Simple Summary
Patients with head and neck cancer undergoing curative treatment with radiation therapy and chemotherapy often experience sleep disturbances that can affect pain and quality of life. We prospectively studied this patient population using sleep symptom questionnaires to assess the impact of sleep disturbances on patient-reported outcomes. We found that at baseline, 39% of patients had subthreshold or greater insomnia and 54% screened positive for OSA. Upon completion of radiation therapy, patients with these sleep disturbances had worse patient-reported quality of life and oral mucositis pain. These findings highlight the importance of managing sleep disturbances as a potential method to improve patient-reported outcomes for patients undergoing radiation therapy for head and neck cancer.
Abstract
Background: Patients with head and neck cancer (HNC) undergoing radiation therapy (RT) often experience sleep disturbances that may contribute to oral mucositis (OM) and quality of life (QOL). Methods: Patients with HNC treated with RT at a single institution were examined. Sleep questionnaires were given on the first day of RT to assess for insomnia and obstructive sleep apnea (OSA). Patient-reported QOL and oral mucositis were assessed during RT. Associations between insomnia and OSA with QOL were assessed using the Mann–Whitney U test. Linear mixed models assessed associations with OM. Results: Among 87 patients, 34 patients (39%) had subthreshold or greater insomnia and 47 patients (54%) screened positive for OSA. Upon RT completion, patients with subthreshold or greater insomnia had worse physical function (p = 0.005), fatigue (p = 0.01), insomnia (p < 0.001), and sticky saliva (p = 0.002). Patients screening positive for OSA had worse physical function (p = 0.01), sticky saliva (p = 0.02), fatigue (p = 0.007), insomnia (p = 0.009), and pain (p = 0.005). Upon linear mixed model evaluation, subthreshold or greater insomnia (p = 0.01) and positive OSA screen (p = 0.002) were associated with worse OM. Conclusion: Insomnia and OSA are highly prevalent in patients with HNC undergoing RT. These sleep disturbances are associated with worse QOL and OM during treatment.
1. Introduction
Oral mucositis (OM) is a painful, dose-limiting side effect of cancer treatment, occurring in >80% of head and neck cancer (HNC) patients who receive radiation therapy (RT) alone or concurrent chemoradiation therapy (CRT) as treatment [1]. These symptoms often disrupt therapy, increasing the risk of hospitalization and mortality [2].
It has previously been shown that the daily time of radiation treatment significantly impacted OM in HNC patients treated with RT [3]. In a follow-up study, genes relevant to OM were found to have diurnal variation in mRNA expression [4]. The involvement of the circadian rhythm with the development of OM led to our interest in the relationship between sleep and OM pain.
While a reciprocal relationship between sleep and pain has been reported in the general population [5], the relationship between sleep and OM pain in cancer patients has yet to be studied. To explore this knowledge gap, we examined the associations of sleep disturbances with RT-induced OM pain and quality of life (QOL) in HNC patients.
2. Materials and Methods
2.1. Patient Cohort
The cohort of patients included in the study was derived from 87 patients who were treated with definitive or adjuvant (C)RT for primary HNC at a single institution from August 2018 to October 2021. Patients included in this study had to meet the following eligibility: (1) primary HNC with pathologic confirmation, (2) definitive or adjuvant intent RT treatment received, (3) completed RT, and (4) had sleep questionnaire, quality of life, and oral mucositis data available. Data were collected under a protocol (EDR 103707) with approval by the Roswell Park Comprehensive Cancer Center Institutional Review Board. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations were followed.
2.2. Clinical Workup and Patient Treatment
All patients completed staging workup with neck and maxillofacial computed tomography (CT) imaging with contrast and/or positron emission tomography-computed tomography (PET/CT). Radiation therapy was delivered to all patients with intensity modulated radiation therapy (IMRT; 60–70 Gy/30–35 fractions to the primary tumor, 54–56 Gy/30–35 fractions to elective lymph nodes), with or without concurrent chemotherapy, as previously described [6]. Concurrent chemotherapy was given as cisplatin 100 mg/m2 intravenously infused once every 3 weeks or cisplatin 40 mg/m2 given once weekly. Chemotherapy was initiated on the first day of RT. Relevant clinicopathologic data were abstracted from the electronic medical record and kept securely in a Research Electronic Data Capture (REDCap) database [7,8]. Further information regarding our standard institutional management of OM has been described previously [9,10].
