The Real-Life Impact of Primary Tumor Resection of Synchronous Metastatic Colorectal Cancer—From a Clinical Oncologic Point of View
Abstract
Simple Summary
Abstract
1. Introduction
2. Method
3. Results
4. Discussion
- Emergency surgery
- 2.
- Elective PTR for potentially curable patients
- 3.
- PTR for patients with no or mild symptoms from the primary tumor
- I
- Arguments against PTR
- II
- Arguments for PTR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- National Cancer Institute SEER Database—Cancer Stat Facts: Colorectal Cancer. Available online: https://seer.cancer.gov/statfacts/html/colorect.html (accessed on 22 November 2023).
- Edwards, M.S.; Chadda, S.D.; Zhao, Z.; Barber, B.L.; Sykes, D.P. A systematic review of treatment guidelines for metastatic colorectal cancer. Color. Dis. 2011, 14, 31–47. [Google Scholar] [CrossRef][Green Version]
- Chibaudel, B.; Tournigand, C.; Andre, T.; de Gramont, A. Therapeutic strategy in unresectable metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2012, 4, 75–89. [Google Scholar] [CrossRef]
- Pécsi, B.; Mangel, L. Real-Life Effectivity of Dose Intensity Reduction of First-Line mFOLFIRI-Based Treatment of Metastatic Colorectal Cancers: Sometimes Less is More. Curr. Oncol. 2023, 30, 908–922. [Google Scholar] [CrossRef]
- Pécsi, B.; Mangel, L. The Real-Life Impact of mFOLFIRI-Based Chemotherapies on Elderly Patients—Should We Let It or Leave It? Cancers 2023, 15, 5146. [Google Scholar] [CrossRef]
- Dorajoo, S.R.; Tan, W.J.H.; Koo, W.S.; Chew, M.H.; Tang, C.L.; Wee, H.L.; Yap, C.W. A scoring model for predicting survival following primary tumour resection in stage IV colorectal cancer patients with unresectable metastasis. Int. J. Color. Dis. 2016, 31, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Köhne, C.H.; Cunningham, D.; Di Costanzo, F.; Glimelius, B.; Blijham, G.; Aranda, E.; Scheithauer, W.; Rougier, P.; Palmer, M.; Wil, J.; et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: Results of a multivariate analysis of 3825 patients. Ann. Oncol. 2012, 13, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Baer, C.; Menon, R.; Bastawrous, S.; Bastawrous, A. Emergency Presentations of Colorectal Cancer. Surg. Clin. N. Am. 2017, 97, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Bayar, B.; Yilmaz, K.B.; Akinci, M.; Sahin, A.; Kolacoglu, H. An evaluation of treatment result of emergency versus elective surgery in colorectal cancer patients. Ulus. Cerrahi Derg. 2016, 32, 11–17. [Google Scholar] [CrossRef]
- Mun, J.-Y.; Kim, J.-E.; Yoo, N.; Cho, H.-M.; Kim, H.; An, H.-J.; Kye, B.-H. Survival Outcomes after Elective or Emergency Surgery for Synchronous Stage IV Colorectal Cancer. Biomedicines 2022, 10, 3114. [Google Scholar] [CrossRef]
- Raphaeli, T.; Menon, R. Current Treatment of Lower Gastrointestinal Hemorrhage. Clin. Colon Rectal Surg. 2012, 25, 219–227. [Google Scholar] [CrossRef]
- Ribeiro, I.B.; de Moura, D.T.H.; Thompson, C.C. Acute abdominal obstruction: Colon stent or emergency surgery? An evidence-based review. World J. Gastrointest. Endosc. 2019, 11, 193–208. [Google Scholar] [CrossRef] [PubMed]
- de Mestier, L.; Manceau, G.; Neuzillet, C.; Bachet, J.B.; Spano, J.P.; Kianmanesh, R.; Vaillant, J.C.; Bouché, O.; Hannoun, L.; Karoui, M. Primary tumor resection in colorectal cancer with unresectable synchronous metastases: A review. World J. Gastrointest. Oncol. 2014, 6, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Siebenhüner, A.R.; Güller, U.; Warschkow, R. Population-based SEER analyis of survival in colorectal cancer patients with or without resection of lung and liver metastases. BMC Cancer 2020, 20, 246. [Google Scholar] [CrossRef]
- Kow, A.W.C. Hepatic metastasis from colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1274–1298. [Google Scholar] [CrossRef]
- Chow, F.C.-L.; Chok, K.S.-H. Colorectal liver metastases: An update on multidisciplinary approach. World J. Hepatol. 2019, 11, 150–172. [Google Scholar] [CrossRef] [PubMed]
- Al Bandar, M.H.; Kim, N.K. Current status and future perspectives on treatment of liver metastasis in colorectal cancer (Review). Oncol. Rep. 2017, 37, 2553–2564. [Google Scholar] [CrossRef]
- Puijk, R.S.; Ruarus, A.H.; Vroomen, L.G.P.H.; van Tilborg, A.A.J.M.; Scheffer, H.J.; Nielsen, K.; de Jong, M.C.; de Vries, J.J.J.; Zonderhuis, B.M.; Eker, H.H.; et al. Colorectal liver metastases: Surgery versus thermal ablation (COLLISION)—A phase III single-blind prospective randomized controlled trial. BMC Cancer 2018, 18, 821. [Google Scholar] [CrossRef]
