The Role of Natural Killer Cells in the Tumor Immune Microenvironment of EBV-Associated Nasopharyngeal Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. EBV-Associated NPC and NK Cells
2.1. EBV-Associated NPC
2.2. NK Cells in NPC
3. NK Subpopulations in NPC
3.1. Surface Markers of NK Cells
3.2. NK Cells in Peripheral Blood of NPC Patients
3.3. NK Cells in the TIME of EBV+ NPC
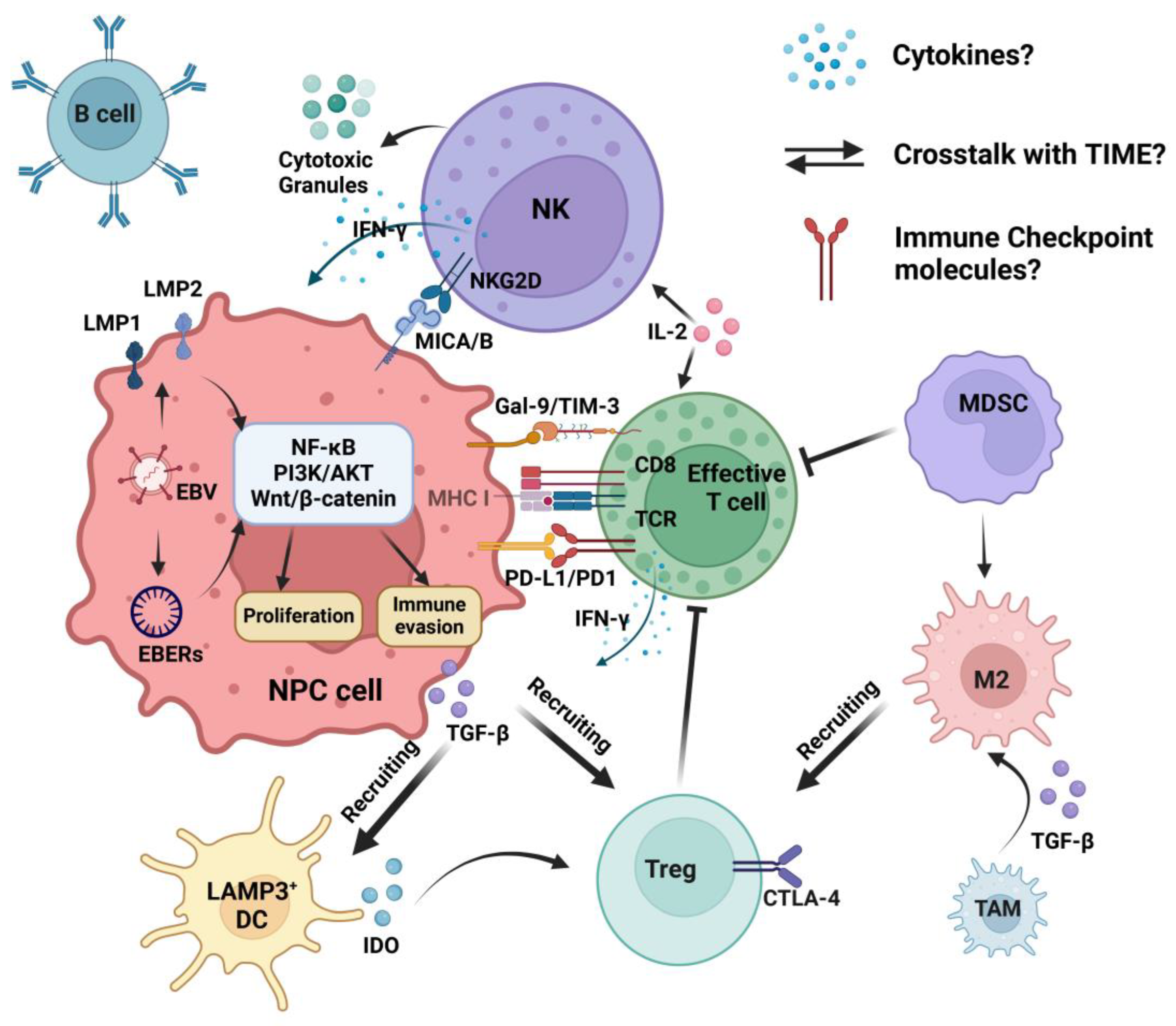
4. The Crosstalk of NK Cells in the TIME
4.1. Activating and Inhibitory Signals of NK Cells
4.2. Cytokines/Chemokines and Immune Cells
4.3. Immune Checkpoint Molecules
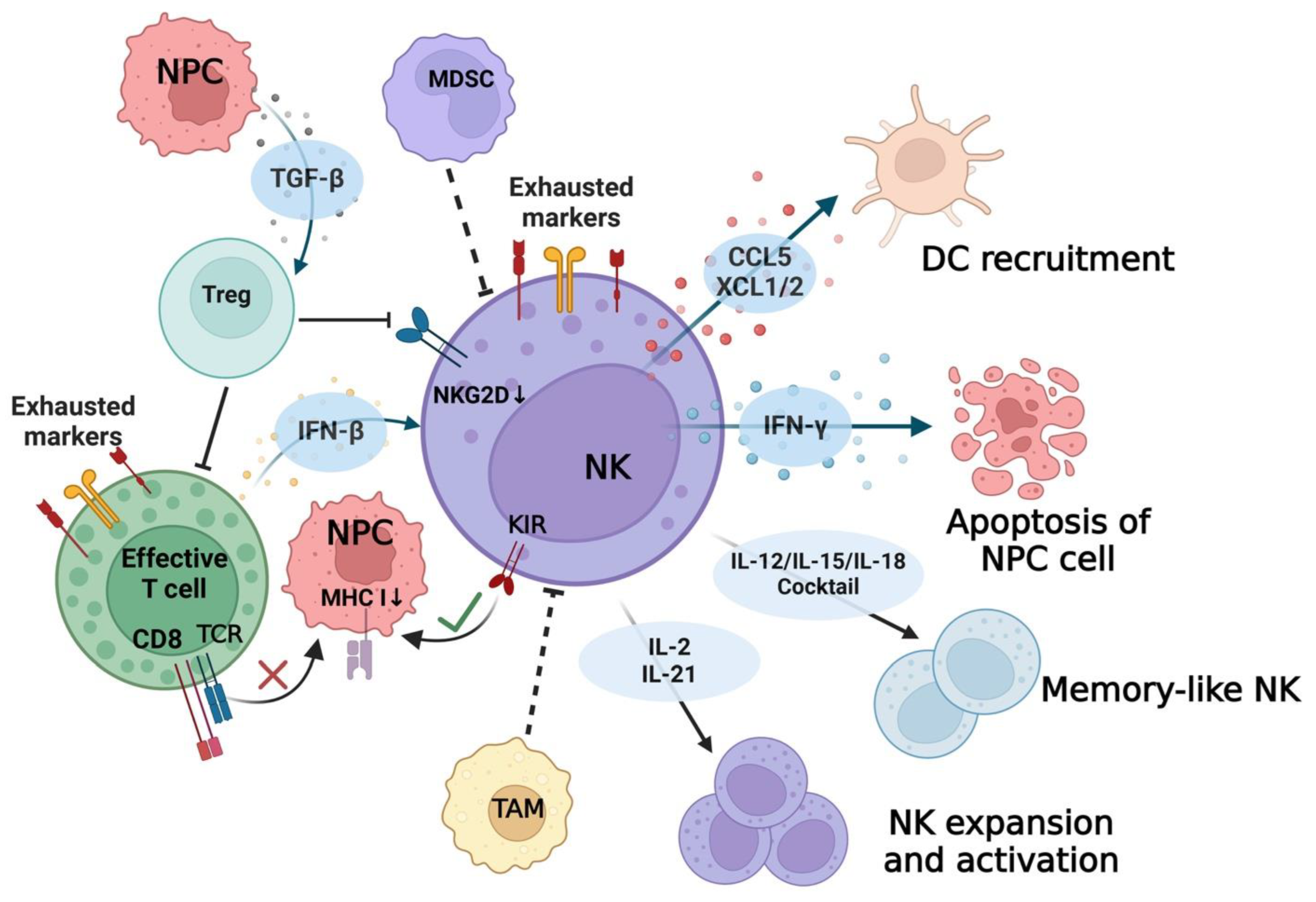
5. NK Cell Cytotoxicity
5.1. NK Cells against EBV Infection
5.2. NK Cells against EBV+ NPC
6. NK Cells in Cancer Therapy
6.1. Pre-Activated/Genetically Modified Adoptive NK Cells
6.2. Adoptive NK Cells Combined with Systemic/Conventional Therapies
6.3. NK Cell Agonists
6.4. NK Cell Engager
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Xia, C.; Yu, X.Q.; Zheng, R.; Zhang, S.; Zeng, H.; Wang, J.; Liao, Y.; Zou, X.; Zuo, T.; Yang, Z.; et al. Spatial and temporal patterns of nasopharyngeal carcinoma mortality in China, 1973–2005. Cancer Lett. 2017, 401, 33–38. [Google Scholar] [CrossRef]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Lo, K.W.; To, K.F.; Huang, D.P. Focus on nasopharyngeal carcinoma. Cancer Cell 2004, 5, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.; Lai, S.Z.; Li, W.F.; Hu, W.H.; Sun, R.; Liu, L.Z.; Zhang, F.; Peng, H.; Du, X.J.; et al. 10-Year Results of Therapeutic Ratio by Intensity-Modulated Radiotherapy versus Two-Dimensional Radiotherapy in Patients with Nasopharyngeal Carcinoma. Oncologist 2019, 24, e38–e45. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yao, Y.; Deng, X.; Huang, Z.; Chen, Y.; Wang, Z.; Hong, H.; Huang, H.; Lin, T. Immunotherapy for nasopharyngeal carcinoma: Current status and prospects (Review). Int. J. Oncol. 2023, 63, 97. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Tsao, S.W.; Tsang, C.M.; Lo, K.W. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372, 20160270. [Google Scholar] [CrossRef]
- Shi, W.; Fijardo, M.; Bruce, J.P.; Su, J.; Xu, W.; Bell, R.; Bissey, P.A.; Hui, A.B.Y.; Waldron, J.; Pugh, T.J.; et al. CD8+ Tumor-Infiltrating Lymphocyte Abundance Is a Positive Prognostic Indicator in Nasopharyngeal Cancer. Clin. Cancer Res. 2022, 28, 5202–5210. [Google Scholar] [CrossRef]
- Peng, W.S.; Zhou, X.; Yan, W.B.; Li, Y.J.; Du, C.R.; Wang, X.S.; Shen, C.Y.; Wang, Q.F.; Ying, H.M.; Lu, X.G.; et al. Dissecting the heterogeneity of the microenvironment in primary and recurrent nasopharyngeal carcinomas using single-cell RNA sequencing. Oncoimmunology 2022, 11, 2026583. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; den Boon, J.A.; Chen, I.H.; Newton, M.A.; Dahl, D.B.; Chen, M.; Cheng, Y.J.; Westra, W.H.; Chen, C.J.; Hildesheim, A.; et al. Genome-wide expression profiling reveals EBV-associated inhibition of MHC class I expression in nasopharyngeal carcinoma. Cancer Res. 2006, 66, 7999–8006. [Google Scholar] [CrossRef]