2.3. Symptom Assessment
Weekly patient evaluations were performed for all patients while undergoing RT through physical exams and patient-reported responses to the Oral Mucositis Weekly Questionnaire-Head and Neck Cancer (OMWQ-HN) survey [11]. On the first day and last day of RT patient-reported quality of life (QOL) was assessed with the European Organization for Research and Treatment of Cancer Quality of Life questionnaire C30 and HNC-specific module (EORTC-QLQ-C30 and H&N35) [12,13]. The EORTC-QLQ-C30 is a 30-item questionnaire composed of multi-item scales and single items that reflect the multidimensionality of the quality-of-life construct [13]. It incorporates five functional scales (physical, role, cognitive, emotional, and social), three symptom scales (fatigue, pain, and nausea and vomiting), a global health and quality of life scale, as well as single items assessing additional symptoms commonly reported by cancer patients (dyspnea, appetite loss, sleep disturbance, constipation, and diarrhea). Sleep disturbance was assessed using one question: “During the past week, have you had trouble sleeping?” with the responses “not at all”, “a little”, “quite a bit”, and “very much”. The EORTC H&N35 includes symptoms of oral pain (4 items), swallowing problems (4 items), sense problems (2 items), speech problems (3 items), trouble with social eating (4 items), trouble with social contact (5 items), and less sexual interest and enjoyment (2 items). Also included are Likert scale single items measuring teeth problems, mouth opening problems, dry mouth, sticky saliva, coughing, and feeling ill. Each symptom scale and Likert scale single item was converted into a score ranging from 0 to 100, according to the EORTC guidelines. A higher score indicates a higher level of symptoms or problems, and a score difference of 10 or greater is considered clinically significant [14]. Figures based on the survey data were generated using Microsoft® Excel® for Microsoft 365 MSO (Version 2205 Build 16.0.15225.20362).
2.4. Sleep Questionnaires
Two sleep questionnaires were given on the first day of RT. The Insomnia Severity Index (ISI) is a valid and reliable 7-item questionnaire that is commonly used in clinic settings and research to rate current insomnia severity. ISI scores range from absence of insomnia (0–7), to subthreshold (8–14), moderate (15–21), and severe insomnia (22–28) [15]. The ISI has also been validated in cancer patients [16].
The Sleep Disorders Symptom Checklist-25 (SDS-CL-25) is a 25-item questionnaire designed for screening multiple sleep disorders including insomnia and obstructive sleep apnea (OSA) [17]. Each question is scored as 0 points for a response of “never”, 1 point for “once a month”, 2 points for “1–3 times a week”, 3 points for “3–5 times a week”, and 4 points for “>5 times a week”. A disorder may be deemed appropriate for follow-up and considered a positive screen if one or more endorsements with a category have response option 3 or 4 checked. The severity of each sleep disorder can be obtained by summing the individual item scores for each category and dividing by the number of items to yield an average score per disorder (maximum range 0–4). Cumulative sleep morbidity was calculated as the sum of all of the endorsements across the 25 items (maximum range 0–100).
2.5. Statistical Analysis
Descriptive statistics were calculated for patient characteristics, treatment information, and sleep assessments. ISI score was examined as both a continuous measure and categorically. The SDS-CL-25 was examined categorically for OSA screening and continuously for OSA severity and cumulative morbidity scores. Associations between baseline subthreshold or greater insomnia and OSA screen status with selected QOL measures on the first and last day of RT were assessed using the Mann–Whitney U test. Differences between patient cohorts based on insomnia and OSA status were assessed with Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables.
To evaluate the impact of baseline insomnia and OSA on OM, the associations between ISI and OSA groupings and longitudinally measured OM outcomes were investigated using linear mixed models (LMMs) with random time effects. The LMMs were fit by restricted maximum likelihood (REML). The statistical significance of each grouping and the time-fixed effects were determined by t-tests using Satterthwaite’s method.
Logistic univariate and multivariate analyses were performed to identify sleep survey factors associated with severe OM development (“quite a lot” or “extreme” reported for the OMWQ-HN mouth and throat soreness item). Patients were grouped in the severe OM category based on their highest reported OM score during RT. Univariate logistic regression was employed to assess for associations between clinicopathologic variables and severe OM. Variables with p-values < 0.25 on univariate analysis were then incorporated into the logistic multivariate regression model. Adjusted odds ratios (aOR) and 95% confidence intervals (CI) were calculated for severe OM and each of the outcomes of interest.