- Zeineddine, F.A.; Zeineddine, M.A.; Yousef, A.; Gu, Y.; Chowdhury, S.; Dasari, A.; Huey, R.W.; Johnson, B.; Kee, B.; Lee, M.S.; et al. Survival improvement for patients with metastatic colorectal cancer over twenty years. Precis. Oncol. 2023, 7, 16. [Google Scholar] [CrossRef]
- Li, H.; Hu, H.; Li, B.; Sun, X.; Sun, Y.; Chen, H. What is the appropriate surgical strategy for pulmonary metastasis of colorectal cancer? Medicine 2020, 99, 30. [Google Scholar] [CrossRef]
- Antonoff, M.B.; Sofocleous, C.T.; Callstrom, M.R.; Nguyen, Q.-N. The roles of surgery, stereotactic radiation, and ablation for treatment of pulmonary metastases. J. Thorac. Cardiovasc. Surg. 2021, 163, 495–502. [Google Scholar] [CrossRef]
- Beckers, P.; Berzenji, L.; Yogeswaran, S.K.; Lauwers, P.; Bilotta, G.; Shkarpa, N.; Hendriks, J.; Van Schil, P.E. Pulmonary metastasectomy in colorectal carcinoma. J. Thorac. Dis. 2021, 13, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Poncet, A.; Combescure, C.; Robert, J.; Ris, H.B.; Gervaz, P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: A systematic review and meta-analysis. Ann. Surg. Oncol. 2013, 20, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Shishido, Y.; Ishii, M.; Maeda, T.; Kokado, Y.; Masuya, D.; Kusama, T.; Fujimoto, K.; Higashiyama, H. Survival outcomes of lung metastases from colorectal cancer treated with pulmonary metastasectomy or modern systemic chemotherapy: A single institution experience. J. Cardiothorac. Surg. 2023, 18, 327. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ohtaki, Y.; Okumura, T.; Boku, N.; Horio, H.; Takenoyama, M.; Yamashita, M.; Hyodo, I.; Mori, K.; Kondo, H. Outcomes and prognostic factors after pulmonary metastasectomy in patients with colorectal cancer with previously resected hepatic metastases. J. Thorac. Cardiovasc. Surg. 2019, 157, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.M.; Muldoon, R. Complications Following Colon Rectal Surgery in the Obese Patient. Clin. Colon Rectal Surg. 2011, 24, 274–282. [Google Scholar] [CrossRef]
- Kirchhoff, P.; Clavien, P.-A.; Hahnloser, D. Complications in colorectal surgery: Risk factors and preventive strategies. Patient Saf. Surg. 2010, 4, 5. [Google Scholar] [CrossRef]
- Kleespies, A.; Fuessl, K.E.; Seeliger, H.; Eichhorn, M.E.; Muller, M.H.; Rentsch, M.; Thasler, W.E.; Angele, M.K.; Kreis, M.E.; Jauch, K.W.; et al. Determinants of morbidity and survival after elective non-curative resection of stage IV colon and rectal cancer. Int. J. Color. Dis. 2009, 24, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Lordick, F.; Fink, C.; Bork, U.; Stange, A.; Jäger, D.; Luntz, S.P.; Englert, S.; Rossion, I.; Koch, M.; et al. Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS—A randomised controlled multicentre trial. BMC Cancer 2012, 12, 142. [Google Scholar] [CrossRef]
- van Eeghen, E.E.; dan Boer, F.C.; Loffeld, R.J.L.F. Thirty days post-operative mortality after surgery for colorectal cancer: A descriptive study. J. Gastroint. Oncol. 2015, 6, 613–617. [Google Scholar]
- Eisenberg, A.; Whelan, R.L.; Neugut, A.I. Survival and symptomatic benefit from palliative primary tumor resection in patients with metastatic colorectal cancer: A review. Int. J. Color. Dis. 2008, 23, 559–568. [Google Scholar] [CrossRef]
- Poultsides, G.A.; Servais, E.L.; Saltz, L.B.; Patil, S.; Kemeny, N.E.; Guillem, J.G.; Weiser, M.; Temple, L.K.; Wong, W.D.; Paty, P.B. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J. Clin. Oncol. 2009, 27, 3379–3384. [Google Scholar] [CrossRef] [PubMed]
- Camilleri-Brennan, J. Introductory Chapter: Challenges in Colorectal Surgery—Identifying Preoperative Risk Factors. In Current Topics in Colorectal Surgery; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Hanna, T.P.; King, W.D.; Thibodeau, S.; Jalink, M.; Paulin, G.A.; Harvey-Jones, E.; O’Sullivan, D.E.; Booth, C.; Sullivan, R.; Aggarwal, A. Mortality due to cancer treatment delay: Systemic review and meta-analysis. BMJ 2020, 371, 4087. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kung, P.T.; Wang, Y.H.; Kuo, W.Y.; Kao, S.L.; Tsai, W.C. Effect of length of time from diagnosis to treatment on colorectal cancer survival: A population-based study. PLoS ONE 2019, 14, e0210465. [Google Scholar] [CrossRef] [PubMed]
