Simple Summary
Aggressive angiomyxoma is a mesenchymal tumor with localized aggressiveness, affecting the connective tissue of the perineum or the lower pelvis. Prevalence in the population is unknown due to its rarity, making management and counseling difficult. The management of angiomyxoma includes multiple types of treatment, such as radical surgery with tumor-free margins, but the probability of local recurrence is high, despite extensive excision with unscathed mar-gins. Considering the low mitotic activity of angiomyxoma, there is not always a rationale for adjuvant radiotherapy and chemotherapy. Given its exceptionally low incidence, optimal management of the disease remains a subject of on-going debate, and a unanimous consensus on treatment strategies has yet to be reached.
Abstract
Aggressive angiomyxoma (AAM) is a rare, locally aggressive, myxoid mesenchymal neoplasm primarily found in the pelvic and perineal regions of young adult females. It is a slow growing and locally infiltrating tumor. Preoperative diagnosis is difficult due to the rarity of these tumors and absence of characteristic signs and symptoms. The primary management is tumor excision. Incomplete excision is common because of the infiltrating nature of the neoplasm and absence of a definite capsule. Other non- surgical modalities have been employed, such as radiotherapy, embolization, GnRH analogues or other anti-estrogenic agents. Local relapses occur in 30–40% of the cases, and often appear many years (sometimes decades) after the first excision. Occasional distant metastasis has also been reported. A limited number of cases have been reported in the literature, mostly in the form of small case series or isolated case reports. Therefore, the aim of this paper by a team of experts from the MITO rare tumors group is to review clinical findings, pathologic characteristics and outcome of patients affected by this rare condition in order to be able to offer up-to-date guidance on the management of these cases.
1. Introduction
Aggressive angiomyxoma (AAM) is a mesenchymal tumor with localized aggressiveness, affecting the connective tissue of the perineum or the lower pelvis [1]. The primary occurrence is among women of reproductive age, with a female-to-male ratio of 6.6/1 [2]. The term “aggressive” is used to highlight its potential infiltrative behavior. Despite its name, AAM does not have an aggressive nature, since its capacity to metastasize and the histologic features of this tumor do not suggest a more aggressive pattern than the commonest vulva carcinoma with also a considerably better prognosis [3,4,5]. Indeed, the pelvis, perineum, vulva, vagina, and bladder are the most common anatomic sites involved [2]. On the other hand, it