To examine the performance of a single sleep item asking about trouble sleeping from the EORTC-QLQ-C30 in terms of categorizing the severity of sleep problems, we calculated percent agreement, Cohen’s kappa in comparison with ISI categories, and Fisher’s exact test. Two-sided p < 0.05 was considered statistically significant. All analyses were performed using R (version 4.2.1, R Project for Statistical Computing, Vienna, Austria). Data analysis was performed from May 2023 to June 2023.
3. Results
A total of 87 patients were included in this study. The median age was 64.6 (IQR, 57.7–70.4) and 70 patients were male (80%). Most patients had tumors of the oropharynx (51%) and received concurrent chemotherapy with radiation (83%). The majority of patients were former smokers (54%) with an ECOG performance status of 0–1 (83%). Table 1 contains complete patient demographic and treatment information.

Table 1.
Patient demographic and treatment information.
3.1. Insomnia and Obstructive Sleep Apnea
Patient sleep outcomes are shown in Table 2. The median ISI score was 6.0 (IQR, 2.0–11.5), with 53 patients (61%) categorized as having no insomnia, 20 patients (23%) with subthreshold insomnia, 11 patients (13%) with moderate insomnia, and 3 patients (3%) with severe insomnia. There were 47 patients (54%) who screened positive for OSA with a median OSA severity score of 0.8 (IQR, 0.3–1.6). Only eight patients had a documented medical history of OSA, of whom seven were included among those who screened positive for OSA. Eight patients reported daily continuous positive airway pressure (CPAP) device use on the SDS-CL-25 survey, with three of these patients overlapping with those who had a documented OSA diagnosis. The median cumulative sleep morbidity score was 15.0 (IQR, 10.0–29.5).

Table 2.
Selected patient sleep questionnaire results.
3.2. Quality of Life
Patients who screened positive for OSA had statistically significantly worse xerostomia (p < 0.001), fatigue (p < 0.001), pain (p = 0.01), and insomnia (p = 0.003) at the start of RT (Table 3) compared to patients that screened negative for OSA. Upon completion of RT, those screening positive for OSA had worse physical function (p = 0.01), sticky saliva (p = 0.02), fatigue (p = 0.007), insomnia (p = 0.009), and pain (p = 0.005) compared to those screening negative for OSA. There were no differences in patient demographics or treatment between those who screened positive and those who screened negative for OSA (Supplementary Table S1). Among the eight patients reporting CPAP use, mean xerostomia scores were lower at the start and end of radiation compared to those screening positive for OSA without CPAP use (scores of 25 and 46, respectively).

Table 3.
Associations between screening positive for obstructive sleep apnea (OSA) and insomnia with selected quality of life (QOL) metrics in patients with head and neck cancer at the start and completion of radiation treatment (RT), which were assessed using the Mann–Whitney U test.
3.3. Oral Mucositis
A total of 51 patients (59%) developed severe OM during treatment. Average weekly mouth and throat soreness scores during RT grouped by subthreshold or greater insomnia score on the ISI and by OSA screen status are shown in Figure 1. Upon linear mixed model evaluation, both subthreshold or greater insomnia (p = 0.01) and positive OSA screen (p = 0.002) were associated with higher mouth and throat soreness scores compared to those without insomnia or negative OSA screen, respectively. Univariate Cox regression identified several factors that met the threshold for inclusion in the multivariate model, which comprised sex, primary tumor site, clinical stage, HPV status, and smoking status (Supplementary Table S3). Upon logistic MVA (Table 4), the development of severe OM was significantly associated with ISI subthreshold or greater insomnia (aOR 3.36 [1.07–11.55], p = 0.04) and trended towards significance for OSA severity score (aOR 1.87 [1.05–3.74], p = 0.05) and cumulative sleep morbidity score (aOR 1.04 [1.00–1.09], p = 0.05). Positive screen for OSA and total ISI score were not associated with the development of severe OM.