- Raulet, D.H.; Marcus, A.; Coscoy, L. Dysregulated cellular functions and cell stress pathways provide critical cues for activating and targeting natural killer cells to transformed and infected cells. Immunol. Rev. 2017, 280, 93–101. [Google Scholar] [CrossRef]
- Cerwenka, A.; Lanier, L.L. Natural killer cells, viruses and cancer. Nat. Rev. Immunol. 2001, 1, 41–49. [Google Scholar] [CrossRef]
- Orange, J.S. Human natural killer cell deficiencies. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 399–409. [Google Scholar] [CrossRef]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e1014. [Google Scholar] [CrossRef] [PubMed]
- Jha, H.C.; Pei, Y.; Robertson, E.S. Epstein-Barr Virus: Diseases Linked to Infection and Transformation. Front. Microbiol. 2016, 7, 1602. [Google Scholar] [CrossRef] [PubMed]
- Zanella, M.C.; Cordey, S.; Kaiser, L. Beyond Cytomegalovirus and Epstein-Barr Virus: A Review of Viruses Composing the Blood Virome of Solid Organ Transplant and Hematopoietic Stem Cell Transplant Recipients. Clin. Microbiol. Rev. 2020, 33, 10-1128. [Google Scholar] [CrossRef]
- Tsao, S.W.; Tsang, C.M.; To, K.F.; Lo, K.W. The role of Epstein-Barr virus in epithelial malignancies. J. Pathol. 2015, 235, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Yap, L.F.; Murray, P.G. Epstein-Barr virus: More than 50 years old and still providing surprises. Nat. Rev. Cancer 2016, 16, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S.; Rickinson, A.B. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 2004, 4, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Pathmanathan, R.; Prasad, U.; Sadler, R.; Flynn, K.; Raab-Traub, N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N. Engl. J. Med. 1995, 333, 693–698. [Google Scholar] [CrossRef]
- Chen, J.; Sathiyamoorthy, K.; Zhang, X.; Schaller, S.; Perez White, B.E.; Jardetzky, T.S.; Longnecker, R. Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. Nat. Microbiol. 2018, 3, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Wang, H.B.; Zhang, A.; Chen, M.L.; Fang, Z.X.; Dong, X.D.; Li, S.B.; Du, Y.; Xiong, D.; et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat. Microbiol. 2018, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; To, K.F.; Lo, K.W.; Ding, M.; Li, X.; Johnson, P.; Huang, D.P. Frequent chromosome 9p losses in histologically normal nasopharyngeal epithelia from southern Chinese. Int. J. Cancer 2002, 102, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; To, K.F.; Lo, K.W.; Mak, K.F.; Pak, W.; Chiu, B.; Tse, G.M.; Ding, M.; Li, X.; Lee, J.C.; et al. High frequency of chromosome 3p deletion in histologically normal nasopharyngeal epithelia from southern Chinese. Cancer Res. 2000, 60, 5365–5370. [Google Scholar] [PubMed]
- Ning, L.; Ko, J.M.; Yu, V.Z.; Ng, H.Y.; Chan, C.K.; Tao, L.; Lam, S.Y.; Leong, M.M.; Ngan, R.K.; Kwong, D.L.; et al. Nasopharyngeal carcinoma MHC region deep sequencing identifies HLA and novel non-HLA TRIM31 and TRIM39 loci. Commun. Biol. 2020, 3, 759. [Google Scholar] [CrossRef]
- Hildesheim, A.; Apple, R.J.; Chen, C.J.; Wang, S.S.; Cheng, Y.J.; Klitz, W.; Mack, S.J.; Chen, I.H.; Hsu, M.M.; Yang, C.S.; et al. Association of HLA class I and II alleles and extended haplotypes with nasopharyngeal carcinoma in Taiwan. J. Natl. Cancer Inst. 2002, 94, 1780–1789. [Google Scholar] [CrossRef]