is important to underline that AAM exhibits an angioinvasive behavior, but with a slow growth and a high rate of local recurrence, and a limited ability to metastasize. The origin is myofibroblastic differentiation of spindle or stellate cells divided by myxoid stroma and abundant vascular constituents. The management of angiomyxoma includes multiple types of treatment, such as radical surgery with tumor-free margins, but the probability of local recurrence is high, despite extensive excision with unscathed margins [6]. Given its exceptionally low incidence, optimal management of the disease remains a subject of ongoing debate, and a unanimous consensus on treatment strategies has yet to be reached [7]. Consequently, we present here a systematic review that may serve as a valuable resource for further discussion and future clinical practice guidelines.
2. Materials and Methods
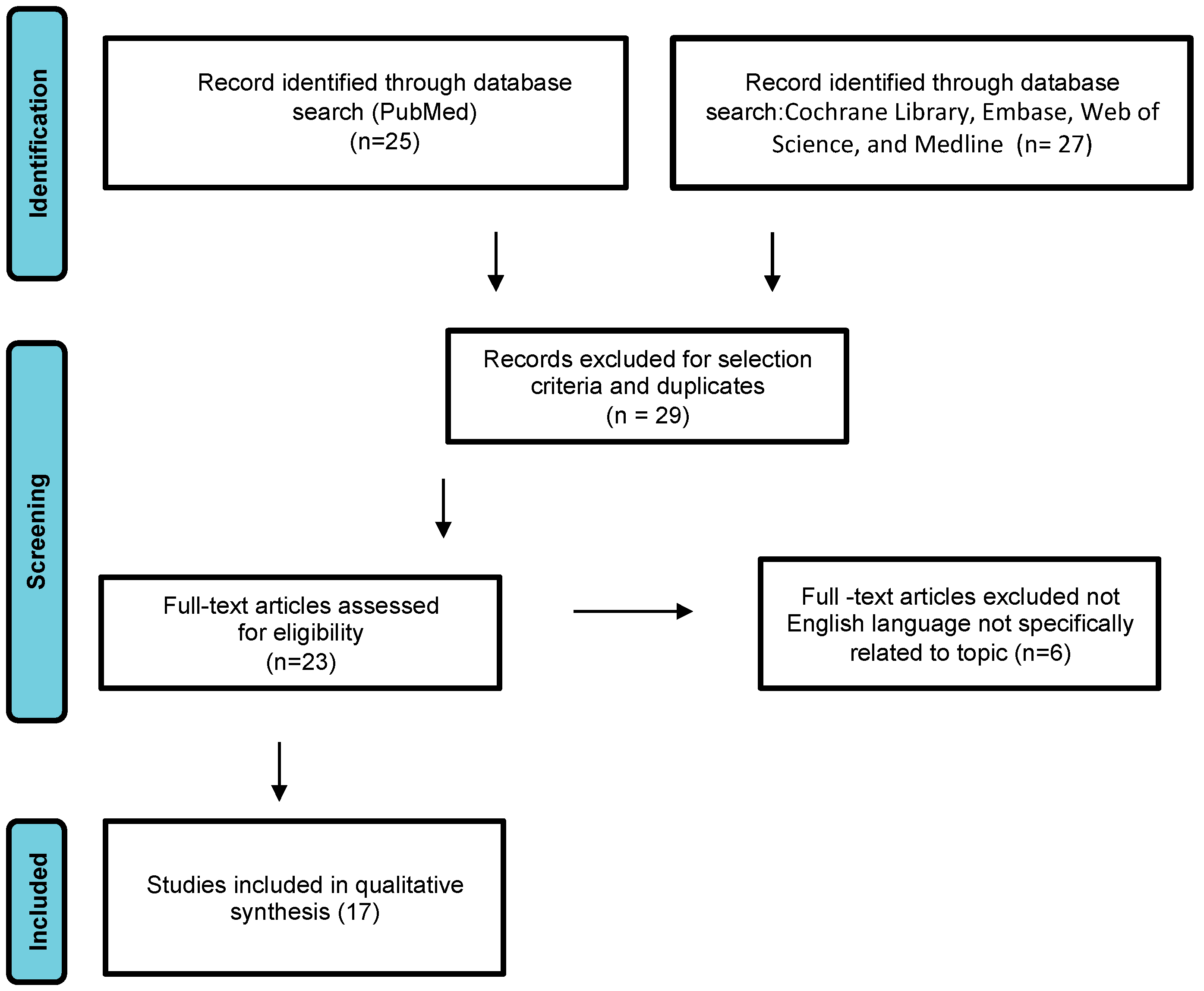
We conducted a systematic review of DAM case reports by searching electronic databases including PubMed, the Cochrane Library, Embase, Web of Science, and Medline. The article research adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Figure 1) [8]. The following search terms were used: “aggressive angiomyxoma”, “deep angiomyxoma”, “vulvar”. No limitations were imposed on the publication timeframe. Specifically, we considered case series and case reports published in English. Three authors (M.D., F.P.M., S.C.) independently reviewed the titles and abstracts of eligible articles, eliminating duplicates. The full texts of potentially suitable studies were then independently evaluated for eligibility by two authors. Any discrepancies were resolved through discussion with two senior reviewers (G.C. and G.M.). Data were collected from articles published between 1983 (when Steeper and Rosai first described a case of aggressive angiomyxoma of the vulva) and February 2023. Articles reporting DAM in pregnant women were excluded.
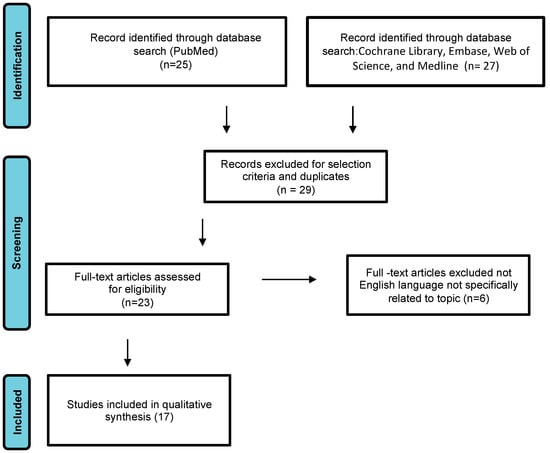
Figure 1.
Study flow diagram: PRISMA flow diagram of identification, screening, and inclusion of articles. Systematic literature reviews were selected with standard methods to be briefly presented in the article.
3. Pathological Examination
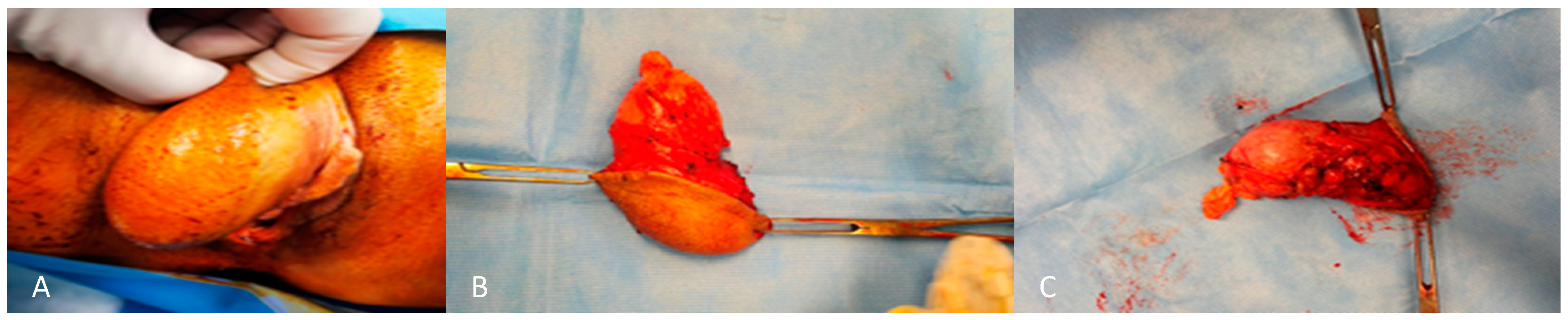
From a macroscopic perspective, these tumors frequently exhibit a smooth surface which is partially or entirely encapsulated. The cut surface presents a shiny, gelatinous appearance with a bluish grey hue, often accompanied by regions of hemorrhage and congestion [9]. Size is variable, although usually the maximum diameter is 10–20 cm. They may produce pressure on adjacent organs. They are usually homogenous in consistency with no obvious nodularity [9] (Figure 2).
