A Systematic Review and Meta-Analysis of 29 Studies Predicting Diagnostic Accuracy of CT, MRI, PET, and USG in Detecting Extracapsular Spread in Head and Neck Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
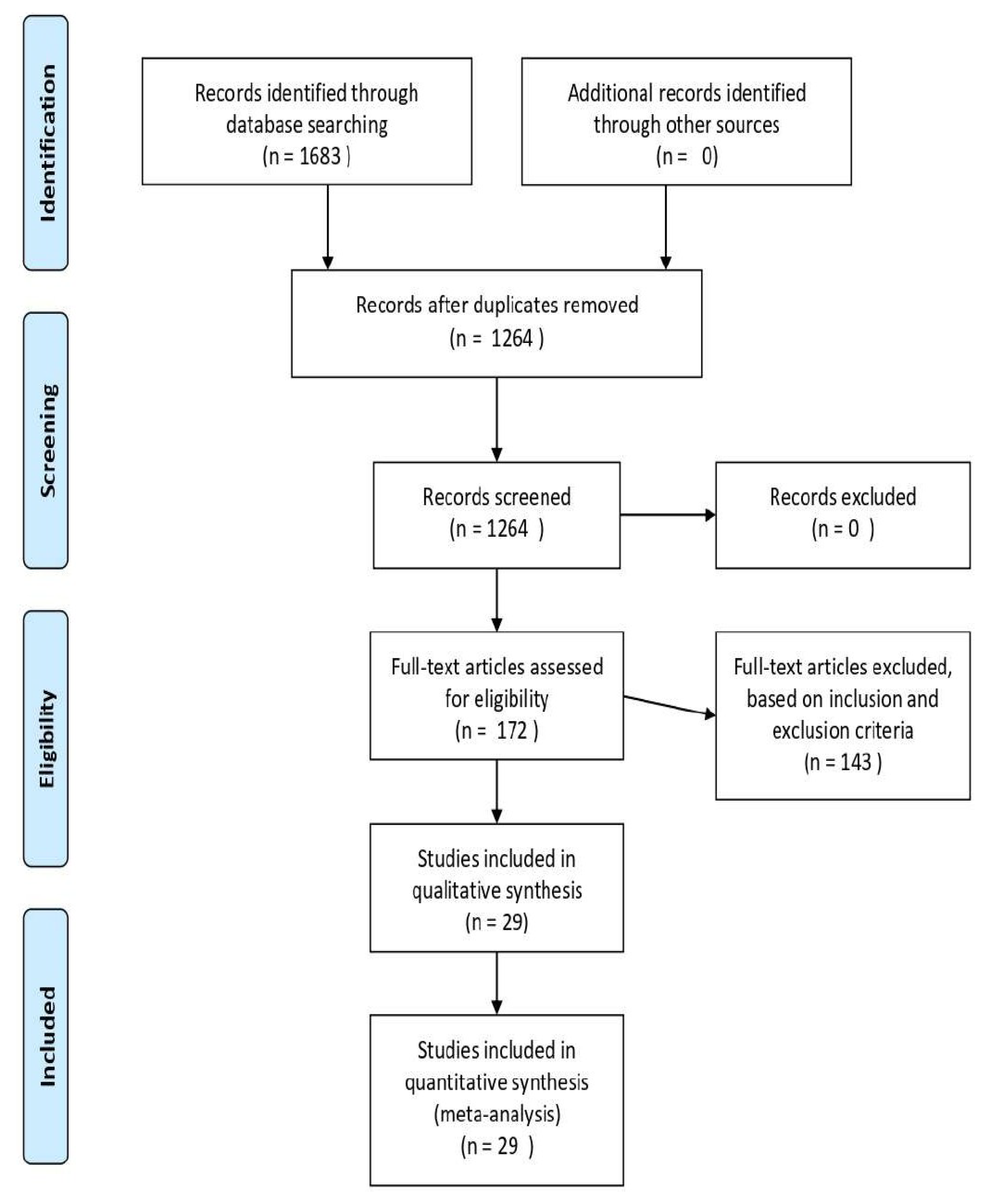
2.1. Search Strategy and Selection Criteria
2.2. Data Extraction and Analysis
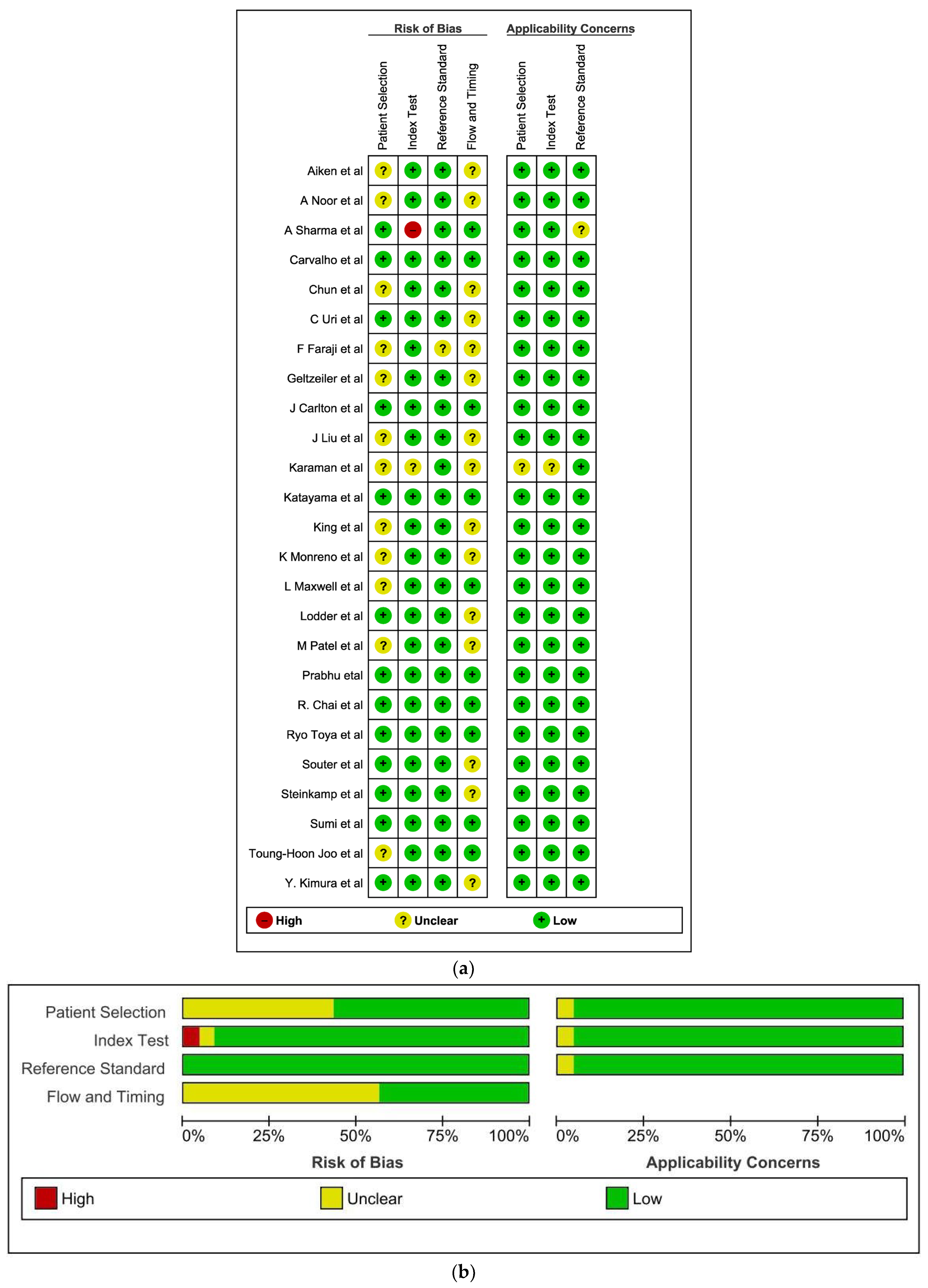
2.3. Assessment of Methodological Quality
2.4. Statistics
3. Results
3.1. Methodological Quality of Included Studies
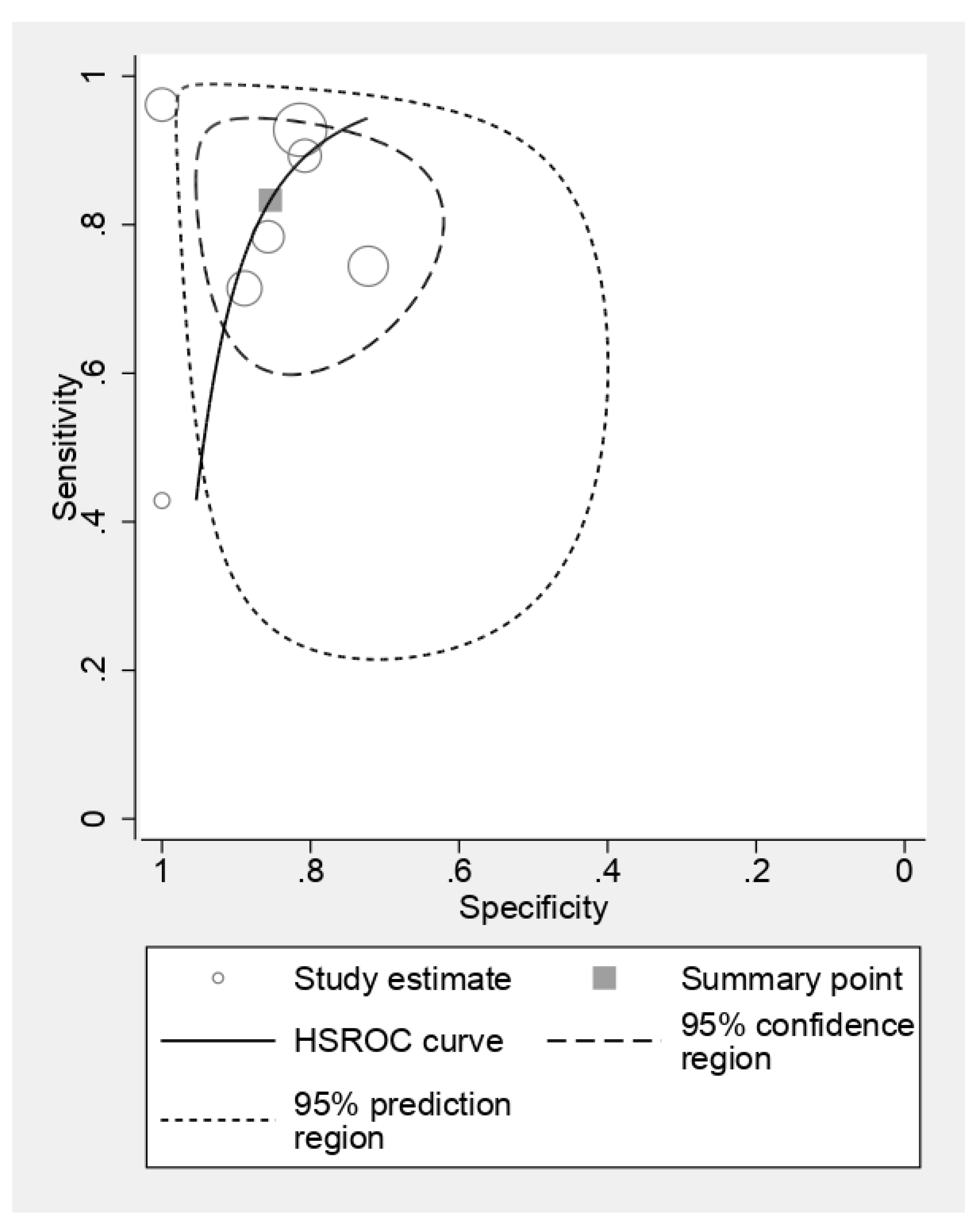
3.2. Findings
3.3. Computed Tomography
3.4. Magnetic Resonance Imaging
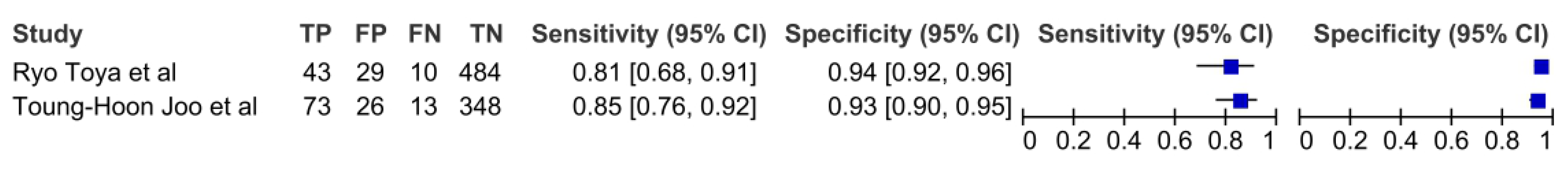
3.5. Positron Emission Tomography
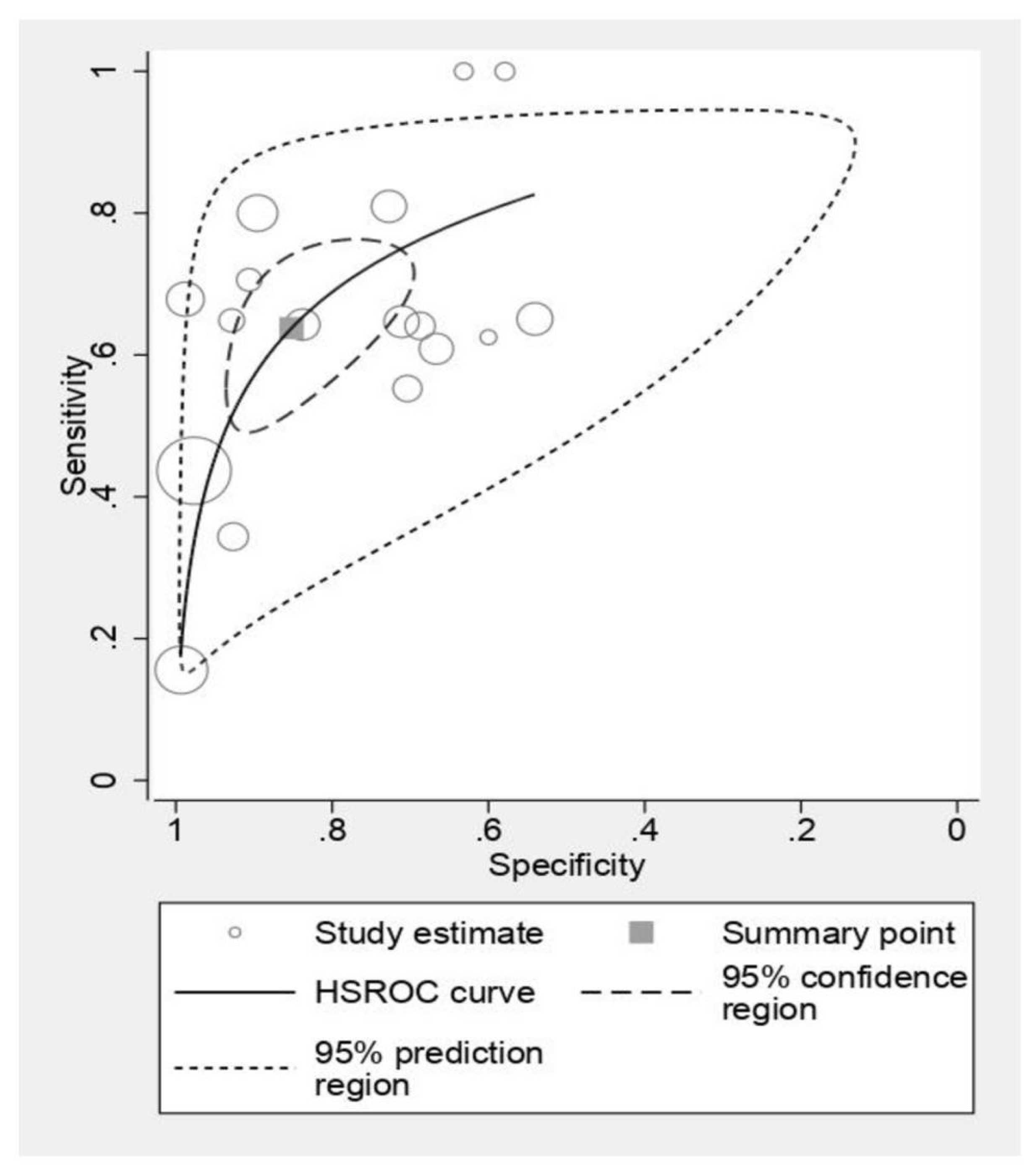
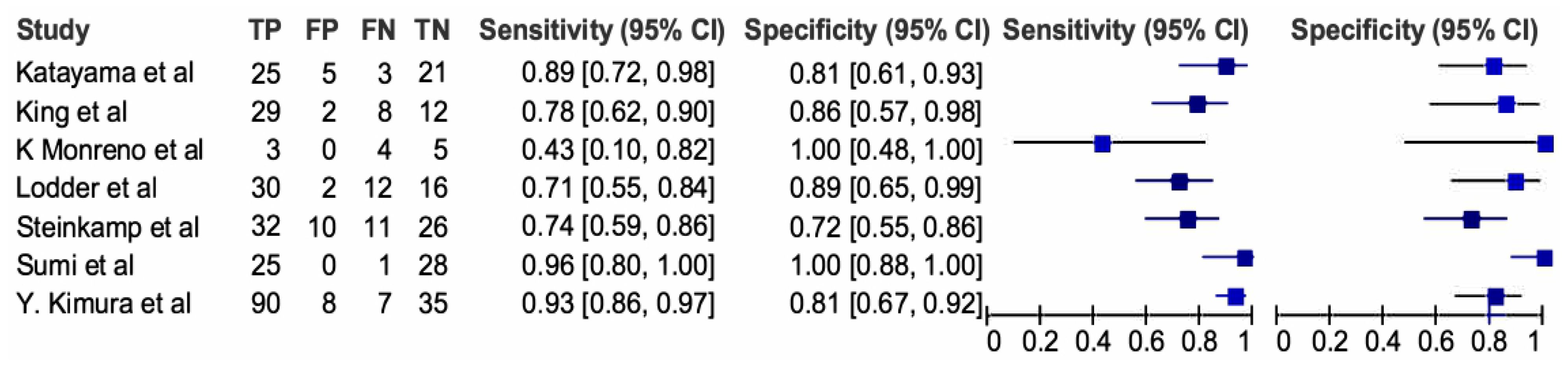
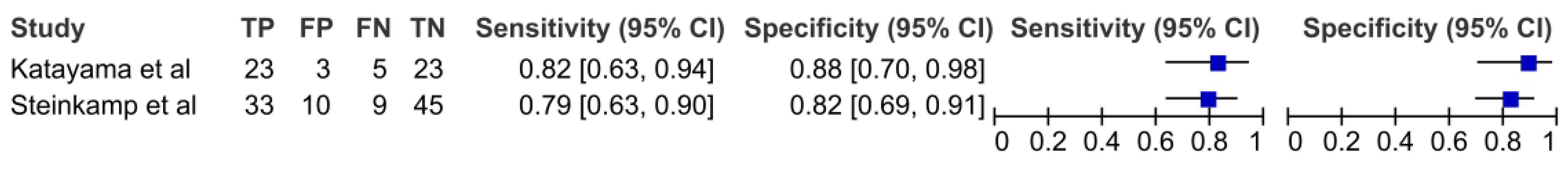
3.6. Ultrasonography
3.7. Comparison of CT and MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pulte, D.; Brenner, H. Changes in Survival in Head and Neck Cancers in the Late 20th and Early 21st Century: A Period Analysis. Oncologist 2010, 15, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Burusapat, C.; Jarungroongruangchai, W.; Charoenpitakchai, M. Prognostic factors of cervical node status in head and neck squamous cell carcinoma. World J. Surg. Oncol. 2015, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, L.; Millan, I.; Torre, A.; Aragon, G.; Otero, J. Prognostic factors for survival and tumor control in cervical lymph node metastases from head and neck cancer: A multivariate study of 492 cases. Cancer 1992, 69, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.P.; Sallah, S.; Karlsson, U.; Antoine, J.E. Combined chemotherapy and radiation therapy for head and neck malignancies: Quality of life issues. Cancer 2002, 94, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Cooper, J.S.; Pajak, T.F.; Van Glabbeke, M.; Bourhis, J.; Forastiere, A.; Ozsahin, E.M.; Jacobs, J.R.; Jassem, J.; Ang, K.-K.; et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005, 27, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Harbord, R. METANDI: Stata Module to Perform Meta-Analysis of Diagnostic Accuracy; Statistical Software Components S456932; Boston College Department of Economics: Chestnut Hill, MA, USA, 2008. [Google Scholar]
- Aiken, A.; Poliashenko, S.; Beitler, J.; Chen, A.; Baugnon, K.; Corey, A.; Magliocca, K.; Hudgins, P. Accuracy of Preoperative Imaging in Detecting Nodal Extracapsular Spread in Oral Cavity Squamous Cell Carcinoma. Am. J. Neuroradiol. 2015, 36, 1776–1781. [Google Scholar] [CrossRef]