- Ansari, M.A.; Singh, V.V.; Dutta, S.; Veettil, M.V.; Dutta, D.; Chikoti, L.; Lu, J.; Everly, D.; Chandran, B. Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J. Virol. 2013, 87, 8606–8623. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Della Vittoria Scarpati, G.; Giuliano, M.; D’Aniello, C.; Gnoni, A.; Cavaliere, C.; Licchetta, A.; Pisconti, S. Epstein-Barr virus infection and nasopharyngeal carcinoma: The other side of the coin. Anti-Cancer Drugs 2015, 26, 1017–1025. [Google Scholar] [CrossRef]
- Li, H.P.; Chang, Y.S. Epstein-Barr virus latent membrane protein 1: Structure and functions. J. Biomed. Sci. 2003, 10, 490–504. [Google Scholar] [CrossRef]
- Fukuda, M.; Longnecker, R. Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway. J. Virol. 2007, 81, 9299–9306. [Google Scholar] [CrossRef]
- Scholle, F.; Bendt, K.M.; Raab-Traub, N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J. Virol. 2000, 74, 10681–10689. [Google Scholar] [CrossRef]
- Morris, M.A.; Dawson, C.W.; Young, L.S. Role of the Epstein-Barr virus-encoded latent membrane protein-1, LMP1, in the pathogenesis of nasopharyngeal carcinoma. Future Oncol. 2009, 5, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.W.; Tramoutanis, G.; Dawson, C.W.; Lo, A.K.; Huang, D.P. The significance of LMP1 expression in nasopharyngeal carcinoma. Semin. Cancer Biol. 2002, 12, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhu, J.; Zhao, Q.; Ma, W.; Xiao, Y.; Xu, G.; Zhang, Z. LMP1 Up-regulates Calreticulin to Induce Epithelial-mesenchymal Transition via TGF-β/Smad3/NRP1 Pathway in Nasopharyngeal Carcinoma Cells. J. Cancer 2020, 11, 1257–1269. [Google Scholar] [CrossRef]
- Martel-Renoir, D.; Grunewald, V.; Touitou, R.; Schwaab, G.; Joab, I. Qualitative analysis of the expression of Epstein-Barr virus lytic genes in nasopharyngeal carcinoma biopsies. J. Gen. Virol. 1995, 76 Pt 6, 1401–1408. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, S.; Sun, R.; Wu, T.; Qian, J. Understanding the interplay between host immunity and Epstein-Barr virus in NPC patients. Emerg. Microbes Infect. 2015, 4, e20. [Google Scholar] [CrossRef]
- Ning, S. Innate immune modulation in EBV infection. Herpesviridae 2011, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Forder, A.; Stewart, G.L.; Telkar, N.; Lam, W.L.; Garnis, C. New insights into the tumour immune microenvironment of nasopharyngeal carcinoma. Curr. Res. Immunol. 2022, 3, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ooft, M.L.; Ipenburg, J.A.v.; Sanders, M.E.; Kranendonk, M.; Hofland, I.; Bree, R.d.; Koljenović, S.; Willems, S.M. Prognostic role of tumour-associated macrophages and regulatory T cells in EBV-positive and EBV-negative nasopharyngeal carcinoma. J. Clin. Pathol. 2018, 71, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Kwong, D.L.; Dai, W.; Wu, P.; Li, S.; Yan, Q.; Zhang, Y.; Zhang, B.; Fang, X.; Liu, L.; et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat. Commun. 2021, 12, 1540. [Google Scholar] [CrossRef] [PubMed]