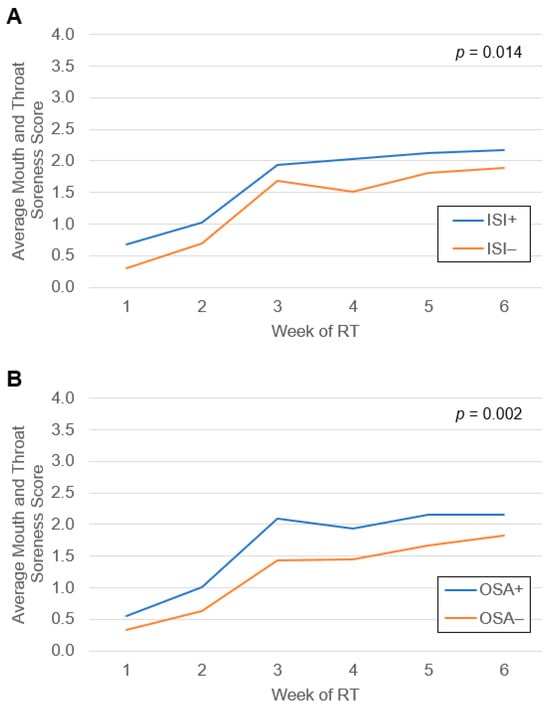
Figure 1.
Average mouth and throat soreness scores from patient-reported responses to the Oral Mucositis Weekly Questionnaire-Head and Neck Cancer survey during radiation therapy for head and neck cancer grouped by (A) baseline subthreshold or greater insomnia score on the Insomnia Severity Index (ISI) and (B) baseline obstructive sleep apnea (OSA) screen status. Differences between groups were assessed by linear mixed models.

Table 4.
Logistic multivariable analysis for clinical characteristics associated with the development of severe oral mucositis.
3.4. Agreement of Sleep Assessment between EORTC-QLQ-C30 and ISI
Trouble sleeping at baseline assessed via the EORTC questionnaire was associated with trouble sleeping at baseline assessed via ISI questionnaires (p < 0.001; Table 5). Patients who reported quite a bit or very much trouble sleeping were more likely to report more severe insomnia. The percent agreement between categories of the two sleep assessments was 49% and the Cohen’s kappa was 0.47, indicating moderate agreement.

Table 5.
Agreement between baseline ISI clinical insomnia categories and the trouble sleeping item from the EORTC QLQ-C30.
4. Discussion
This investigation of sleep disturbances and symptom burden in patients with HNC undergoing RT or CRT yielded several novel findings. First, both baseline subthreshold or greater insomnia scores on the Insomnia Severity Index (ISI) and baseline obstructive sleep apnea (OSA) screen status are common and significantly impacted the average patient-reported mouth and throat soreness scores during all weeks of radiation therapy. Second, both baseline insomnia and positive OSA screen predicted significantly worse QOL across multiple domains at the beginning and end of treatment. Third, baseline ISI was significantly associated development of severe oral mucositis. Fourth, the EORTC trouble sleeping question was significantly correlated with ISI. These findings underscore the importance of sleep evaluation for this patient population to target sleep improvement as a method of mitigating RT symptom burden.
Baseline insomnia was identified in 39% of patients using the ISI survey, which is similar to what has been previously reported for patients with HNC. In a systematic review of sleep disturbances in HNC patients, Santoso et al. have shown the prevalence of insomnia to be 29% before treatment and 45% during treatment [18]. This study also noted baseline hypersomnolence and sleep-related breathing disturbances were prevalent in this population, with rates of 16% and 66%, respectively [18]. Notably, our study found no difference in EORTC insomnia score from baseline to end of RT for the entire cohort (35.2 and 33.7, respectively). Another study has demonstrated 44% of HNC patients have poor sleep quality before treatment, with female sex identified as an associated factor which was similarly found in our study [19].
OSA screen was positive in 54% of patients. Consistent with our findings, a systematic review of OSA in HNC patients has shown the baseline incidence of OSA in this population to be 60% [20]. The incidence of poor sleep quality in the general population is 27–36%, suggesting that HNC patients may be at higher risk for insomnia [21,22]. These findings highlight the high prevalence of sleep disturbances in HNC patients. Given this prevalence of and dramatic association of baseline sleep disturbances with OM and QOL outcomes, clinical trials of HNC patients treated with RT should consider documenting sleep measures.