- Noor, A.; Mintz, J.; Patel, S.; Bajic, N.; Boase, S.; Sethi, N.; Foreman, A.; Krishnan, S.; Hodge, J. Predictive value of computed tomography in identifying extracapsular spread of cervical lymph node metastases in p16 positive oropharyngeal squamous cell carcinoma. J. Med. Imaging Radiat. Oncol. 2019, 63, 500–509. [Google Scholar] [CrossRef]
- Sharma, A.; Jaiswal, A.A.; Umredkar, G.; Barle, R.; Sharma, N.; Banerjee, P.K.; Garg, A.K.; Membally, R. Lymph Node Central Necrosis on the Computed Tomography as the Predictor of the Extra Capsular Spread in Metastatic Head and Neck Squamous Cell Carcinoma. Indian J. Otolaryngol. Head Neck Surg. 2017, 69, 323–332. [Google Scholar] [CrossRef]
- Carvalho, P.; Baldwin, D.; Carter, R.; Parsons, C. Accuracy of CT in detecting squamous carcinoma metastases in cervical lymph nodes. Clin. Radiol. 1991, 44, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Url, C.; Schartinger, V.; Riechelmann, H.; Glückert, R.; Maier, H.; Trumpp, M.; Widmann, G. Radiological detection of extracapsular spread in head and neck squamous cell carcinoma (HNSCC) cervical metastases. Eur. J. Radiol. 2013, 82, 1783–1787. [Google Scholar] [CrossRef]
- Faraji, F.; Gaba, R.C. Radiologic Modalities and Response Assessment Schemes for Clinical and Preclinical Oncology Imaging. Front. Oncol. 2019, 9, 471. [Google Scholar] [CrossRef]
- Geltzeiler, M.; Clayburgh, D.; Gleysteen, J.; Gross, N.D.; Hamilton, B.; Andersen, P.; Brickman, D. Predictors of extracapsular extension in HPV-associated oropharyngeal cancer treated surgically. Oral Oncol. 2017, 65, 89–93. [Google Scholar] [CrossRef]
- A Carlton, J.; Maxwell, A.W.; Bauer, L.B.; McElroy, S.M.; Layfield, L.J.; Ahsan, H.; Agarwal, A. Computed tomography detection of extracapsular spread of squamous cell carcinoma of the head and neck in metastatic cervical lymph nodes. Neuroradiol. J. 2017, 30, 222–229, Correction in Neuroradiol. J. 2018, 31, NP1. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.T.; Kann, B.H.; De, B.; Buckstein, M.; Bakst, R.L.; Genden, E.M.; Posner, M.R.; Som, P.M.; Gupta, V. Prognostic value of radiographic extracapsular extension in locally advanced head and neck squamous cell cancers. Oral Oncol. 2016, 52, 52–57. [Google Scholar] [CrossRef] [PubMed]
- King, A.D.; Ahuja, A.T.; Leung, S.-F.; Lam, W.W.; Teo, P.; Chan, Y.-L.; Metreweli, C. Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck 2000, 22, 275–281. [Google Scholar] [CrossRef]