- Azzi, T.; Lünemann, A.; Murer, A.; Ueda, S.; Béziat, V.; Malmberg, K.J.; Staubli, G.; Gysin, C.; Berger, C.; Münz, C.; et al. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood 2014, 124, 2533–2543. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Huang, J. CD8+ T Cells and NK Cells: Parallel and Complementary Soldiers of Immunotherapy. Curr. Opin. Chem. Eng. 2018, 19, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, H.; Dong, Y.; Wu, Q.; Jiang, N.; Luo, Q.; Chen, F. Distribution of CD8 T Cells and NK Cells in the Stroma in Relation to Recurrence or Metastasis of Nasopharyngeal Carcinoma. Cancer Manag. Res. 2022, 14, 2913–2926. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Yin, J.H.; Li, W.F.; Li, H.J.; Chen, D.P.; Zhang, C.J.; Lv, J.W.; Wang, Y.Q.; Li, X.M.; Li, J.Y.; et al. Single-cell transcriptomics reveals regulators underlying immune cell diversity and immune subtypes associated with prognosis in nasopharyngeal carcinoma. Cell Res. 2020, 30, 1024–1042. [Google Scholar] [CrossRef]
- Lu, J.; Chen, X.M.; Huang, H.R.; Zhao, F.P.; Wang, F.; Liu, X.; Li, X.P. Detailed analysis of inflammatory cell infiltration and the prognostic impact on nasopharyngeal carcinoma. Head Neck 2018, 40, 1245–1253. [Google Scholar] [CrossRef]
- Wolf, N.K.; Kissiov, D.U.; Raulet, D.H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 2023, 23, 90–105. [Google Scholar] [CrossRef]
- Dianat-Moghadam, H.; Rokni, M.; Marofi, F.; Panahi, Y.; Yousefi, M. Natural killer cell-based immunotherapy: From transplantation toward targeting cancer stem cells. J. Cell. Physiol. 2018, 234, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Rancan, C.; Ma, W.; Toth, M.; Senda, T.; Carpenter, D.J.; Kubota, M.; Matsumoto, R.; Thapa, P.; Szabo, P.A.; et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell 2020, 180, 749–763.e713. [Google Scholar] [CrossRef]
- Raulet, D.H.; Gasser, S.; Gowen, B.G.; Deng, W.; Jung, H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013, 31, 413–441. [Google Scholar] [CrossRef] [PubMed]
- Obiedat, A.; Charpak-Amikam, Y.; Tai-Schmiedel, J.; Seidel, E.; Mahameed, M.; Avril, T.; Stern-Ginossar, N.; Springuel, L.; Bolsée, J.; Gilham, D.E.; et al. The integrated stress response promotes B7H6 expression. J. Mol. Med. 2020, 98, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.S.; Bix, M.; Zijlstra, M.; Jaenisch, R.; Raulet, D. MHC class I deficiency: Susceptibility to natural killer (NK) cells and impaired NK activity. Science 1991, 253, 199–202. [Google Scholar] [CrossRef]
- Ljunggren, H.G.; Kärre, K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 1990, 11, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef]
- Angelo, L.S.; Banerjee, P.P.; Monaco-Shawver, L.; Rosen, J.B.; Makedonas, G.; Forbes, L.R.; Mace, E.M.; Orange, J.S. Practical NK cell phenotyping and variability in healthy adults. Immunol. Res. 2015, 62, 341–356. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, R.; Huang, C.; Zhang, M.; Li, J.; Zong, J.; Qiu, S.; Lin, S.; Chen, H.; Ye, Y.; et al. Analysis of the Expression of Surface Receptors on NK Cells and NKG2D on Immunocytes in Peripheral Blood of Patients with Nasopharyngeal Carcinoma. Asian Pac. J. Cancer Prev. 2018, 19, 661–665. [Google Scholar] [CrossRef]
