs and TMAO. Physiology 2020, 35, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Reid, E.; Suneja, G.; Ambinder, R.F.; Ard, K.; Baiocchi, R.; Barta, S.K.; Carchman, E.; Cohen, A.; Gupta, N.; Johung, K.L. Cancer in people living with HIV, version 1.2018, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 986–1017. [Google Scholar] [CrossRef] [PubMed]
- Reneeta, N.P.; Thiyonila, B.; Aathmanathan, V.S.; Ramya, T.; Chandrasekar, P.; Subramanian, N.; Prajapati, V.K.; Krishnan, M. Encapsulation and Systemic Delivery of 5-Fluorouracil Conjugated with Silkworm Pupa Derived Protein Nanoparticles for Experimental Lymphoma Cancer. Bioconjugate Chem. 2018, 29, 2994–3009. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, H.; Chen, X.; Chen, W.; Song, W.; Li, Z. Mutual regulation between glycosylation and transforming growth factor-β isoforms signaling pathway. Int. J. Biol. Macromol. 2023, 236, 123818. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.M.; Howarth, G.S.; Butler, R.N. Short-chain fatty acid modulation of apoptosis in the Kato III human gastric carcinoma cell line. Cancer Biol. Ther. 2007, 6, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.M.; Howarth, G.S.; Butler, R.N. Short-Chain Fatty Acids Induce Apoptosis in Colon Cancer Cells Associated with Changes to Intracellular Redox State and Glucose Metabolism. Chemotherapy 2012, 58, 102–109. [Google Scholar] [CrossRef]
- Ohara, T.; Mori, T. Antiproliferative Effects of Short-chain Fatty Acids on Human Colorectal Cancer Cells via Gene Expression Inhibition. Anticancer. Res. 2019, 39, 4659–4666. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Suzutani, T. Intake of Bifidobacterium longum and Fructo-oligosaccharides prevents Colorectal Carcinogenesis. Euroasian J. Hepato-Gastroenterol. 2018, 8, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.M. Short-Chain Fatty Acid Modulation of Apoptosis in Gastric and Colon Cancer Cells. Ph.D. Thesis, The University of Adelaide, Adelaide, SA, Australia, 2007. [Google Scholar]
- Shin, H.; Lee, Y.S.; Lee, Y.C. Sodium butyrate-induced DAPK-mediated apoptosis in human gastric cancer cells. Oncol. Rep. 2012, 27, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Mikami, D.; Uwada, J.; Yazawa, T.; Kamiyama, K.; Kimura, H.; Taniguchi, T.; Iwano, M. A short-chain fatty acid, propionate, enhances the cytotoxic effect of cisplatin by modulating GPR41 signaling pathways in HepG2 cells. Oncotarget 2018, 9, 31342–31354. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.P.; Hermine, O.; Small, T.; Suarez, F.; O’Reilly, R.; Boulad, F.; Fingeroth, J.; Askin, M.; Levy, A.; Mentzer, S.J. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus–associated lymphoid malignancies. Blood 2007, 109, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Angelakis, E.; Drissi, F.; Raoult, D. Occam’s razor and probiotics activity on Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 2013, 110, E1. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Merhej, V.; Raoult, D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect. Dis. 2013, 13, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Drissi, F.; Merhej, V.; Angelakis, E.; El Kaoutari, A.; Carrière, F.; Henrissat, B.; Raoult, D. Comparative genomics analysis of Lactobacillus species associated with weight gain or weight protection. Nutr. Diabetes 2014, 4, e109. [Google Scholar] [CrossRef] [PubMed]
- Sorbara, M.T.; Pamer, E.G. Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol. 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Ahmadi, S.; Ghollasi, M.; Hosseini, H.M. The apoptotic impact of nisin as a potent bacteriocin on the colon cancer cells. Microb. Pathog. 2017, 111, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, P.; Hayami, T.; Matte, B.; Liu, Y.; Danciu, T.; Ramamoorthy, A.; Worden, F.; Kapila, S.; Kapila, Y. Nisin ZP, a bacteriocin and food preservative, inhibits head and neck cancer tumorigenesis and prolongs survival. PLoS ONE 2015, 10, e0131008. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, Z.; Salimi, A.; Halabian, R.; Fahimi, H. Nisin, a potent bacteriocin and anti-bacterial peptide, attenuates expression of metastatic genes in colorectal cancer cell lines. Microb. Pathog. 2018, 123, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Mandal, M. Induction of apoptosis of azurin synthesized from P. aeruginosa MTCC 2453 against Dalton’s lymphoma ascites model. Biomed. Pharmacother. 2011, 65, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.E.; Ritchie, K.; Kamarajan, P.; Miao, D.; Kapila, Y.L. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC 1. Cancer Med. 2012, 1, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; MWachsman, B.; Knoetze, H.; Meincken, M.; Dicks, L.M. An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soya beans. Int. J. Antimicrob. Agents 2005, 25, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, M.B.; Castilla, V.; de Ruiz Holgado, A.P.; de Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antivir. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Férir, G.; Petrova, M.I.; Andrei, G.; Huskens, D.; Hoorelbeke, B.; Snoeck, R.; Vanderleyden, J.; Balzarini, J.; Bartoschek, S.; Brönstrup, M. The lantibiotic peptide labyrinthopeptin A1 demonstrates broad anti-HIV and anti-HSV activity with potential for microbicidal applications. PLoS ONE 2013, 8, e64010. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.I.; Noll, K.S.; Xu, S.; Li, J.; Huang, Q.; Sinko, P.J.; Wachsman, M.B.; Chikindas, M.L. Safety, formulation and in vitro antiviral activity of the antimicrobial peptide subtilosin against herpes simplex virus type 1. Probiotics Antimicrob. Proteins 2013, 5, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Torres, A.G.; de Pouplana, L.R. Inosine in Biology and Disease. Genes 2021, 12, 600. [Google Scholar] [CrossRef]