- Maxwell, J.H.; Rath, T.J.; Byrd, J.K.; Albergotti, W.G.; Wang, H.; Duvvuri, U.; Kim, S.; Johnson, J.T.; Branstetter, B.F.; Ferris, R.L. Accuracy of computed tomography to predict extracapsular spread in p16-positive squamous cell carcinoma. Laryngoscope 2015, 125, 1613–1618. [Google Scholar] [CrossRef]
- Patel, M.R.; Hudgins, P.A.; Beitler, J.J.; Magliocca, K.R.; Griffith, C.C.; Liu, Y.; Bougnon, K.; El-Deiry, M.; Saba, N.F.; Aiken, A.H. Radiographic Imaging Does Not Reliably Predict Macroscopic Extranodal Extension in Human Papilloma Virus-Associated Oropharyngeal Cancer. ORL J. Otorhinolaryngol. Relat. Spec. 2018, 80, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.S.; Magliocca, K.R.; Hanasoge, S.; Aiken, A.H.; Hudgins, P.A.; Hall, W.A.; Chen, S.A.; Eaton, B.R.; Higgins, K.A.; Saba, N.F.; et al. Accuracy of Computed Tomography for Predicting Pathologic Nodal Extracapsular Extension in Patients With Head-and-Neck Cancer Undergoing Initial Surgical Resection. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.L.; Rath, T.J.; Johnson, J.T.; Ferris, R.L.; Kubicek, G.J.; Duvvuri, U.; Branstetter, B.F. Accuracy of Computed Tomography in the Prediction of Extracapsular Spread of Lymph Node Metastases in Squamous Cell Carcinoma of the Head and Neck. JAMA Otolaryngol. Neck Surg. Head. 2013, 139, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, H.J.; Hoeck, E.; Böck, J.C.; Felix, R. Kapseldurchbrüche zervikaler Lymphknotenmetastasen: Diagnostischer Stellenwert der Computertomographie [The extracapsular spread of cervical lymph node metastases: The diagnostic value of computed tomography]. Rofo 1999, 170, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Souter, M.A.; Allison, R.S.; Clarkson, J.H.; Cowan, I.A.; Coates, M.H.; Wells, J.E. Sensitivity and specificity of computed tomography for de-tection of extranodal spread from metastatic head and neck squamous cell carcinoma. J. Laryngol. Otol. 2009, 123, 778–782. [Google Scholar] [CrossRef]
- Karaman, Z.F.; Çağlı, S.; Yüce, İ.; Ozturk, M.A.; Güney, E.; Ozcan, N. Characterization of cervical lymph nodes with 16 slice multislice computed tomography and histopathologic correlation. Erciyes Med. J. 2009, 31, 169–175. [Google Scholar]
- Moreno, K.F.; Cornelius, R.S.; Lucas, F.V.; Meinzen-Derr, J.; Patil, Y.J. Using 3 Tesla magnetic resonance imaging in the pre-operative evaluation of tongue carcinoma. J. Laryngol. Otol. 2017, 131, 793–800. [Google Scholar] [CrossRef]