- Murchie, P.; Raja, E.A.; Brewster, D.H.; Campbell, N.C.; Ritchie, L.D.; Robertson, R.; Samuel, L.; Gray, N.; Lee, A.J. Time from first presentation in primary care to treatment of symptomatic colorectal cancer: Effect on disease stage and survival. Br. J. Cancer 2014, 111, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Escofet, X.; Karandikar, S.S.; Stamatakis, J.D. Outcomes of resection and non-resection strategies in management of patients with advanced colorectal cancer. World J. Surg. Oncol. 2009, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Cirocchi, R.; Trastulli, S.; Abraha, I.; Vettoretto, N.; Boselli, C.; Montedori, A.; Parisi, A.; Noya, G.; Platell, C. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst. Rev. 2012, 8, CD008997. [Google Scholar] [CrossRef] [PubMed]
- Bakkerus, L.; Buffart, L.M.; Buffart, T.E.; Meyer, Y.M.; Zonderhuis, B.M.; Haasbeek, C.J.A.; Versteeg, S.; Loosveld, O.L.J.; de Groot, J.W.B.; Hendriks, M.P.; et al. Health-Related Quality of Life in Patients with Metastatic Colorectal Cancer Undergoing Systemic Therapy with or without Maximal Tumor Debulking. J. Natl. Compr. Cancer Netw. 2023, 21, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.Y.; Ziogas, A.; Lis, B.S.; Seery, T.E.; Karnes, W.; Stamos, M.J.; Zell, J.A. Role of Primary Tumor Resection among Chemotherapy-Treated Patients with Synchronous Stage IV Colorectal Cancer: A Survival Analysis. J. Gastrointest. Surg. 2014, 18, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Karoui, M.; Koubaa, W.; Delbaldo, C.; Charachon, A.; Laurent, A.; Piedbois, P.; Cherqui, D.; Tran Van Nhieu, J. Chemotherapy also has an effect on primary tumor in colon carcinoma. Ann. Surg. Oncol. 2008, 15, 3440–3446. [Google Scholar] [CrossRef]
- Venderbosch, S.; de Wilt, J.H.; Teerenstra, S.; Loosveld, O.J.; van Bochove, A.; Sinnige, H.A.; Creemers, G.J.; Tesselaar, M.E.; Mol, L.; Punt, C.J.; et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: Retrospective analysis of two randomized studies and a review of the literature. Ann. Surg. Oncol. 2011, 18, 3252–3260. [Google Scholar] [CrossRef]
- Peeters, C.F.; Westphal, J.R.; de Waal, R.M.; Ruiter, D.J.; Wobbes, T.; Ruers, T.J. Vascular density in colorectal liver metastases increases after removal of the primary tumor in human cancer patients. Int. J. Cancer 2004, 112, 554–559. [Google Scholar] [CrossRef]
- Cook, A.D.; Single, R.; McCahill, L.E. Surgical Resection of Primary Tumors in Patients Who Present with Stage IV Colorectal Cancer: An Analysis of Surveillance, Epidemiology, and End Results Data, 1988 to 2000. Ann. Surg. Oncol. 2005, 12, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, F.; Malka, D.; Bourredjem, A.; Allonier, C.; Bouché, O.; Louafi, S.; Boige, V.; Mousseau, M.; Raoul, J.L.; Bedenne, L.; et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: Results from the multicenter, randomised trial Fédération Francophone de Cancérologie Digestive 9601. Eur. J. Cancer 2013, 49, 90–97. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Liu, Y.; Zhang, C.; Li, H.; Lai, B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med. 2020, 9, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Ruo, L.; Gougoutas, C.; Paty, P.B.; Guillem, J.G.; Cohen, A.M.; Wong, D.W. Elective bowel resection for incurable stage IV colorectal cancer: Prognostic variables for asymptomatic patients. J. Am. Coll. Surg. 2003, 196, 722–728. [Google Scholar] [CrossRef]
- Stillwell, A.P.; Buettner, P.G.; Ho, Y.H. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J. Surg. 2010, 34, 797–807. [Google Scholar] [CrossRef]
- Ahmed, S.; Leis, A.; Fields, A.; Chandra-Kanthan, S.; Haider, K.; Alvi, R.; Reeder, B.; Pahwa, P. Survival impact of surgical resection of primary tumor in patients with stage IV colorectal cancer: Results from a large population-based cohort study. Cancer 2014, 120, 683–691. [Google Scholar] [CrossRef]
- Ergun, Y.; Bal, O.; Dogan, G.; Dirikoc, M.; Acikgoz, Y.; Bacaksiz, F.; Uncu, D. Does primary resection contribute to overall survival in unresectable synchronous metastatic colorectal cancer. J. Res. Med. Sci. 2020, 25, 14. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.; Burke, J.P.; Barry, M.; Kalady, M.F.; Calvin Coffey, J. A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann. Surg. Oncol. 2014, 21, 3900–3908. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Taieb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; Martinelli, E. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Mangel, L.C.; Tornóczky, T.; Zemplényi, A.; Boncz, I. The possible role of the timing of the first oncological treatment on he survival rate of cancer diseases (Hungarian). Orvosi Hetil. 2018, 159, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Gbolahan, O.; Hashemi-Sadraei, N.; Yash, S.; Williams, G.; Ramachandran, R.; Kim, Y.-I.; Paluri, R.; Outlaw, D.; El-Rayes, B.; Nabell, L. Time to treatment initiation and its impact on real-world survival in metastatic colorectal cancer and pancreatic cancer. Cancer Med. 2022, 12, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Helewa, R.M.; Turner, D.; Park, J.; Wirtzfeld, D.; Czaykowski, P.; Hochman, D.; Singh, H.; Shu, E.; McKay, A. Longer waiting times for patients undergoing colorectal cancer surgery are not associated with decreased survival. J. Surg. Oncol. 2013, 108, 378–384. [Google Scholar] [CrossRef]