- Zheng, Y.; Cao, K.Y.; Ng, S.P.; Chua, D.T.; Sham, J.S.; Kwong, D.L.; Ng, M.H.; Lu, L.; Zheng, B.J. Complementary activation of peripheral natural killer cell immunity in nasopharyngeal carcinoma. Cancer Sci. 2006, 97, 912–919. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Y.; Liang, Z.; Feng, H.; Xu, S.E. MACC1 facilitates the escape of nasopharyngeal carcinoma cells from killing by natural killer cells. Biotechnol. Biotechnol. Equip. 2019, 33, 579–588. [Google Scholar] [CrossRef]
- Jin, S.; Li, R.; Chen, M.Y.; Yu, C.; Tang, L.Q.; Liu, Y.M.; Li, J.P.; Liu, Y.N.; Luo, Y.L.; Zhao, Y.; et al. Single-cell transcriptomic analysis defines the interplay between tumor cells, viral infection, and the microenvironment in nasopharyngeal carcinoma. Cell Res. 2020, 30, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Lu, W.; Hu, S.; Zhao, K. Differentially Infiltrated Identification of Novel Diagnostic Biomarkers Associated with Immune Infiltration in Nasopharyngeal Carcinoma. Dis. Markers 2022, 2022, 3934704. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, C.; Pang, R.; Liu, H.; Yin, W.; Chen, J.; Tao, L. Comprehensive single-cell transcriptomic and proteomic analysis reveals NK cell exhaustion and unique tumor cell evolutionary trajectory in non-keratinizing nasopharyngeal carcinoma. J. Transl. Med. 2023, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Duan, X.; Deng, X.; Huang, Y.; Zhou, X.; Zhang, S.; Zhang, X.; Liu, P.; Yang, C.; Liu, G.; et al. EBV-Upregulated B7-H3 Inhibits NK cell-Mediated Antitumor Function and Contributes to Nasopharyngeal Carcinoma Progression. Cancer Immunol. Res. 2023, 11, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Banerjee, S. Downregulation of HLA-ABC expression through promoter hypermethylation and downmodulation of MIC-A/B surface expression in LMP2A-positive epithelial carcinoma cell lines. Sci. Rep. 2020, 10, 5415. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Tang, Y.; Wang, J.; Xiong, F.; Guo, C.; Wang, Y.; Xiang, B.; Zhou, M.; Li, X.; Wu, X.; et al. The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J. Cancer 2018, 9, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.S.; Chen, S.; Zhang, M.J.; Chan, J.Y.; Gao, W. Epstein-Barr virus-encoded microRNA BART7 downregulates major histocompatibility complex class I chain-related peptide A and reduces the cytotoxicity of natural killer cells to nasopharyngeal carcinoma. Oncol. Lett. 2018, 16, 2887–2892. [Google Scholar] [CrossRef]
- Wang, W.; Tian, W.; Zhu, F.; Li, L.; Cai, J.; Wang, F.; Liu, K.; Jin, H.; Wang, J. MICA Gene Deletion in 3411 DNA Samples from Five Distinct Populations in Mainland China and Lack of Association with Nasopharyngeal Carcinoma (NPC) in a Southern Chinese Han population. Ann. Hum. Genet. 2016, 80, 319–326. [Google Scholar] [CrossRef]
- Tse, K.P.; Su, W.H.; Yang, M.L.; Cheng, H.Y.; Tsang, N.M.; Chang, K.P.; Hao, S.P.; Yao Shugart, Y.; Chang, Y.S. A gender-specific association of CNV at 6p21.3 with NPC susceptibility. Hum. Mol. Genet. 2011, 20, 2889–2896. [Google Scholar] [CrossRef]