- Lodder, W.L.; Lange, C.A.; van Velthuysen, M.-L.F.; Hauptmann, M.; Balm, A.J.; Brekel, M.W.v.D.; Pameijer, F.A. Can extranodal spread in head and neck cancer be detected on MR imaging. Oral Oncol. 2013, 49, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Sumi, M.; Nakamura, T. Extranodal spread in the neck: MRI detection on the basis of pixel-based time-signal intensity curve analysis. J. Magn. Reson. Imaging 2011, 33, 830–838. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumi, M.; Sakihama, N.; Tanaka, F.; Takahashi, H.; Nakamura, T. MR Imaging Criteria for the Prediction of Extranodal Spread of Metastatic Cancer in the Neck. Am. J. Neuroradiol. 2008, 29, 1355–1359. [Google Scholar] [CrossRef]
- Steinkamp, H.J.; Beck, A.; Werk, M.; Felix, R. Extracapsular spread of cervical lymph node metastases: Diagnostic value of magnetic resonance imaging. Rofo 2002, 174, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, H.J.; Beck, A.; Werk, M.; Rademaker, J.; Felix, R. Extracapsular spread of cervical lymph node metastases: Diagnostic relevance of ultrasound examinations. Ultraschall Med. 2003, 24, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Zoumalan, R.A.; Kleinberger, A.J.; Morris, L.G.T.; Ranade, A.; Yee, H.; DeLacure, M.D.; Myssiorek, D. Lymph node central necrosis on computed tomography as predictor of extracapsular spread in metastatic head and neck squamous cell carcinoma: Pilot study. J. Laryngol. Otol. 2010, 124, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Katayama, I.; Sasaki, M.; Kimura, Y.; Hotokezaka, Y.; Eida, S.; Tashiro, S.; Sumi, M.; Nakamura, T. Comparison between ultrasonography and MR imaging for discriminating squamous cell carcinoma nodes with extranodal spread in the neck. Eur. J. Radiol. 2012, 81, 3326–3331. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.-H.; Yoo, I.-R.; Cho, K.-J.; Park, J.-O.; Nam, I.-C.; Kim, C.-S.; Kim, M.-S. Relationship between extracapsular spread and FDG PET/CT in oropharyngeal squamous cell carcinoma. Acta Oto-Laryngol. 2013, 133, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.; Yoo, I.; Cho, K.; Park, J.; Nam, I.; Kim, M. Extracapsular spread and FDG PET/CT correlations in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2013, 42, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.H.; Yoo, I.R.; Cho, K.J.; Park, J.O.; Nam, I.C.; Kim, M.S. Extracapsular spread in hypopharyngeal squamous cell carcinoma: Di-agnostic value of FDG PET/CT. Head Neck 2013, 35, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Choi, Y.J.; Roh, J.L.; Kim, J.S.; Lee, J.H.; Cho, K.J.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. Preoperative Contrast-Enhanced CT Versus 18F-FDG PET/CT Evaluation and the Prognostic Value of Extranodal Extension for Surgical Patients with Head and Neck Squamous Cell Carcinoma. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.J.; Yoo, I.R.; Joo, Y.H.; Nam, I.C.; Cho, J.H.; Kim, C.S.; Cho, K.-J.; Kim, M.-S. The efficacy of 18F-FDG PET/CT imaging for extracapsular spread of the laryngeal squamous cell carcinoma. Head Neck 2014, 38, 290–293. [Google Scholar] [CrossRef]
- Dequanter, D.; Shahla, M.; Aubert, C.; Deniz, Y.; Lothaire, P. Prognostic value of FDG PET/CT in head and neck squamous cell carcinomas. OncoTargets Ther. 2015, 26, 2279–2283. [Google Scholar] [CrossRef]
- Randall, D.R.; Lysack, J.T.; Hudon, M.E.; Guggisberg, K.; Nakoneshny, S.C.; Matthews, T.W.; Dort, J.C.; Chandarana, S.P. Diagnostic utility of central node necrosis in predicting extracapsular spread among oral cavity squamous cell carcinoma. Head Neck 2013, 37, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Toya, R.; Saito, T.; Matsuyama, T.; Kai, Y.; Shiraishi, S.; Murakami, D.; Yoshida, R.; Watakabe, T.; Sakamoto, F.; Tsuda, N.; et al. Diagnostic Value of FDG-PET/CT for the Identification of Extranodal Extension in Patients With Head and Neck Squamous Cell Carcinoma. Anticancer. Res. 2020, 40, 2073–2077. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, S.C.; Giger, R.; Bojaxhiu, B.; Sachpekidis, C.; Dammann, F.; Dettmer, M.S.; Arnold, A.; Wartenberg, J.; Nisa, L. Multimodal Imaging with Positron Emission Tomography/Computed Tomography and Magnetic Resonance Imaging to Detect Extracapsular Extension in Head and Neck Cancer. Laryngoscope 2020, 131, E163–E169. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; O’Sullivan, B.; Su, J.; Bartlett, E.; Kim, J.; Waldron, J.N.; Ringash, J.; de Almeida, J.R.; Bratman, S.; Hansen, A.; et al. Prognostic importance of radiologic extranodal extension in HPV-positive oropharyngeal carcinoma and its potential role in refining TNM-8 cN-classification. Radiother. Oncol. 2020, 144, 13–22. [Google Scholar] [CrossRef]
- Lydiatt, W.; O’sullivan, B.; Patel, S. Major Changes in Head and Neck Staging for 2018. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Duan, Z.; Pan, W.; Wu, C.; Jia, Y.; Han, B.; Li, C. Predicting extracapsular spread of head and neck cancers using different imaging techniques: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2015, 45, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Hu, Y.; Xiao, Y.; Guo, Q.; Huang, S.H.; O’Sullivan, B.; Fang, Y.; Zong, J.; Chen, Y.; Lin, S.; et al. Prognostic value of radiologic extranodal extension and its potential role in future N classification for nasopharyngeal carcinoma. Oral Oncol. 2019, 99, 104438. [Google Scholar] [CrossRef]