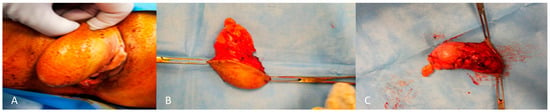
Figure 2.
External (A) and internal (B,C) macroscopic aspect of angiomyxoma of the right labium.
Microscopically, the tumor exhibits spindle and stellate-shaped cells within a myxoid matrix characterized by delicate wavy collagen fibrils [10]. Additionally, there is a notable presence of vascular structures of various sizes [10]. The cells have abundant wispy pink cytoplasm with bland nuclei [10]. There is no cytological atypia, no atypical mitotic features or discernible mitotic activity, nor any evidence of coagulative tumor cell necrosis [11]. Immunohistochemical analyses typically reveal positive reactivity for desmin, smooth muscle actin (SMA), muscle specific actin, vimentin, CD34, estrogen, and progestin receptors in the majority of these tumors [11]. Conversely, the S100 protein is invariably negative. The vast majority of these neoplasms exhibit positivity for estrogen and progesterone receptors, indicating that AA is likely a hormone-dependent tumor. This is supported by observations of rapid growth and recurrence during pregnancy [12]. Recurrent tumors usually show similar histological characteristics. Diagnostic problems may arise when the pathologist is dealing with uncommon morphological features [13], deposition with or without hyalinized blood vessels, or neurofibroma-like appearance [12].
4. Clinical Features
The clinical aspects described in the world literature are of a bulky and soft tumor which looks like a cutaneous mass, or of an ulcerated or polypoid/pedunculated tumor, sometimes associated with bleeding [14]. The areas most affected are the labia majora, vulva and pubis, perineum, medial gluteal region, and the periurethral region [15,16]. Their size ranges from 1 to 60 cm, but the chances of recurrence do not depend on the size [15]. Almost all patients report a soft mass on the pelvic region, sometimes bilobated, mobile, with slow growth, pelvic fullness, perineal bulge, and discomfort/dyspareunia [14]. The clinical presentation is similar to other benign lesions such as Bartholin duct cysts, lipoma, vulvar masses or abscesses, or Gartner duct cysts. Consequently, AAM can often be misdiagnosed [15].
5. Management: Treatment of Aggressive Angiomyxoma of the Vulva
Several therapy modalities have been described for the treatment of angiomyxoma. Radical surgery with tumor-free margins is the treatment of choice [17]. The literature has highlighted how the probability of local recurrence is very high even with wide local excision. On the other hand, we see how adjuvant radiotherapy and chemotherapy have limited indications considering the low mitotic activity of angiomyxoma [18]. Since most of these tumors have positive estrogen and progesterone receptors, they may respond to hormone treatment with gonadotropin-releasing hormone (GnRH), raloxifene, and tamoxifen agonists, both preoperatively and after relapse [14]. Therefore, several studies have published the use of GnRH agonists as the sole treatment or as adjuvant hormone therapy after surgery for the management of these malignancies. However, the duration is not clearly defined GnRH agonist or antihormone therapy (tamoxifen) for correlation with hormone-based proliferation are indicated as emerging therapies [8]. This treatment is indicated either as an adjuvant approach for residual masses or before surgery to minimize the size of the tumor and promote the possibility of complete excision [15]. However, after approximately 10 days of treatment, receptors undergo down-regulation through internalization, leading to decreased hormone levels [8]. Indeed, some reports have documented complete radiological resolution of tumors with the administration of GnRH agonists, both in cases of primary and recurrent tumors. For this reason, some authors advocate for the inclusion of GnRH agonists in the treatment protocol to potentially avoid the necessity for radical pelvic surgery in hormone receptor-positive patients [18]. More recently, evidence has emerged to support the use of an aromatase inhibitor in angiomyxoma treatment; a study reported successful tumor shrinkage when the inhibitor was administered prior to resection [17]. This treatment approach works by blocking the aromatase enzyme from synthesizing estrogen. Clearly, adjuvant therapy in raloxifene, tamoxifen, or GnRH agonists like leuprolide acetate and goserelin have demonstrated efficacy in cases where the tumor exhibits sensitivity to estrogen and progesterone receptors [8]. It must be considered, however, that these drugs have multiple side effects including bone depletion and menopausal-like manifestations [17]. It is also highlighted that following the discontinuation of the drug, there is the subsequent growth of the tumor [14], therefore, these drugs do not represent a definitive treatment [15]. An alternative option in postmenopausal women is to use oral hormone therapy based on aromatase inhibitors [17]. Finally, angiographic embolization or chemoembolization has been described [14]. Extensive surgical excision with tumor-free margins remains the gold standard. It is necessary to underline the need for long-term follow-up due to the high recurrence frequency of between 36 and 72% which can be found at the same site as the initial resection [15]. Therefore, the widest margin of excision as possible is recommended without undue morbidity [17]. In fact, growth is usually slow and locally infiltrative, extending insidiously into adjacent soft tissues [14]. The presence of an infiltrative capacity of this tumor and the absence of a well-defined capsule limits the incomplete removal of the neoplasm and contributes to the high rate of tumor recurrence [18]. Distant metastases are very rare thanks to the low mitotic index, and so the prognosis is excellent [17]. Radiological investigations are fundamental for diagnosis, monitoring of recurrences, and planning of surgery in order to examine the actual extent of the tumor [17]. Treatment alternatives are angiographic embolization, mainly as adjuvant therapy or in cases where surgery is contraindicated [15]. The role of chemotherapy and radiotherapy has limited indications [15]. For example, the use of radiotherapy can be proposed in cases of multiple recurrent diseases after poor results with surgical excision [15,17,18]. Indeed, to date there is no unequivocal consensus in the literature on the appropriate treatment method. Therefore, treatment should be tailored on a case-by-case basis after discussion in a multidisciplinary and experienced team.