Remarkably, ISI subthreshold or greater insomnia patients had worse QOL across all domains at the start of RT. This finding persisted for most patients upon treatment completion. While the data are conflicting, both female sex [23,24,25] and smoking [26,27,28] have been implicated in worse OM. For patients screening positive for OSA, worse QOL was seen at treatment start and completion for fatigue, pain, and insomnia. This is consistent with a previous study that showed patients with OSA have a reduction in QOL proportional to severity, including the domains of bodily pain, vitality, and physical functioning [29].
Interventions to improve sleep quality in HNC patients may have the potential to delay the onset of OM pain. Sleep deprivation has the potential to elicit hyperalgesic changes that can increase pain sensitivity and vulnerability to pain, causing a vicious cycle whereby worsening pain makes sleep more difficult [30]. Supporting this, a large population-based study has shown that the frequency and severity of insomnia are associated with pain sensitivity in a dose-response manner [31].
Though over half of the patients screened positive for OSA, fewer than 10 percent were actually being treated with continuous positive airway pressure (CPAP). Exploratory analysis of the patients using CPAP found that they had lower xerostomia scores than those screening positive for OSA. Adherence to CPAP therapy in patients with OSA has been linked to improved QOL and patient symptoms including dry mouth upon awakening, excessive fatigue, and decreased energy [32,33]. Treatment of OSA via CPAP with humidification may have the additional benefit for patients with HNC undergoing RT of directly benefitting mucositis by improving mucosal hydration [34].
There was significant agreement for trouble sleeping at baseline assessed via the EORTC and ISI questionnaires. Patients who reported quite a bit or very much trouble sleeping were more likely to report clinically significant insomnia with moderate agreement. These findings indicate that the EORTC question is an excellent screening question to determine if the ISI should be administered. Omitting the ISI in patients who answered “Not at all” to the EORTC sleeping item would have failed to detect only 3 out of 34 patients with subclinical or greater insomnia, with these 3 missed patients having subclinical insomnia. To minimize the survey burden in clinics or clinical trials, the EORTC item could be used to screen patients to receive the full ISI. The ISI is well correlated with a similar single item from the Patient Health Questionnaire-9 survey [35].
The strengths of this study include the use of validated sleep questionnaires (ISI and SDS-CL-25), longitudinal collection of QOL and oral pain data, comprehensive adjustment of potential confounding factors, and being the first to examine the relationship between sleep and OM pain in HNC patients undergoing RT. This study is limited by sample size and lack of objective sleep data (through actigraphy or formal sleep monitoring) to analyze alongside the patient-reported data.
5. Conclusions
Insomnia and OSA are highly prevalent in patients with HNC undergoing RT. These sleep disturbances are associated with worse QOL and OM during treatment. Documentation and treatment of baseline insomnia and OSA should be considered in clinical trials of OM in HNC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16071335/s1, Table S1: Patient characteristics of patients screening positive and negative for OSA, Table S2: Patient characteristics of those scored as having subthreshold or greater insomnia on the Insomnia Severity Index at the start of RT, Table S3: Univariate Cox regression of clinicopathologic factors associated with the development of severe oral mucositis.
Author Contributions
A.J.I.: Data Curation, Investigation, Formal Analysis, Writing—Original Draft. K.S.: Data Curation. H.Y.: Formal Analysis, Writing—Review and Editing. M.A.K.: Writing—Review and Editing. C.R.J.: Writing—Review and Editing. A.D.R.: Writing—Review and Editing. M.K.F.: Writing—Review and Editing. F.G.: Conceptualization, Formal Analysis, Writing—Review and Editing. A.K.S.: Conceptualization, Validation, Funding Acquisition, Supervision, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Cancer Institute Cancer Center Support Grant (P30CA016056). The funding sources had no role in the preparation of this manuscript.
Institutional Review Board Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Roswell Park Comprehensive Cancer Center (EDR 103707).