- Cao, W.; Xi, X.; Hao, Z.; Li, W.; Kong, Y.; Cui, L.; Ma, C.; Ba, D.; He, W. RAET1E2, a soluble isoform of the UL16-binding protein RAET1E produced by tumor cells, inhibits NKG2D-mediated NK cytotoxicity. J. Biol. Chem. 2007, 282, 18922–18928. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tian, W.; Wang, W.; Liu, K.; Wang, J.; Jin, H.; Cai, J.; Wang, J. NKG2C copy number variations in five distinct populations in mainland China and susceptibility to nasopharyngeal carcinoma (NPC). Hum. Immunol. 2015, 76, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Butsch Kovacic, M.; Martin, M.; Gao, X.; Fuksenko, T.; Chen, C.J.; Cheng, Y.J.; Chen, J.Y.; Apple, R.; Hildesheim, A.; Carrington, M. Variation of the killer cell immunoglobulin-like receptors and HLA-C genes in nasopharyngeal carcinoma. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2673–2677. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.B.; Han, H.Q.; Bei, J.X.; Liu, C.C.; Lei, J.J.; Cui, Q.; Feng, Q.S.; Wang, H.Y.; Zhang, J.X.; Liang, Y.; et al. Expression of human leukocyte antigen G is associated with prognosis in nasopharyngeal carcinoma. Int. J. Biol. Sci. 2012, 8, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Yang, H.; Ying, S.; Lu, H.; Wang, W.; Lv, J.; Xiong, H.; Hu, W. High HLA-F Expression Is a Poor Prognosis Factor in Patients with Nasopharyngeal Carcinoma. Anal. Cell. Pathol. 2018, 2018, 7691704. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, T.Y.; Chen, L.; Liu, Y.; Dian, M.J.; Hao, W.C.; Lin, X.L.; Li, X.Y.; Li, Y.L.; Lian, M.; et al. miR-19 regulates the expression of interferon-induced genes and MHC class I genes in human cancer cells. Int. J. Med. Sci. 2020, 17, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.Y.; Mei, J.Z.; Yao, K.T. Immunoediting of natural killer cells by human nasopharyngeal carcinoma cell line: Altered expression of KIRs and NKG2D receptors leads to reduction of natural killer cell-mediated cytolysis. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2007, 27, 247–249. [Google Scholar]
- Makowska, A.; Meier, S.; Shen, L.; Busson, P.; Baloche, V.; Kontny, U. Anti-PD-1 antibody increases NK cell cytotoxicity towards nasopharyngeal carcinoma cells in the context of chemotherapy-induced upregulation of PD-1 and PD-L1. Cancer Immunol. Immunother. CII 2021, 70, 323–336. [Google Scholar] [CrossRef]
- Makowska, A.; Lelabi, N.; Nothbaum, C.; Shen, L.; Busson, P.; Tran, T.T.B.; Eble, M.; Kontny, U. Radiotherapy Combined with PD-1 Inhibition Increases NK Cell Cytotoxicity towards Nasopharyngeal Carcinoma Cells. Cells 2021, 10, 2458. [Google Scholar] [CrossRef]
- Liou, A.K.; Soon, G.; Tan, L.; Peng, Y.; Cher, B.M.; Goh, B.C.; Wang, S.; Lim, C.M. Elevated IL18 levels in Nasopharyngeal carcinoma induced PD-1 expression on NK cells in TILS leading to poor prognosis. Oral Oncol. 2020, 104, 104616. [Google Scholar] [CrossRef]
- Yang, J.; Hu, M.; Bai, X.; Ding, X.; Xie, L.; Ma, J.; Fan, B.; Yu, J. Plasma levels of soluble programmed death ligand 1 (sPD-L1) in WHO II/III nasopharyngeal carcinoma (NPC): A preliminary study. Medicine 2019, 98, e17231. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A.; Ohashi, M.; Yoshiyama, H.; Ohba, Y. The Role of Transforming Growth Factor β in Cell-to-Cell Contact-Mediated Epstein-Barr Virus Transmission. Front. Microbiol. 2018, 9, 984. [Google Scholar] [CrossRef] [PubMed]