6. Prognostic Factors
AAM usually exhibit a slow, insidious growth pattern, a capacity for local infiltration, and a marked tendency for repeated local recurrence [8,19]. The reported local recurrence rate stands at around 30% and may occur months to several years after excision (2 months to 15 years) [18]. Multiple recurrences are not infrequent 3–6, and more than half of the presenting lesions and all relapsing lesions involve adjacent organs, with 71% of recurrences occurring within three years of resection [18].
Despite a quite high relapse rate, distant metastasis and death from disease are extremely rare [18]. Two cases of distant metastasis have been reported in the literature [18]:
- -
- a case of an aggressive angiomyxoma of the pelvis, with massive bilateral pulmonary, mediastinal, iliac, and aortic lymph node and peritoneal metastases ending in death described by Siassi et al. in 1999 [14].
- -
- another case, a 34-year-old woman developed several local recurrences after primary resection of an AAM and subsequently died from multiple lung metastases [17].
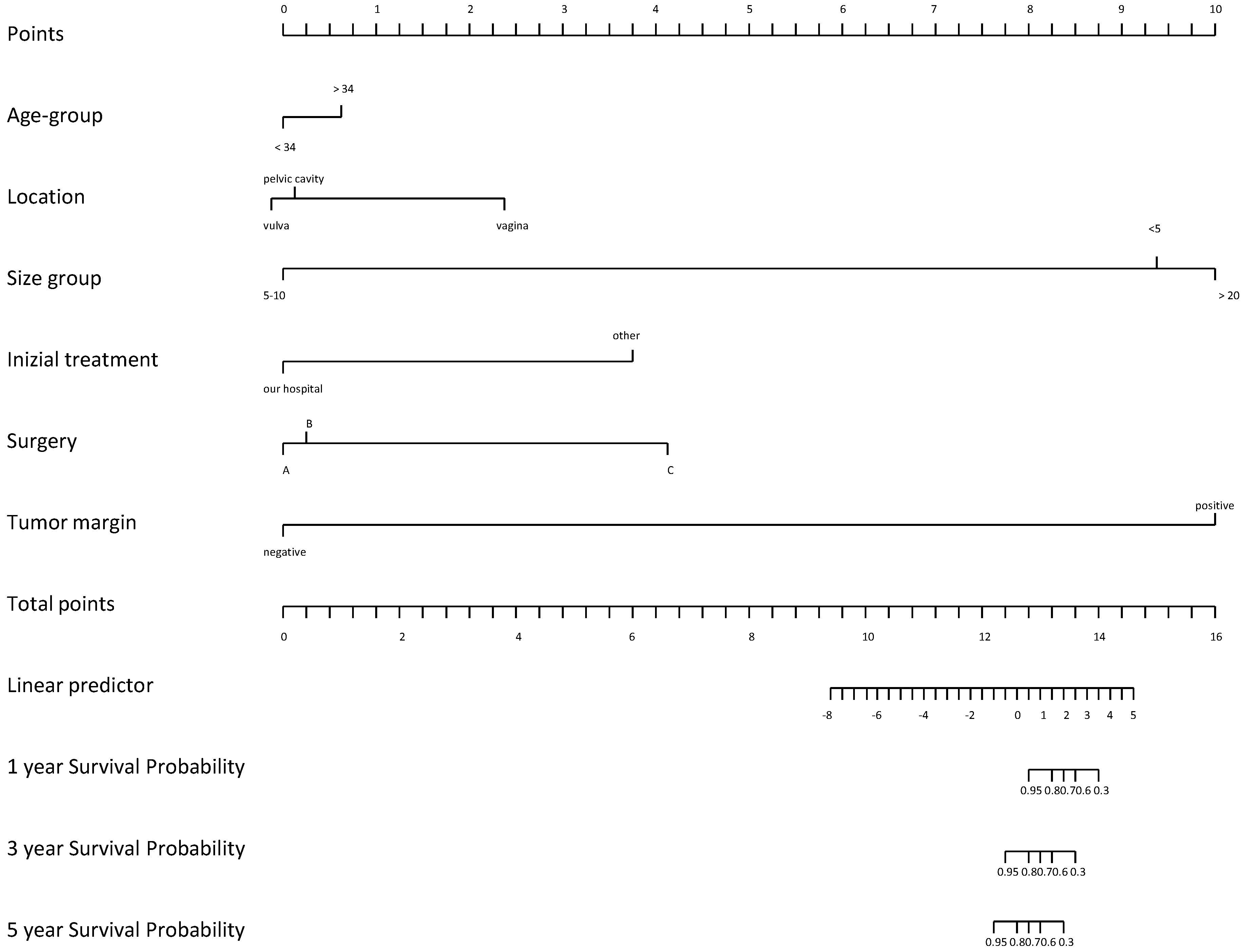
Notwithstanding its capacity to metastasize, the histologic features of this tumor do not suggest a more aggressive pattern and prognosis is considerably good [15]. Most relapses were related to incomplete resection. However, these tumors often invade surrounding tissues and the visceral peritoneum, making complete resection quite challenging [18]. Except for positive surgical margins, there are no clinical or histological predictors for tumor recurrence. Nonetheless, Chan et al. found that patients with negative resection margins have similar chances of remaining disease-free compared with those having positive resection margins, 50% vs. 40% in 10 years [8]. Moreover, a review of over 100 cases observed that those with positive margins are as likely to have recurrences as those with negative margins [18]. Recently, several studies have assessed that incomplete or partial resection could be acceptable, especially when high operative morbidity is anticipated and preservation of fertility represents an issue [20]. In a recent study by Li et al., outcomes of 14 females with AA were analyzed during a median follow-up of 78.8 months [21]. Univariate Cox regression analysis identified tumor margin (p = 0.012) and initial treatment site (p = 0.039) as being associated with disease-free survival (DFS) [21]. Patients with positive tumor margins had a significantly lower probability of survival with DFS than those with negative margins (HR = 3.41, CI: 2.73–15.74, p = 0.012). The authors hypothesized that tumor location, tumor margin, surgical procedure, and tumor size had a greater effect on patient outcomes (Figure 3).