- Wilkinson, K.J.; Chua, W.; Ng, W.; Roohullah, A. Management of asymptomatic primary tumours in stage IV colorectal cancer: Review of outcomes. World J. Gastrointest. Oncol. 2015, 7, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.W.J.; Shapiro, J.D.; Tran, B.; Wong, H.-L.; Wong, S.F.; Richardson, G.E.; Harold, M.; Gibbs, P. Tumor burden (TB) as a prognostic indicator in patients with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2014, 32, 572. [Google Scholar] [CrossRef]
- Travers, A.; Jalali, A.; Begbie, S.; Semira, C.; Kosmider, S.; Ananda, S.; Wong, R.; Lee, M.; Shapiro, J.; Burge, M.; et al. Real-World Treatment and Outcomes of Metastatic Colorectal Cancer Patients with a Poor or Very Poor Performance Status. Clin. Color. Cancer 2020, 20, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Seruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocana, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Braun, L.H.; Baumann, D.; Zwirner, K.; Eipper, E.; Hauth, F.; Peter, A.; Zips, D.; Gani, C. Neutrophil-to-Lymphocyta Ratio in Rectal Cancer—Novel Biomarker of Tumor Immunogenicity During Radiotherapy or Confounding Variable? Int. J. Mol. Sci. 2019, 10, 2448. [Google Scholar] [CrossRef]
- Turner, N.; TranTran, P.V.; Sinnathamby, M.; Wong, H.-L.; Jones, I.; Croxford, M.; Desai, J.; Tie, J.; Field, K.M.; Kosmider, S.; et al. Primary Tumor Resection in Patients with Metastatic Colorectal Cancer Is Associated with Reversal of Systemic Inflammation and Improved Survival. Clin. Color. Cancer Other Gastrointest. Malig. 2015, 14, 185–191. [Google Scholar] [CrossRef]
- Macrae, F.A. Colorectal cancer: Epidemiology, risk factors, and protective factors. UpToDate. 2021. Available online: https://www.uptodate.com/contents/epidemiology-and-risk-factors-for-colorectal-cancer (accessed on 22 November 2023).
- van der Pool, A.E.; Damhuis, R.A.; Ijzermans, J.N.; de Wilt, J.H.; Eggermont, A.M.; Kranse, R.; Verhoef, C. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: A population-based series. Color. Dis. 2012, 14, 56–61. [Google Scholar] [CrossRef]
- Haller, D.G. Safety of Oxaliplatin in the Treatment of Colorectal Cancer. Oncology 2000, 14, 15–20. [Google Scholar] [PubMed]
- Saltz, L.B.; Cox, J.V.; Blanke, C.; Rosen, L.S.; Fehrenbacher, L.; Moore, M.J.; Maroun, J.A.; Ackland, S.P.; Locker, P.K.; Pirotta, N.; et al. Irinotecan Plus Fluorouracil and Leucovorin for Metastatic Colorectal Cancer. NEJM 2000, 343, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Weycker, D.; Li, X.; Edelsberg, J.; Barron, R.; Kartashov, A.; Xu, H.; Lyman, G.H. Risk and Consequences of Chemotherapy-Induced Febrile Neutropenia With Metastatic Solid Tumors. J. Oncol. Pract. 2014, 11, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Blayney, D.W.; Schwartzberg, L. Chemotherapy-induced neutropenia and emerging agents for prevention and treatment: A review. Cancer Treat. Rev. 2022, 109, 102427. [Google Scholar] [CrossRef] [PubMed]
- Harary, M.; Jolissaint, J.S.; Tavakkoli, A. Outcomes of GI Operations in Neutropenic Patients. In Proceedings of the Academic Surgical Congress 2018, Abstract, Jacksonville, FL, USA, 30 January–1 February 2018. [Google Scholar]
- Ledere, A.-K.; Bartsch, F.; Moehler, M.; Gassmann, P.; Lang, H. Morbidity and Mortality of Neutropenic Patients in Visceral Surgery: A Narrative Review. Cells 2022, 11, 3314. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tian, X.; Yu, C.; Hong, J.; Fang, J.; Chen, H. Increased risk of hemorrhage in metastatic colorectal cancer patients treated with bevacizumab. Medicine 2016, 95, 34. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.; Wang, C.; Dehkordi, S.H.H.; Sears, J.J.; Hu, Z.I. Bleeding and thrombotic events in patients with colorectal cancer treated with bevacizumab and receiving novel oral anticoagulants and antiplatelet medications. J. Clin. Oncol. 2023, 41, 104. [Google Scholar] [CrossRef]
- EMA Product Information: Avastin (Bevacizumab). 2023. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/avastin (accessed on 22 November 2023).