- Lasek, W.; Janyst, M.; Wolny, R.; Zapała, Ł.; Bocian, K.; Drela, N. Immunomodulatory effects of inosine pranobex on cytokine production by human lymphocytes. Acta Pharm. 2015, 65, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Tsang, K.Y.; Fudenberg, H.H.; Pan, J.F.; Gnagy, M.J.; Bristow, C.B. An in vitro study on the effects of isoprinosine on immune responses in cancer patients. Int. J. Immunopharmacol. 1983, 5, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Newman, A.S.; O’Daly, J.; Duffy, S.; Grafton, G.; Brady, C.A.; Curnow, S.J.; Barnes, N.M.; Gordon, J. Inosine Acedoben Dimepranol promotes an early and sustained increase in the natural killer cell component of circulating lymphocytes: A clinical trial supporting anti-viral indications. Int. Immunopharmacol. 2017, 42, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Bekesi, J.; Tsang, P.; Wallace, J.; Roboz, J. Immunorestorative properties of isoprinosine in the treatment of patients at high risk of developing ARC or AIDS. J. Clin. Lab. Immunol. 1987, 24, 155–161. [Google Scholar] [PubMed]
- Tsang, K.Y.; Pan, J.F.; Swanger, D.L.; Fudenberg, H.H. In vitro restoration of immune responses in aging humans by isoprinosine. Int. J. Immunopharmacol. 1985, 7, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.A.; Selma, M.V.; García-Conesa, M.T.; Espín, J.C. Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol. Nutr. Food Res. 2017, 61, 1500901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.W.; Tao, L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol. Med. 2014, 11, 92–100. [Google Scholar] [PubMed]

| Cell Type/Cancer | Gut Microbial Metabolites | Study Type | Mechanisms | Reference |
|---|---|---|---|---|
| Epithelial and lymphoid cells (Raji and Rael) | Butyric acid—SCFA | In vitro | The administration of nisin resulted in the stimulation of inflammatory and apoptotic reactions within tumor cells. The activation of the n-butyric gene was observed to decline when the cell membrane transporters MCT-1 and MCT-4 were downregulated through siRNA. | Astakhova et al. [98] |
| T-lymphoma cells | Propionate—SCFA | In vitro and in vivo | Inhibited the growth of T-lymphoma cells. | Mukovozov et al. [99] |
| Epstein–Barr virus (EBV)—lymphoma | Isoprinosine (IP)—Inosine complex | In vivo | After two weeks of treatment, IP resulted in elevated levels of virus-neutralizing antibodies, leukocytes, and neutrophils | Janíčková et al. [100] |
| Jurkat lymphoma cells | Nisin—Bacteriocins | In vitro | Induced apoptosis and inhibited their growth. | Kaur and Kaur [101] |
| Lymphoma cells | Enterocin CRL35—Bacteriocins | In vivo | Induced apoptosis in Dalton’s lymphoma-bearing cells and significantly inhibited their growth. | Baindara et al. [102] |
| Human anaplastic large lymphoma cell lines (KARPAS-299 and MAC-2A) and human leukemia cell lines (MOLT-4 and HL-60) | Urolithin A (UA) | In vitro | Inhibited the growth of lymphoma cells and induced apoptosis. Inhibited the activation of the NF-κB signaling pathway, which is involved in the survival of lymphoma cells. | Okumura et al. [103] |
| B-cell lymphoma | Urolithin B (UB) | In vitro | Inhibited the NF-κB signaling pathway and the activity of an enzyme STAT3, which lead to the growth inhibition and induction of apoptosis in lymphoma cells. | Lv et al. [104] |
| Human T-cells lymphoma | UA | In vitro | Inhibited the growth of lymphoma cells and induced apoptosis through the inhibition of the Akt enzyme activity, which is involved in cell survival and proliferation. | Lu et al. [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khazaleh, A.K.; Chang, D.; Münch, G.W.; Bhuyan, D.J. The Gut Connection: Exploring the Possibility of Implementing Gut Microbial Metabolites in Lymphoma Treatment. Cancers 2024, 16, 1464. https://doi.org/10.3390/cancers16081464
Al-Khazaleh AK, Chang D, Münch GW, Bhuyan DJ. The Gut Connection: Exploring the Possibility of Implementing Gut Microbial Metabolites in Lymphoma Treatment. Cancers. 2024; 16(8):1464. https://doi.org/10.3390/cancers16081464
Chicago/Turabian StyleAl-Khazaleh, Ahmad K., Dennis Chang, Gerald W. Münch, and Deep Jyoti Bhuyan. 2024. "The Gut Connection: Exploring the Possibility of Implementing Gut Microbial Metabolites in Lymphoma Treatment" Cancers 16, no. 8: 1464. https://doi.org/10.3390/cancers16081464
APA StyleAl-Khazaleh, A. K., Chang, D., Münch, G. W., & Bhuyan, D. J. (2024). The Gut Connection: Exploring the Possibility of Implementing Gut Microbial Metabolites in Lymphoma Treatment. Cancers, 16(8), 1464. https://doi.org/10.3390/cancers16081464