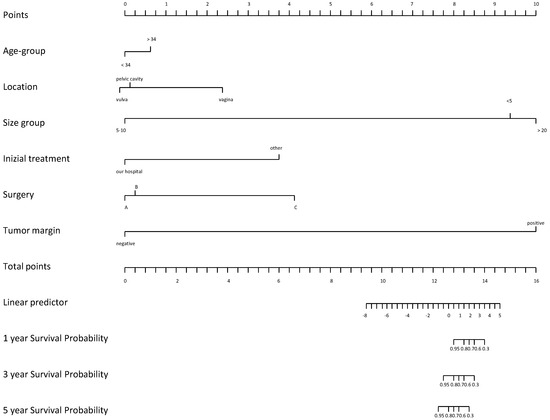
Figure 3.
Nomogram on DFS according to initial treatment site (A), surgery procedure (B) and tumor location (C).
In particular, the prognosis of patients varied depending on the tumor location. For instance, patients with pelvic tumors had the worst prognosis, while patients with vaginal tumors had a better prognosis [22]. In addition, Li and colleagues observed that patients with tumor sizes in the range of 5–10 cm had the poorest probability of DFS survival, while those with tumor size less than 5 cm had the greatest probability of DFS survival. Risk of relapse was demonstrated to be higher among patients older than 34 years (p = 0.67) [21].
7. Results
From the literature search, we identified 17 articles reporting a total of 19 cases listed in Table 1.

Table 1.
Overview of cases of aggressive angiomyxoma of the lower female genital tract: literature reviewed until February 2023.
8. Discussion
Soft tissue myxoid tumors constitute a diverse array of lesions characterized by varying degrees of extracellular myxoid matrix [22]. Steeper and Rosai were the first to describe nine cases of a distinct, infiltrative, locally aggressive but non-metastasizing fibro-myxoid soft tissue tumors arising in the pelvic and perineal regions of young female patients, which they designated aggressive angiomyxoma [1]. This category of tumors displays considerable variability in their biological behavior and includes benign lesions, tumors prone to recurrence but lacking metastatic potential, and outright malignant neoplasms [31]. In general, angiomyxomas are classified as either superficial (also referred to as cutaneous myxoma) or AA. Superficial angiomyxoma is commonly associated with the Carney complex [46]. This type of lesion predominantly affects middle-aged adults and can manifest in superficial tissues throughout the body, but mostly it occurs in the trunk, lower extremities, and head and neck regions [23]. Clinically, most lesions emerge as slow growing polypoid cutaneous lesions and often have to be differentiated from angiomyoblastoma, myxoid neurofibroma, myxoma, spindle cell lipoma, myxoid liposarcoma, leiomyosarcoma, and botryoid rhabdo-myosarcoma [24]. Aggressive angiomyxoma typically displace neighboring organs without direct invasion, yet their locally infiltrative nature can eventually result in the invasion of adjacent organs over time, culminating in the development of a large tumor that occupies the abdominal and pelvic cavities [18]. The recurrence rate has been reported to be as high as 70%; with the majority of cases recurring within two years. However, recurrence can occur as early as a few months or as late as 20 years after initial treatment [22,26]. Evidence suggests that AAM affects almost exclusively the genital, perineal, and pelvic regions in women of reproductive age, implicating especially the vulva [27]; less frequently, the buttocks, retroperioneum, and inguinal area may be involved. AAM are infrequently observed in men, with the scrotum being the primary site of involvement [28]. The prevailing theory regarding AAM pathogenesis suggests that the lesion originates from a primitive multipotent mesenchymal cell found in the lower female genital tract, with the capacity for diverse differentiation pathways. [20].