- Zhang, H.; Huang, Z.; Zou, X.; Liu, T. Bevacizumab and wound-healing complications: A systematic review and meta-analysis of randomized controlled trials. Oncotarget 2016, 7, 82473–82481. [Google Scholar] [CrossRef]
- D’Alterio, C.; Scala, S.; Sozzi, G.; Roz, L.; Bertolini, G. Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin. Cancer Biol. 2020, 60, 351–361. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Condeelis, J.S.; Oktay, M.H. Chemotherapy-induced metastasis: Mechanisms and translational opportunities. Clin. Exp. Metastasis 2018, 35, 269–284. [Google Scholar] [CrossRef]
- Kranenburg, O.; van der Speeten, K.; de Hingh, I. Peritoneal Metastases From Colorectal Cancer: Defining and Addressing the Challenges. Front. Oncol. 2021, 11, 650098. [Google Scholar] [CrossRef]
- Inci, K.; Nilsson, B.; Lindskog, S.; Giglio, D. Palliative resection of the primary tumor improves survival in incurable metastatic colorectal cancer. ANZ J. Surg. 2023, 93, 2680–2685. [Google Scholar] [CrossRef] [PubMed]
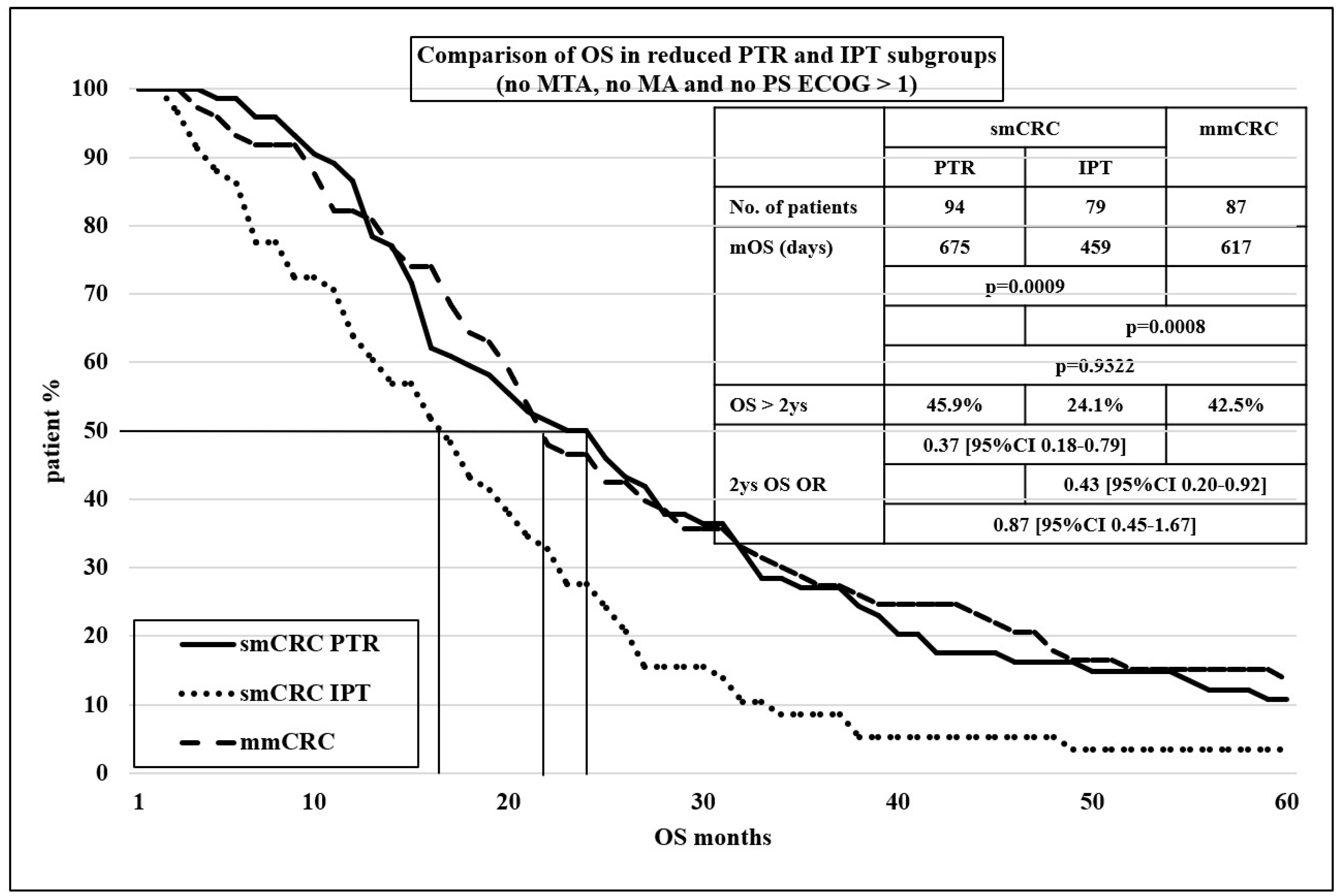
| smCRC | mmCRC | ||
|---|---|---|---|
| PTR | IPT | ||
| No. of patients | 285 | 164 | 215 |
| Male/female rate | 166/119 (58.2%/41.8%) | 94/70 (57.3%/42.7%) | 128/87 (59.5%/40.5%) |
| Age (median) | 65.7 (30.1–85.6) | 64.0 (31.0–92.7) | 67.1 (26.2–85.4) |
| PS (average) (ECOG) | 0.98 | 1.1 | 1.04 |
| p = 0.0456 | |||
| RCC | 74 (25.9%) | 28 (17.1%) | 40 (18.6%) |
| p = 0.0243 | |||