Informed Consent Statement
Patient consent was waived due to this study being a single-institution analysis of a de-identified patient database.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Conflicts of Interest
Author Fangyi Gu was employed by the company Bristol Myers Squibb. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Vera-Llonch, M.; Oster, G.; Hagiwara, M.; Sonis, S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer 2006, 106, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Trotti, A.; Bellm, L.A.; Epstein, J.B.; Frame, D.; Fuchs, H.J.; Gwede, C.K.; Komaroff, E.; Nalysnyk, L.; Zilberberg, M.D. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother. Oncol. 2003, 66, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Farrugia, M.K.; Duncan, W.D.; Feng, Y.; Hutson, A.D.; Schlecht, N.F.; Repasky, E.A.; Antoch, M.P.; Miller, A.; Platek, A.; et al. Daily Time of Radiation Treatment Is Associated with Subsequent Oral Mucositis Severity during Radiotherapy in Head and Neck Cancer Patients. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Gomez, E.C.; Chen, J.; Buas, M.F.; Schlecht, N.F.; Hulme, K.; Kulkarni, S.V.; Singh, P.K.; O’Connor, R.; Ambrosone, C.B.; et al. Genes Relevant to Tissue Response to Cancer Therapy Display Diurnal Variation in mRNA Expression in Human Oral Mucosa. J. Circadian Rhythms 2021, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.R.; Almeida, D.M.; Klick, B.; Haythornthwaite, J.A.; Smith, M.T. Duration of sleep contributes to next-day pain report in the general population. Pain® 2008, 137, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Fung-Kee-Fung, S.D.; Hackett, R.; Hales, L.; Warren, G.; Singh, A.K. A prospective trial of volumetric intensity-modulated arc therapy vs conventional intensity modulated radiation therapy in advanced head and neck cancer. World J. Clin. Oncol. 2012, 3, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Judge, L.F.; Farrugia, M.K.; Singh, A.K. Narrative review of the management of oral mucositis during chemoradiation for head and neck cancer. Ann. Transl. Med. 2021, 9, 916. [Google Scholar] [CrossRef]
- Iovoli, A.J.; Turecki, L.; Qiu, M.L.; Khan, M.; Smith, K.; Yu, H.; Ma, S.J.; Farrugia, M.K.; Singh, A.K. Severe Oral Mucositis After Intensity-Modulated Radiation Therapy for Head and Neck Cancer. JAMA Netw. Open 2023, 6, e2337265. [Google Scholar] [CrossRef]
- Epstein, J.B.; Beaumont, J.L.; Gwede, C.K.; Murphy, B.; Garden, A.S.; Meredith, R.; Le, Q.T.; Brizel, D.; Isitt, J.; Cella, D. Longitudinal evaluation of the oral mucositis weekly questionnaire-head and neck cancer, a patient-reported outcomes questionnaire. Cancer 2007, 109, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Bjordal, K.; de Graeff, A.; Fayers, P.M.; Hammerlid, E.; van Pottelsberghe, C.; Curran, D.; Ahlner-Elmqvist, M.; Maher, E.J.; Meyza, J.W.; Bredart, A.; et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. EORTC Quality of Life Group. Eur. J. Cancer 2000, 36, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.F.; Blackford, A.L.; Sussman, J.; Bainbridge, D.; Howell, D.; Seow, H.Y.; Carducci, M.A.; Wu, A.W. Identifying changes in scores on the EORTC-QLQ-C30 representing a change in patients’ supportive care needs. Qual. Life Res. 2015, 24, 1207–1216. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallieres, A.; Morin, C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Savard, M.H.; Savard, J.; Simard, S.; Ivers, H. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology 2005, 14, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Klingman, K.J.; Jungquist, C.R.; Perlis, M.L. Questionnaires that screen for multiple sleep disorders. Sleep Med. Rev. 2017, 32, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Santoso, A.M.; Jansen, F.; de Vries, R.; Leemans, C.R.; van Straten, A.; Verdonck-de Leeuw, I.M. Prevalence of sleep disturbances among head and neck cancer patients: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 47, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Santoso, A.M.M.; Jansen, F.; Lissenberg-Witte, B.I.; Baatenburg de Jong, R.J.; Langendijk, J.A.; Leemans, C.R.; Smit, J.H.; Takes, R.P.; Terhaard, C.H.J.; van Straten, A.; et al. Poor sleep quality among newly diagnosed head and neck cancer patients: Prevalence and associated factors. Support. Care Cancer 2021, 29, 1035–1045. [Google Scholar] [CrossRef]