Interesting molecular studies have detected a significant clonal aberration of chromosome 12, in the region 12q13–15, with rearrangement of the HMGIC gene (high-mobility group protein isoform I-C) linked with AAM [29]. Thus, AAM is molecularly part of the benign group of mesenchymal tumors showing multiple aberration region involvement [30]. Immunohistochemically, AA cells show diffuse staining for estrogen receptor (ER), progesterone receptor (PR), desmin, smooth muscle actin, and vimentin. Numerous tumors are also CD34-positive [31]. Furthermore, AAM seems to grow in size in pregnant women due to the positivity of progesterone receptors, underlying a concrete hormonal correlation between the tumor and female hormonal status [20]. Hormonal correlation suggests that antihormonal therapy (e.g., Tamoxifen), gonadotrophin-releasing hormone (GnRH) agonist, or aromatase inhibitors could be considered feasible emerging options in AA treatment [28]. Moreover, a neo-adjuvant treatment to reduce tumor size before surgery, facilitating complete excision, or an adjuvant approach for incompletely resectable/residual mass could be investigated [29]. However, the adverse effects of long-term use of the GnRH agonist (e.g., menopausal symptoms and bone loss) and tumor regrowth after drug interruption do not allow us to approve it as a best choice of treatment [32,33,45]. Large surgical excision with tumor-free margins remains the gold standard management, but it necessitates long-term follow-up because of the high relapse rate (between 36 and 72%) [32]. Imaging is important to establish the effective extension of the tumor, tailor surgery, and monitor relapse. MRI with contrast enhancement by gadolinium is an optimum radiological possibility for diagnosis, especially on T2-weighted images, since it show the lesion as hyperintense relative to muscle [33]. On the other hand, ultrasound can be useful in follow-up monitoring as well. In conclusion, AMM should be evaluated as a differential diagnosis whenever a patient presents with a soft tissue tumor in the vulva–vaginal region, perineum, or pelvis since timely diagnosis with following surgical management (wide surgical excision possibly with free margins) are crucial for the prognosis of these women.
9. Conclusions
Soft tissue myxoid tumors constitute a diverse array of lesions characterized by varying degrees of extracellular myxoid matrix. This category of tumors displays considerable variability in their biological behavior and includes benign lesions, tumors prone to recurrence but lacking metastatic potential, and outright malignant neoplasms. Clinically, most lesions emerge as slowly growing polypoid cutaneous lesions and often have to be differentiated from angiomyoblastoma, myxoid neurofibroma, myxoma, spindle cell lipoma, myxoid liposarcoma, leiomyosarcoma and botryoid rhabdo-myosarcoma. Aggressive angiomyxoma typically displace neighboring organs without direct invasion, yet their locally infiltrative nature can eventually result in the invasion of adjacent organs over time, culminating in the development of a large tumor that occupies the abdominal and pelvic cavities. Evidence suggests that AAM affects almost exclusively the genital, perineal, and pelvic regions in women of reproductive age, implicating especially the vulva. Imaging is important to establish the effective extension of the tumor, tailor surgery, and monitor relapse. MRI with contrast enhancement by gadolinium is an optimum radiological possibility for diagnosis, especially on T2-weighted images, since it shows the lesion as hyperintense relative to muscle. Large surgical excision with tumor-free margins remains the gold standard management, but it necessitates long-term follow-up because of the high relapse rate. In conclusion, AAM should be evaluated as a differential diagnosis whenever a patient presents with a soft tissue tumor in the vulva–vaginal region, perineum, or pelvis since timely diagnosis with following surgical management are crucial for the prognosis of these women.
Author Contributions
Conceptualization, M.D. and F.M.; methodology, F.M. and S.C.; resources, S.P. and L.D.; data curation, G.M. and L.D.; writing—original draft preparation, M.D., L.D., F.M., S.C. and S.T.M.; writing—review and editing, S.P., G.C. and G.M.; visualization, G.M.; supervision, G.C.; project administration, G.C.; funding acquisition, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Steeper, T.A.; Rosai, J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am. J. Surg. Pathol. 1983, 7, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Graadt van Roggen, J.F.; Hogendoorn, P.C.; Fletcher, C.D. Myxoid tumours of soft tissue. Histopathology 1999, 35, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Bucchi, L.; Micheletti, L.; Privitera, S.; Corazza, M.; Cosma, S.; Gallio, N.; Borghi, A.; Bevilacqua, F.; Benedetto, C. Four-decade trends in lymph node status of patients with vulvar squamous cell carcinoma in northern Italy. Sci. Rep. 2021, 11, 5661. [Google Scholar] [CrossRef] [PubMed]