- Richards, P.S. The role of ultrasound in the detection of cervical lymph node metastases in clinically N0 squamous cell carcinoma of the head and neck. Cancer Imaging 2007, 7, 167–178. [Google Scholar] [CrossRef]

| Author | Year of Publication | Prospective/Retrospective | Number of Patients | Unit Considered | Mean Age | Male | Female | Site Included | HPV-Positive Only or Combined or Not Known | Imaging Modality Studied | Reference Standard | Threshold |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carvalho et al. [14] | 1991 | retrospective | 28 | Necks (31) | NK | NK | NK | NK | NK | CT | histology | capsule irregularity |
| Steinkamp et al. [25] | 1999 | retrospective | 97 | Patients | NK | NK | NK | NK | NK | CT, USG | histology | infiltration (CT) and capsule irregularity and necrosis (USG) |
| Steinkamp et al. [33] | 2002 | retrospective | 79 | Patients | NK | NK | NK | NK | NK | MRI | histology | necrosis |
| King et al. [20] | 2004 | retrospective | 17 | Nodes (51) | 62.4 | 16 | 1 | o, op, hp, l | NK | CT, MRI | histology | fat stranding and margin irregularity (CT) and necrosis (MRI) |
| Y. Kimura et al. [31] | 2008 | retrospective | 109 | Patients | 66 | 89 | 20 | o, op, np, hp, l | NK | MRI | histology | NK |
| Karaman et al. [27] | 2009 | retrospective | 140 | Patients | 55 | 4 | o, hp | NK | CT | histology | infiltration | |
| Souter et al. [26] | 2009 | retrospective | 127 | Patients | NK | NK | NK | NK | NK | CT | histology | necrosis |
| Zoumalan et al. [34] | 2010 | retrospective | 17 | 61 nodes | 57 | 17 | 0 | o, op, hp, l | NK | CT | histology | necrosis |
| Sumi et al. [30] | 2011 | retrospective | 43 | Nodes (54) | 62 | 37 | 6 | o, op, np, hp, l | NK | MRI | histology | capsule irregularity and infiltration |
| Katayama et al. [35] | 2012 | retrospective | 50 | Nodes (54) | NK | NK | NK | o, op, hp | NK | MRI, USG | histology | necrosis (MRI) and infiltration (USG) |
| C Url et al. [15] | 2013 | retrospective | 49 | Patients | 60 | 44 | 5 | o, op, hp, l | NK | CT | histology | infiltration |
| Lodder et al. [29] | 2013 | retrospective | 39 | Nodes (60) | 63 | 24 | 15 | o, op, hp, l | NK | MRI | histology | necrosis |
| Joo et al. [36] | 2013 | retrospective | 78 | 106 level | NK | NK | NK | op | NK | PET | histology | SUV max 3.85 |
| Joo et al. [37] | 2013 | retrospective | 80 | 71 level | NK | NK | NK | o | NK | PET | histology | SUV max 2.25 |