- Makowska, A.; Wahab, L.; Braunschweig, T.; Kapetanakis, N.I.; Vokuhl, C.; Denecke, B.; Shen, L.; Busson, P.; Kontny, U. Interferon beta induces apoptosis in nasopharyngeal carcinoma cells via the TRAIL-signaling pathway. Oncotarget 2018, 9, 14228–14250. [Google Scholar] [CrossRef] [PubMed]
- Makowska, A.; Franzen, S.; Braunschweig, T.; Denecke, B.; Shen, L.; Baloche, V.; Busson, P.; Kontny, U. Interferon beta increases NK cell cytotoxicity against tumor cells in patients with nasopharyngeal carcinoma via tumor necrosis factor apoptosis-inducing ligand. Cancer Immunol. Immunother. 2019, 68, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Shibuya, K.; Mui, A.; Zonin, F.; Murphy, E.; Sana, T.; Hartley, S.B.; Menon, S.; Kastelein, R.; Bazan, F.; et al. IGIF does not drive Th1 development but synergizes with IL-12 for interferon-gamma production and activates IRAK and NFkappaB. Immunity 1997, 7, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Strowig, T.; Brilot, F.; Arrey, F.; Bougras, G.; Thomas, D.; Muller, W.A.; Münz, C. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-gamma. PLoS Pathog. 2008, 4, e27. [Google Scholar] [CrossRef] [PubMed]
- Jud, A.; Kotur, M.; Berger, C.; Gysin, C.; Nadal, D.; Lünemann, A. Tonsillar CD56brightNKG2A+ NK cells restrict primary Epstein-Barr virus infection in B cells via IFN-γ. Oncotarget 2017, 8, 6130–6141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wen, B.; Anton, O.M.; Yao, Z.; Dubois, S.; Ju, W.; Sato, N.; DiLillo, D.J.; Bamford, R.N.; Ravetch, J.V.; et al. IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc. Natl. Acad. Sci. USA 2018, 115, E10915–E10924. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Investig. 2017, 127, 4042–4058. [Google Scholar] [CrossRef]
- Romee, R.; Schneider, S.E.; Leong, J.W.; Chase, J.M.; Keppel, C.R.; Sullivan, R.P.; Cooper, M.A.; Fehniger, T.A. Cytokine activation induces human memory-like NK cells. Blood 2012, 120, 4751–4760. [Google Scholar] [CrossRef]
- Parrish-Novak, J.; Dillon, S.R.; Nelson, A.; Hammond, A.; Sprecher, C.; Gross, J.A.; Johnston, J.; Madden, K.; Xu, W.; West, J.; et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000, 408, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Wu, C.Y.; Chen, M.Y.; Liu, S.X.; Yan, S.M.; Kang, Y.F.; Sun, C.; Grandis, J.R.; Zeng, M.S.; Zhong, Q. PD-1+CXCR5−CD4+ Th-CXCL13 cell subset drives B cells into tertiary lymphoid structures of nasopharyngeal carcinoma. J. Immunother. Cancer 2021, 9, e002101. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Feng, L.; Zhang, S.; Zhang, H.; Zhang, X.; Qi, X.; Zhang, Y.; Feng, Q.; Xiang, T.; Zeng, Y.X. Induction of chemokine (C-C motif) ligand 5 by Epstein-Barr virus infection enhances tumor angiogenesis in nasopharyngeal carcinoma. Cancer Sci. 2018, 109, 1710–1722. [Google Scholar] [CrossRef]
- Makowska, A.; Braunschweig, T.; Denecke, B.; Shen, L.; Baloche, V.; Busson, P.; Kontny, U. Interferon β and Anti-PD-1/PD-L1 Checkpoint Blockade Cooperate in NK Cell-Mediated Killing of Nasopharyngeal Carcinoma Cells. Transl. Oncol. 2019, 12, 1237–1256. [Google Scholar] [CrossRef] [PubMed]