- Ralli, M.; Campo, F.; Angeletti, D.; Allegra, E.; Minni, A.; Polimeni, A.; Greco, A.; de Vincentiis, M. Obstructive Sleep Apnoea in Patients Treated for Head and Neck Cancer: A Systematic Review of the Literature. Medicina 2020, 56, 399. [Google Scholar] [CrossRef]
- Hinz, A.; Glaesmer, H.; Brahler, E.; Loffler, M.; Engel, C.; Enzenbach, C.; Hegerl, U.; Sander, C. Sleep quality in the general population: Psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Med. 2017, 30, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liao, Y.; Kelly, B.C.; Xie, L.; Xiang, Y.T.; Qi, C.; Pan, C.; Hao, W.; Liu, T.; Zhang, F.; et al. Gender and Regional Differences in Sleep Quality and Insomnia: A General Population-based Study in Hunan Province of China. Sci. Rep. 2017, 7, 43690. [Google Scholar] [CrossRef] [PubMed]
- Gebri, E.; Kiss, A.; Toth, F.; Hortobagyi, T. Female sex as an independent prognostic factor in the development of oral mucositis during autologous peripheral stem cell transplantation. Sci. Rep. 2020, 10, 15898. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B.; et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Vokurka, S.; Bystricka, E.; Koza, V.; Scudlova, J.; Pavlicova, V.; Valentova, D.; Visokaiova, M.; Misaniova, L. Higher incidence of chemotherapy induced oral mucositis in females: A supplement of multivariate analysis to a randomized multicentre study. Support. Care Cancer 2006, 14, 974–976. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Gao, J.; Qian, L.; Huang, Y.; Zhou, Y.; Yang, L.; He, J.; Yang, J.; Wang, R.; Zhang, Y. Factors associated with acute oral mucosal reaction induced by radiotherapy in head and neck squamous cell carcinoma: A retrospective single-center experience. Medicine 2017, 96, e8446. [Google Scholar] [CrossRef] [PubMed]
- Vatca, M.; Lucas, J.T., Jr.; Laudadio, J.; D’Agostino, R.B.; Waltonen, J.D.; Sullivan, C.A.; Rouchard-Plasser, R.; Matsangou, M.; Browne, J.D.; Greven, K.M.; et al. Retrospective analysis of the impact of HPV status and smoking on mucositis in patients with oropharyngeal squamous cell carcinoma treated with concurrent chemotherapy and radiotherapy. Oral Oncol. 2014, 50, 869–876. [Google Scholar] [CrossRef]
- Wuketich, S.; Hienz, S.A.; Marosi, C. Prevalence of clinically relevant oral mucositis in outpatients receiving myelosuppressive chemotherapy for solid tumors. Support. Care Cancer 2012, 20, 175–183. [Google Scholar] [CrossRef]
- Lopes, C.; Esteves, A.M.; Bittencourt, L.R.; Tufik, S.; Mello, M.T. Relationship between the quality of life and the severity of obstructive sleep apnea syndrome. Braz. J. Med. Biol. Res. 2008, 41, 908–913. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Kundermann, B.; Krieg, J.C. Sleep deprivation and pain perception. Sleep Med. Rev. 2006, 10, 357–369. [Google Scholar] [CrossRef]
- Sivertsen, B.; Lallukka, T.; Petrie, K.J.; Steingrimsdottir, O.A.; Stubhaug, A.; Nielsen, C.S. Sleep and pain sensitivity in adults. Pain 2015, 156, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Avlonitou, E.; Kapsimalis, F.; Varouchakis, G.; Vardavas, C.I.; Behrakis, P. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath 2012, 16, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Coman, A.C.; Borzan, C.; Vesa, C.S.; Todea, D.A. Obstructive sleep apnea syndrome and the quality of life. Clujul Med. 2016, 89, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Macann, A.; Fauzi, F.; Simpson, J.; Sasso, G.; Krawitz, H.; Fraser-Browne, C.; Manitz, J.; Raith, A. Humidification mitigates acute mucosal toxicity during radiotherapy when factoring volumetric parameters. Trans Tasman Radiation Oncology Group (TROG) RadioHUM 07.03 substudy. Oral Oncol. 2017, 75, 75–80. [Google Scholar] [CrossRef]
- Schulte, T.; Hofmeister, D.; Mehnert-Theuerkauf, A.; Hartung, T.; Hinz, A. Assessment of sleep problems with the Insomnia Severity Index (ISI) and the sleep item of the Patient Health Questionnaire (PHQ-9) in cancer patients. Support. Care Cancer 2021, 29, 7377–7384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).