| R. Chai et al. [24] | 2013 | retrospective | 100 | Patients | 62 | 79 | 21 | o, op, l | NK | CT | histology | necrosis, fat stranding |
| young-Hoon Joo et al. [38] | 2013 | retrospective | 57 | Nodes (460) | 61 | 55 | 2 | hp | NK | PET | histology | SUV max 2.65 |
| Prabhu et al. [23] | 2014 | retrospective | 432 | Patients | 60 | NK | NK | o, op, l | NK | CT | histology | infiltration |
| Aiken [11] | 2015 | prospective | 111 | Patients | NK | NK | NK | o | NK | CT | histology | necrosis (irregular borders, fat stranding, invasion) |
| Lee et al. [39] | 2015 | retrospective | 263 | Patients | NK | NK | NK | o, op, hp, l | NK | CT, PET | histology | necrosis (CT) SUV max 4.9 (PET) |
| Chun et al. [40] | 2015 | retrospective | 89 | Nodes (524) | NK | NK | NK | l | NK | PET | histology | SUV max 2.85 |
| D Dequanter et al. [41] | 2015 | retrospective | 54 | Patients | NK | NK | NK | o, op, hp, l | NK | PET | histology | SUV max 4.15 |
| L Maxwell et al. [21] | 2015 | retrospective | 65 | Patients | 55.9 | 60 | 5 | Combined | CT | histology | margin irregularity | |
| Randall et al. [42] | 2015 | retrospective | 40 | 77 nodes | NK | 29 | 11 | o | NK | CT | histology | necrosis |
| J Liu et al. [19] | 2016 | retrospective | 96 | Patients | 58 | 116 | 24 | o, op, np, hp, l | NK | CT | histology | (thick wall, enhancing margin, loss of nodal margin, infiltration) |
| A Sharma et al. [13] | 2017 | prospective | 30 | Patients | 52.9 | 24 | 6 | o | NK | CT | histology | central necrosis |
| Geltzeiler [17] | 2017 | prospective | 100 | Patients | NK | NK | NK | op | HPV +ve | CT | histology | infiltration, matte nodes |
| J Carlton et al. [18] | 2017 | prospective | 93 | Patients | 61 | 58 | 25 | o, op, np, l | NK | CT | histology | central necrosis |
| K Moreno et al. [28] | 2017 | prospective | 20 | Neck (34) and nodes (12) | 58 | 12 | 8 | o | NK | MRI | histology | NK |
| M Patel et al. [22] | 2018 | prospective | 27 | Patients | 57 | 27 | 0 | op, np | HPV +ve | CT | histology | necrosis, lobular pattern (perinodal stranding, matted appearance, invasion) |
| A Noor et al. [12] | 2019 | prospective | 80 | Nodes (91) | 58 | 68 | 12 | op | NK | CT | histology | perinodal fat stranding (capsule contour, invasion) |
| Ryo Toya et al. [43] | 2020 | retrospective | 94 | Nodes (566) | NK | NK | NK | o, op, hp, l | NK | PET | histology | SUV max 2.3 |
| Sheppard et al. [44] | 2020 | retrospective | 176 | Patients | NK | NK | NK | o, op, hp, l | NK | PET | histology | SUV max 10 |