- Dellino, M.; Cicogna, S.; Falcone, F.; Mitidieri, M.; Mazzeo, R.; Pignata, S.; Mangili, G.; Cormio, G. “Intestinal-Type” Vulvar Adenocarcinoma: A Review of the MITO Rare Tumors Group. Cancers 2022, 14, 5171. [Google Scholar] [CrossRef] [PubMed]
- Cicogna, S.; Dellino, M.; Miano, S.T.; Magazzino, F.; Domenici, L.; Pignata, S.; Mangili, G.; Cormio, G. Aggressive Angiomyxoma of the Lower Female Genital Tract in Pregnancy: A Review of the MITO Rare Tumors Group. Cancers 2023, 15, 3403. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.A.; Headington, J.T.; Su, W.P. Cutaneous myxomas. A major component of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Arch. Dermatol. 1986, 122, 790–798. [Google Scholar] [CrossRef]
- Behranwala, K.A.; Latifaj, B.; Blake, P.; Barton, D.P.; Shepherd, J.H.; Thomas, J.M. Vulvar soft tissue tumors. Int. J. Gynecol. Cancer 2004, 14, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.M.; Hon, E.; Ngai, S.W.; Ng, T.Y.; Wong, L.C. Aggressive angiomyxoma in females: Is radical resection the only option? Acta Obstet. Gynecol. Scand. 2000, 79, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Zizi-Sermpetzoglou, A.; Myoteri, D.; Koulia, K.; Kontostolis, V.; Moschouris, H.; Dellaportas, D. Aggressive angiomyxoma of the vulva: A bizarre perineal lesion. Case Rep. Oncol. Med. 2015, 2015, 292304. [Google Scholar] [CrossRef]
- van Roggen, J.F.; van Unnik, J.A.; Briaire-de Bruijn, I.H.; Hogendoorn, P.C. Aggressive angiomyxoma: A clinicopathological and immunohistochemical study of 11 cases with long-term follow-up. Virchows Arch. 2005, 446, 157–163. [Google Scholar] [CrossRef]
- Micci, F.; Panagopoulos, I.; Bjerkehagen, B.; Heim, S. Deregulation of HMGA2 in an aggressive angiomyxoma with t(11;12)(q23;q15). Virchows Arch. 2006, 448, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.R.; Weremowicz, S.; Neskey, D.M.; Sornberger, K.; Tallini, G.; Morton, C.C.; Quade, B.J. Chromosomal translocation t(8;12) induces aberrant HMGIC expression in aggressive angiomyxoma of the vulva. Genes Chromosomes Cancer 2001, 32, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Pisacane, A.; Cascardi, E.; Berrino, E.; Polidori, A.; Sarotto, I.; Casorzo, L.; Panero, M.; Boccaccio, C.; Verginelli, F.; Benvenuti, S. Real-world histopathological approach to malignancy of undefined primary origin (MUO) to diagnose cancers of unknown primary (CUPs). Virchows Arch. 2023, 482, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Siassi, R.M.; Papadopoulos, T.; Matzel, K.E. Metastasizing aggressive angiomyxoma. N. Engl. J. Med. 1999, 341, 1772. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.Q.; Liu, J.; Feng, J.J. Clinicopathological analysis of aggressive angiomyxoma. Zhonghua Yi Xue Za Zhi 2012, 92, 1553–1555. [Google Scholar] [PubMed]
- Pinto, V.; Dellino, M.; Cicinelli, R.; Micheletti, L.; Ingravallo, G.; Cazzato, G.; Cascardi, E.; Cicinelli, E. Multiple Vulvar Polyps in Pregnancy: A Benign Disease With a Challenging Diagnosis. J. Low. Genit. Tract Dis. 2023, 27, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Gulino, F.A.; Gulisano, M.; Ettore, C.; Giorlandino, A.; Russo, E.; Ettore, G. Aggressive Angiomyxoma of the Vulva: Which Is the Best Management Strategy? Description of a Case Report and Review of Literature of the Last Ten Years. J. Clin. Med. 2023, 12, 1726. [Google Scholar] [CrossRef]
- Xu, H.; Sun, P.; Xu, R.; Wang, L.; Shi, Y. Aggressive angiomyxoma in pregnancy: A case report and literature review. J. Int. Med. Res. 2020, 48, 0300060520936414. [Google Scholar] [CrossRef]
- Dellino, M.; Gargano, G.; Tinelli, R.; Carriero, C.; Minoia, C.; Tetania, S.; Silvestris, E.; Loizzi, V.; Paradiso, A.; Casamassima, P.; et al. A strengthening the reporting of observational studies in epidemiology (STROBE): Are HE4 and CA 125 suitable to detect a Paget disease of the vulva? Medicine 2021, 100, e24485. [Google Scholar] [CrossRef]
- Faraj, W.; Houjeij, M.; Haydar, A.; Nassar, H.; Nounou, G.; Khalife, M. Aggressive angiomyxoma presenting with back and perineal bulge; a complex surgical approach: A case report. Int. J. Surg. Case Rep. 2016, 24, 211–214. [Google Scholar] [CrossRef]
- Cohen-Cole, S.A. Training outcome in liaison psychiatry. Literature review and methodological proposals. Gen. Hosp. Psychiatry 1980, 2, 282–288. [Google Scholar] [CrossRef]
- Choi, H.; Park, C.; Ji, Y.-I. Alternative surgical approaches for aggressive angiomyxoma at different sites in the pelvic cavity. Obstet. Gynecol. Sci. 2015, 58, 525–529. [Google Scholar] [CrossRef]
- Salman, M.C.; Kuzey, G.M.; Dogan, N.U.; Yuce, K. Aggressive angiomyxoma of vulva recurring 8 years after initial diagnosis. Arch. Gynecol. Obstet. 2009, 280, 485–487. [Google Scholar] [CrossRef]