| LCC | 118 (41.4%) | 50 (30.5%) | 72 (33.5%) |
| p = 0.0213 | |||
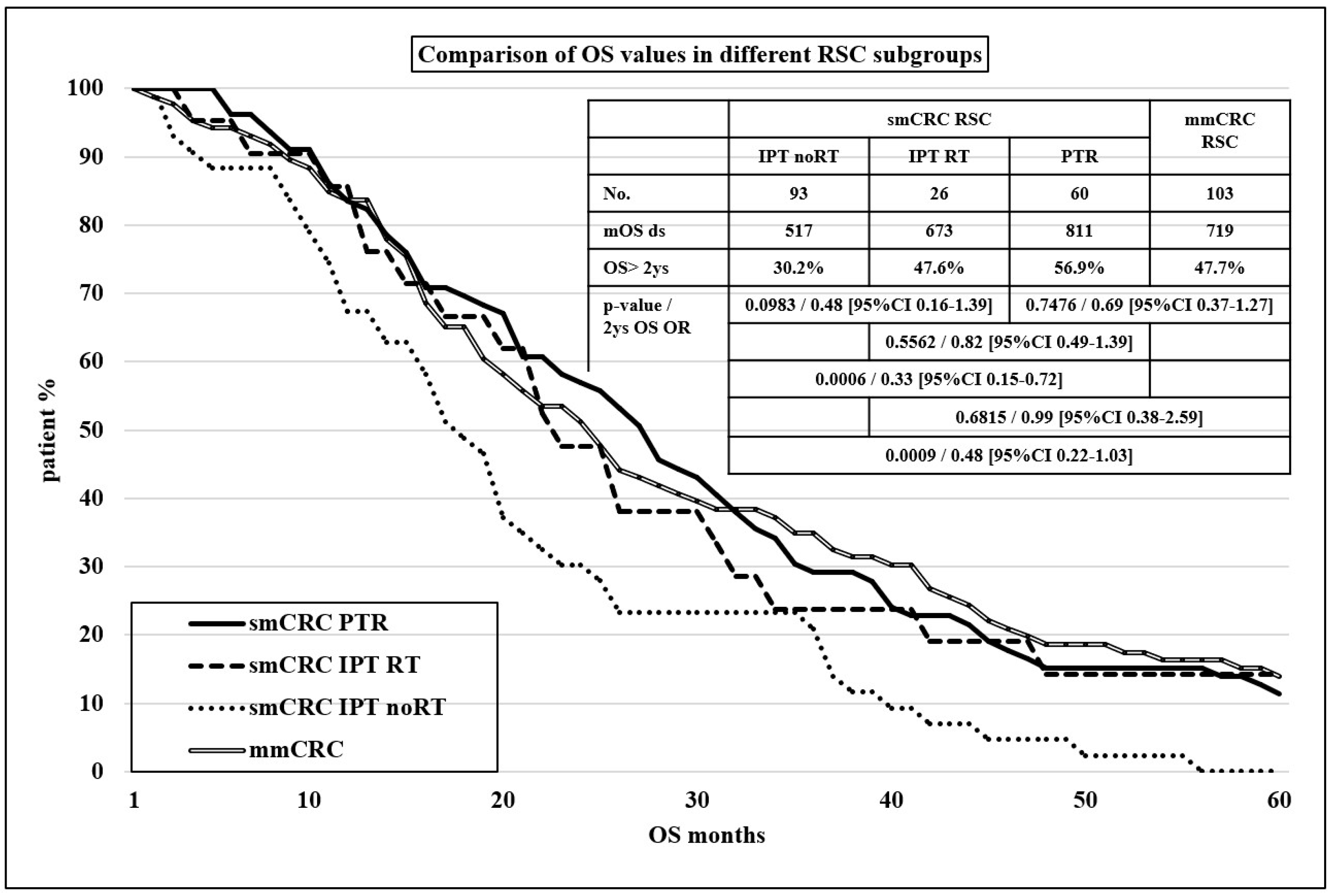
| RSC | 93 (32.6%) | 86 (52.4%) | 103 (47.9%) |
| p < 0.0001 | |||
| Extrapelvic vs. intrapelvic primary tumor | 67.4% | 47.6% | |
| p < 0.0001 | |||
| Mono-/multiorgan metastases | 216/69 (75.8%/24.2%) | 103/61 (62.8%/37.2%) | 168/47 (78.1%/21.9%) |
| p = 0.0034 | |||
| smCRC | mmCRC | ||
|---|---|---|---|
| PTR | IPT | ||
| Chemotherapy | |||
| Monotherapy (FP) | 11.6% | 13.4% | 13.1% |
| p = 0.5688 | |||
| Doublet therapy | 88.4% | 86.6% | 86.9% |
| IRI/OXA | 96.0%/4.0% | 95.8%/4.2% | 93.6%/6.4% |
| 5FU CI/CAP | 96.4%/3.6% | 96.5%/3.5% | 87.8%/12.2% |
| Doublet therapy + MTA | 45.3% | 28.7% | 31.6% |
| p = 0.0005 | |||
| VEGFi/EGFRi | 70.5%/29.5% | 59.6%/40.4% | 61.8%/38.2% |
| Metastasis ablation | 62 (21.8%) | 2 (1.2%) | 58 (26.9%) |
| p < 0.0001 | |||
| smCRC | mmCRC | ||
|---|---|---|---|
| PTR | IPT | ||
| Progression-free survival 1 | 301 | 259 | 273 |
| p < 0.0001 | |||
| 1 year PFS | 39.2% | 26.6% | 36.8% |
| OR 0.56 (95% CI 0.36–0.87] | |||
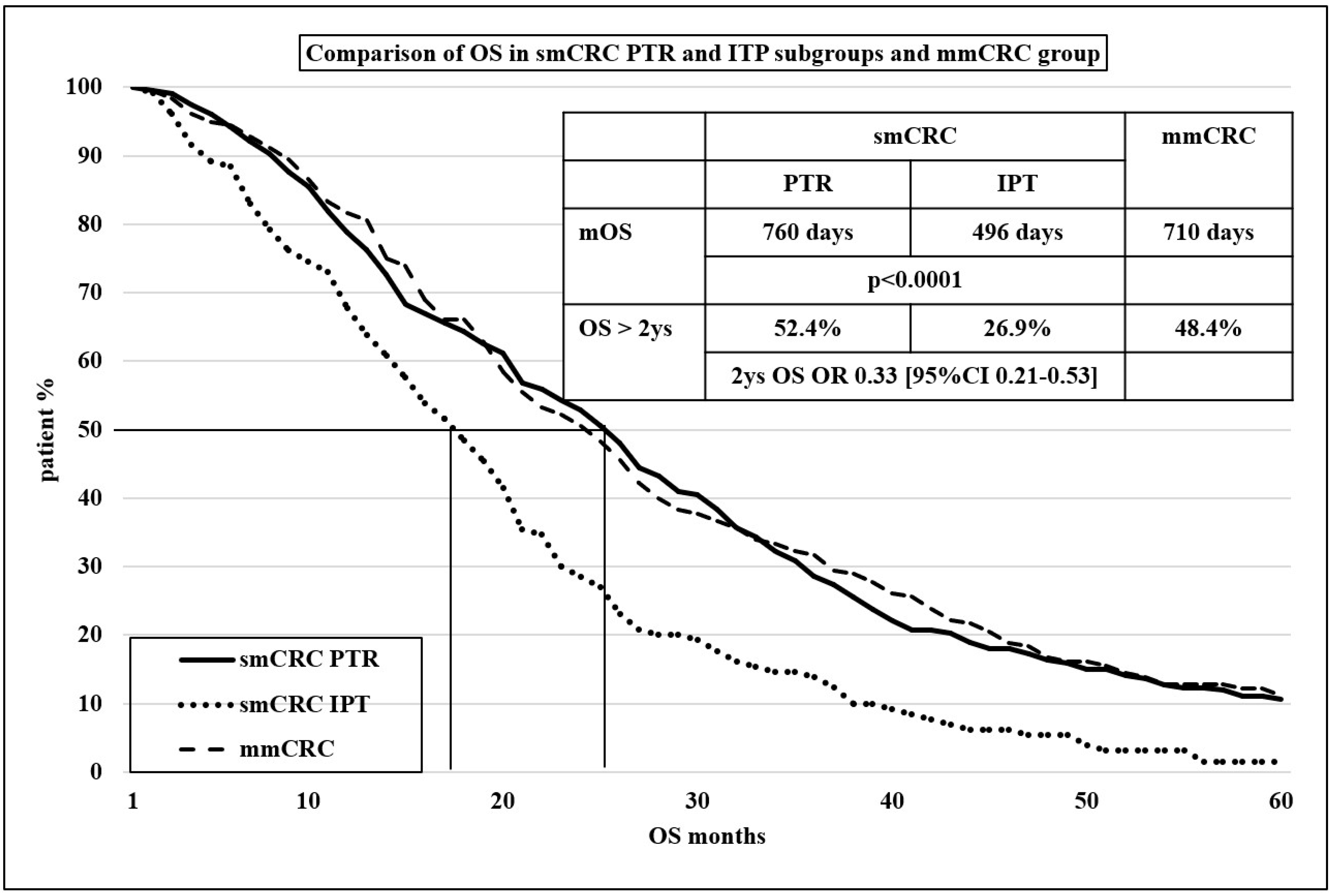
| Overall survival | 760 | 495 | 710 |
| p < 0.0001 | |||
| 2 y OS | 52.4% | 26.9% | 48.4% |
| OR 0.33 (95% CI 0.21–0.53] | |||
| PTR | IPT | |||
|---|---|---|---|---|
| Metastases | Monoorganic | Multiorganic | Monoorganic | Multiorganic |
| No. of patients | 68 | 26 | 50 | 29 |
| OS (days) | 732 | 422 | 463 | 371 |