| Sheppard et al. [44] | 2020 | retrospective | 166 | Patients | NK | NK | NK | o, op, hp, l | NK | MRI | histology | margin irregularity |
| Imaging | Pooled Sensitivity (95% Confidence Interval) | Pooled Specificity (95% Confidence Interval) | Likelihood Ratios +ve (95% Confidence Interval) | Likelihood Ratios −ve (95% Confidence Interval) | Diagnostic Odds Ratio (95% Confidence Interval) |
|---|---|---|---|---|---|
| CT | 0.63 [95% CI = 0.53–0.73] | 0.85 [95% CI = 0.74–0.91] | 4.33 [95% CI = 2.63–7.05] | 0.425 [95% CI = 0.33–0.53]. | 10.1 [95% CI = 5.89–17.42] |
| MRI | 0.83 [95% CI = 0.71–0.90] | 0.85 [95% CI = 0.73–0.92] | 5.7 [95% CI = 2.96–10.99] | 0.19 [95% CI = 0.10–0.35] | 29.18 [95% CI = 9.79–86.94] |
| PET | 0.80 [95% CI = 0.74–0.85] | 0.93 [95% CI = 0.92–0.94] | 12.3 [95% CI = 9.9–15.26] | 0.21 [95% CI = 0.15–0.29]. | 57.75 [95% CI = 37.37–89.25] |
| USG | 0.80 [95% CI = 0.68–0.88] | 0.84 [95% CI = 0.74–0.91] | 4.83 [95% CI = 2.89–8.07] | 0.23 [95% CI = 0.14–0.38] | 20.69 [95% CI = 8.9–48.08] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mair, M.; Singhavi, H.; Pai, A.; Khan, M.; Conboy, P.; Olaleye, O.; Salha, R.; Ameerally, P.; Vaidhyanath, R.; Chaturvedi, P. A Systematic Review and Meta-Analysis of 29 Studies Predicting Diagnostic Accuracy of CT, MRI, PET, and USG in Detecting Extracapsular Spread in Head and Neck Cancers. Cancers 2024, 16, 1457. https://doi.org/10.3390/cancers16081457
Mair M, Singhavi H, Pai A, Khan M, Conboy P, Olaleye O, Salha R, Ameerally P, Vaidhyanath R, Chaturvedi P. A Systematic Review and Meta-Analysis of 29 Studies Predicting Diagnostic Accuracy of CT, MRI, PET, and USG in Detecting Extracapsular Spread in Head and Neck Cancers. Cancers. 2024; 16(8):1457. https://doi.org/10.3390/cancers16081457
Chicago/Turabian StyleMair, Manish, Hitesh Singhavi, Ameya Pai, Mariya Khan, Peter Conboy, Oladejo Olaleye, Rami Salha, Phil Ameerally, Ram Vaidhyanath, and Pankaj Chaturvedi. 2024. "A Systematic Review and Meta-Analysis of 29 Studies Predicting Diagnostic Accuracy of CT, MRI, PET, and USG in Detecting Extracapsular Spread in Head and Neck Cancers" Cancers 16, no. 8: 1457. https://doi.org/10.3390/cancers16081457
APA StyleMair, M., Singhavi, H., Pai, A., Khan, M., Conboy, P., Olaleye, O., Salha, R., Ameerally, P., Vaidhyanath, R., & Chaturvedi, P. (2024). A Systematic Review and Meta-Analysis of 29 Studies Predicting Diagnostic Accuracy of CT, MRI, PET, and USG in Detecting Extracapsular Spread in Head and Neck Cancers. Cancers, 16(8), 1457. https://doi.org/10.3390/cancers16081457