- McCluggage, W.; Jamieson, T.; Dobbs, S.; Grey, A. Aggressive angiomyxoma of the vulva: Dramatic response to gonadotropin-releasing hormone agonist therapy. Gynecol. Oncol. 2006, 100, 623–625. [Google Scholar] [CrossRef]
- Yuan, R.; Zhuo, R.; Xiao, T.; Dai, X.; Wang, Z.; Le, A. Five cases’ female aggressive angiomyxoma experience. Eur. J. Gynaecol. Oncol. 2017, 38, 715–719. [Google Scholar]
- Schwartz, P.E.; Hui, P.; McCarthy, S. Hormonal therapy for aggressive angiomyxoma: A case report and proposed management algorithm. J. Low. Genit. Tract Dis. 2014, 18, E55–E61. [Google Scholar] [CrossRef]
- Dahiya, K.; Jain, S.; Duhan, N.; Nanda, S.; Kundu, P. Aggressive angiomyxoma of vulva and vagina: A series of three cases and review of literature. Arch. Gynecol. Obstet. 2011, 283, 1145–1148. [Google Scholar] [CrossRef]
- Raptin, C.; Lucot, J.-P.; Bassil, A.; Poncelet, E.; Prolongeau, J.-F.; Phalippou, J. Aggressive angiomyxoma of the perineal region. SAGE Open Med. Case Rep. 2019, 7, 2050313X19843391. [Google Scholar] [CrossRef]
- Blandamura, S.; Cruz, J.; Vergara, L.F.; Puerto, I.M.; Ninfo, V. Aggressive angiomyxoma: A second case of metastasis with patient’s death. Hum. Pathol. 2003, 34, 1072–1074. [Google Scholar] [CrossRef]
- Zamani, M.; Mollabashi, M.; Mehrabi, N.; Alizadeh, S. Aggressive angiomyxoma of vulva in 28-years old patient: A case report of second recurrence. Ann. Med. Surg. 2021, 69, 102706. [Google Scholar] [CrossRef]
- Wiser, A.; Korach, J.; Gotlieb, W.; Fridman, E.; Apter, S.; Ben-Baruch, G. Importance of accurate preoperative diagnosis in the management of aggressive angiomyxoma: Report of three cases and review of the literature. Abdom. Imaging 2006, 31, 383–386. [Google Scholar] [CrossRef]
- Shinohara, N.; Nonomura, K.; Ishikawa, S.; Seki, H.; Koyanagi, T. Medical management of recurrent aggressive angiomyxoma with gonadotropin-releasing hormone agonist. Int. J. Urol. 2004, 11, 432–435. [Google Scholar] [CrossRef]
- Bhandari, R.N.; Dragun, A.E.; Aguero, E.G.; Sharma, A.K. External beam radiotherapy for perirectal angiomyxoma results in a dramatic clinical response and allows a patient to avoid abdominoperineal resection. Am. J. Clin. Oncol. 2006, 29, 318–319. [Google Scholar] [CrossRef]
- Begin, L.R.; Clement, P.B.; Kirk, M.E.; Jothy, S.; McCaughey, W.T.; Ferenczy, A. Aggressive angiomyxoma of pelvic soft parts: A clinicopathologic study of nine cases. Hum. Pathol. 1985, 16, 621–628. [Google Scholar] [CrossRef]
- Fetsch, J.F.; Laskin, W.B.; Tavassoli, F.A. Superficial angiomyxoma (cutaneous myxoma): A clinicopathologic study of 17 cases arising in the genital region. Int. J. Gynecol. Pathol. 1997, 16, 325–334. [Google Scholar] [CrossRef]
- Granter, S.R.; Nucci, M.R.; Fletcher, C.D. Aggressive angiomyxoma: Reappraisal of its relationship to angiomyofibroblastoma in a series of 16 cases. Histopathology 1997, 30, 3–10. [Google Scholar] [CrossRef]
- York, D.; Saikumar, S.; Patel, P.; Edwards, C.; Garcia, G.; Naqvi, H. A Paraurethral Aggressive (Deep) Angiomyxoma. Case Rep. Obstet. Gynecol. 2022, 2022, 5604460. [Google Scholar] [CrossRef]
- Han-Geurts, I.J.; van Geel, A.N.; van Doorn, L.; Bakker, M.d.; Eggermont, A.M.; Verhoef, C. Aggressive angiomyxoma: Multimodality treatments can avoid mutilating surgery. Eur. J. Surg. Oncol. 2006, 32, 1217–1221. [Google Scholar] [CrossRef]
- Foust-Wright, C.; Allen, A.; Shobeiri, S.A. Periurethral aggressive angiomyxoma: A case report. Int. Urogynecol. J. 2013, 24, 877–880. [Google Scholar] [CrossRef]
- Srivastava, V.; Jha, P.K.; Verma, A.K.; Ansari, M.A. Vulvar aggressive angiomyxoma: A surgical challenge. BMJ Case Rep. 2021, 14, e240687. [Google Scholar] [CrossRef]
- Wahid, A.; Hakeem, A.; Khan, S. Aggressive angiomyxoma in the ischiorectal fossa. J. Pak. Med. Assoc. 2020, 70, 1304–1306. [Google Scholar] [CrossRef]
- Ayati, E.; Pesikhani, M.D.; Karamali, M.; Borhan, A.; Pourali, L. A deep giant aggressive angiomyxoma of the labia majora: A case report. Int. J. Surg. Case Rep. 2022, 96, 107313. [Google Scholar] [CrossRef]
- Padmavathy, L.; Rao, L.L.; Lakshmi, M.D.; Sylvester, N. Aggressive angiomyxoma. Indian Dermatol. Online J. 2014, 5, 151–153. [Google Scholar] [CrossRef]
- Li, J.; You, L.; Wang, C.; Zhao, H.; Guo, W.; Yu, J.; Yuan, Z.; Qi, S.; Huang, Y. Clinicopathological characteristics and prognosis analysis of Aggressive angiomyxoma: A Retrospective Study. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Siddiqui, S.F. Rare case of metastatic aggressive angiomyxoma-first case of renal metastasis. Gynecol. Obstet. Case Rep. 2020, 6, 27. [Google Scholar]
- Goyal, L.D.; Garg, P.; Badyal, R.; Bhalla, S. Aggressive (deep) angiomyxoma of the vulva: A case report. J. Med. Case Rep. 2022, 16, 71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).