| p = 0.0045 | p = 0.5089 | |||
| p = 0.3344 | ||||
| p = 0.0017 | ||||
| 2 y OS | 50.9% | 33.3% | 23.1% | 26.3% |
| OR 0.48 (95% CI 0.17–1.38) | OR 0.84 (95% CI 0.24–2.97) | |||
| OR 0.29 (95% CI 0.12–0.72) | ||||
| OR 0.71 (95% CI 0.18–2.80) | ||||
| (A) | ||||||||
| PTR | IPT | |||||||
| Primary tumor | RCC | LCC | RSC | RCC | LCC | RSC | ||
| No. of patients | 74 | 118 | 93 | 28 | 50 | 86 | ||
| 26.0% | 41.4% | 32.5% | 17.1% | 30.5% | 52.4% | |||
| mOS (days) | 552 | 818 | 811 | 376 | 436 | 584 | ||
| p = 0.3487 | p = 0.8611 | |||||||
| 0.7134 | p = 0.0049 | |||||||
| p = 0.2299 | p = 0.0419 | |||||||
| 2 y OS | 36.8% | 58.2% | 56.9% | 22.7% | 15.9% | 35.9% | ||
| OR 0.42 (95% CI 0.21–0.83) | OR 0.64 (95% CI 0.18–2.32) | |||||||
| OR 0.95 (95% CI 0.52–1.75) | OR 0.34 (95% CI 0.13–0.88) | |||||||
| OR 0.44 (95% CI 0.21–0.89) | OR 0.52 (95% CI 0.17–1.61) | |||||||
| (B) | ||||||||
| Primary Tumor | Extrapelvic | Intrapelvic | Extrapelvic | Intrapelvic | ||||
| No. of patients | 70 | 24 | 40 | 39 | ||||
| mOS (days) | 680 | 656 | 361 | 644 | ||||
| p = 0.7496 | p = 0.0070 | |||||||
| p < 0.0001 | ||||||||
| p = 0.3004 | ||||||||
| 2 y OS | 46.2% | 45.5% | 9.1% | 44.0% | ||||
| OR 0.97 (95% CI 0.36–2.64) | OR 0.13 (95% CI 0.03–0.53) | |||||||
| OR 0.12 (95% CI 0.03–0.43) | ||||||||
| OR 0.94 (95% CI 0.30–2.98) | ||||||||
| smCRC | mmCRC | ||
|---|---|---|---|
| PTR | IPT | ||
| PS (average) (ECOG) | 0.98 | 1.1 | 1.04 |
| p = 0.0456 | |||
| RCC | 25.9% | 17.1% | 18.6% |
| p = 0.0243 | |||
| LCC | 41.4% | 30.5% | 33.5% |
| p = 0.0192 | |||
| RSC | 32.6% | 52.4% | 47.9% |
| p < 0.0001 | |||
| Extrapelvic primary tumor | 67.4% | 47.6% | 52.1% |
| p < 0.0001 | |||
| Monoorganic metastasis | 75.8% | 62.8% | 52.1% |
| p = 0.0034 | |||
| Doublet therapy + MTA | 45.3% | 28.7% | 31.6% |
| p = 0.0005 | |||
| Metastasis ablation | 21.8% | 1.2% | 26.9% |
| p < 0.0001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pécsi, B.; Mangel, L.C. The Real-Life Impact of Primary Tumor Resection of Synchronous Metastatic Colorectal Cancer—From a Clinical Oncologic Point of View. Cancers 2024, 16, 1460. https://doi.org/10.3390/cancers16081460
Pécsi B, Mangel LC. The Real-Life Impact of Primary Tumor Resection of Synchronous Metastatic Colorectal Cancer—From a Clinical Oncologic Point of View. Cancers. 2024; 16(8):1460. https://doi.org/10.3390/cancers16081460
Chicago/Turabian StylePécsi, Balázs, and László Csaba Mangel. 2024. "The Real-Life Impact of Primary Tumor Resection of Synchronous Metastatic Colorectal Cancer—From a Clinical Oncologic Point of View" Cancers 16, no. 8: 1460. https://doi.org/10.3390/cancers16081460
APA StylePécsi, B., & Mangel, L. C. (2024). The Real-Life Impact of Primary Tumor Resection of Synchronous Metastatic Colorectal Cancer—From a Clinical Oncologic Point of View. Cancers, 16(8), 1460. https://doi.org/10.3390/cancers16081460