- Malarkannan, S. Molecular mechanisms of FasL-mediated ‘reverse-signaling’. Mol. Immunol. 2020, 127, 31–37. [Google Scholar] [CrossRef] [PubMed]
- López-Montañés, M.; Alari-Pahissa, E.; Sintes, J.; Martínez-Rodríguez, J.E.; Muntasell, A.; López-Botet, M. Antibody-Dependent NK Cell Activation Differentially Targets EBV-Infected Cells in Lytic Cycle and Bystander B Lymphocytes Bound to Viral Antigen-Containing Particles. J. Immunol. 2017, 199, 656–665. [Google Scholar] [CrossRef]
- Wang, J.; Ge, J.; Wang, Y.; Xiong, F.; Guo, J.; Jiang, X.; Zhang, L.; Deng, X.; Gong, Z.; Zhang, S.; et al. EBV miRNAs BART11 and BART17-3p promote immune escape through the enhancer-mediated transcription of PD-L1. Nat. Commun. 2022, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Zamai, L.; Ahmad, M.; Bennett, I.M.; Azzoni, L.; Alnemri, E.S.; Perussia, B. Natural killer (NK) cell-mediated cytotoxicity: Differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 1998, 188, 2375–2380. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Ahmad, F.; Hong, H.S.; Eberhard, J.; Lu, I.N.; Ballmaier, M.; Schmidt, R.E.; Jacobs, R.; Meyer-Olson, D. FcγRIII (CD16)-mediated ADCC by NK cells is regulated by monocytes and FcγRII (CD32). Eur. J. Immunol. 2014, 44, 3368–3379. [Google Scholar] [CrossRef]
- Wang, R.; Jaw, J.J.; Stutzman, N.C.; Zou, Z.; Sun, P.D. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J. Leukoc. Biol. 2012, 91, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Chijioke, O.; Müller, A.; Feederle, R.; Barros, M.H.; Krieg, C.; Emmel, V.; Marcenaro, E.; Leung, C.S.; Antsiferova, O.; Landtwing, V.; et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013, 5, 1489–1498. [Google Scholar] [CrossRef]
- Orange, J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013, 132, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Hatton, O.; Strauss-Albee, D.M.; Zhao, N.Q.; Haggadone, M.D.; Pelpola, J.S.; Krams, S.M.; Martinez, O.M.; Blish, C.A. NKG2A-Expressing Natural Killer Cells Dominate the Response to Autologous Lymphoblastoid Cells Infected with Epstein-Barr Virus. Front. Immunol. 2016, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.R.; Quinn, L.L.; Rowe, M.; Zuo, J. Induction of the Lytic Cycle Sensitizes Epstein-Barr Virus-Infected B Cells to NK Cell Killing That Is Counteracted by Virus-Mediated NK Cell Evasion Mechanisms in the Late Lytic Cycle. J. Virol. 2016, 90, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Jochum, S.; Moosmann, A.; Lang, S.; Hammerschmidt, W.; Zeidler, R. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog. 2012, 8, e1002704. [Google Scholar] [CrossRef] [PubMed]
- Mancusi, A.; Ruggeri, L.; Velardi, A. Haploidentical hematopoietic transplantation for the cure of leukemia: From its biology to clinical translation. Blood 2016, 128, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.M.; Wagner, J.A.; Jewell, B.A.; Schappe, T.; Leong, J.W.; Abdel-Latif, S.; Schneider, S.E.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef]
- Berrien-Elliott, M.M.; Foltz, J.A.; Russler-Germain, D.A.; Neal, C.C.; Tran, J.; Gang, M.; Wong, P.; Fisk, B.; Cubitt, C.C.; Marin, N.D.; et al. Hematopoietic cell transplantation donor-derived memory-like NK cells functionally persist after transfer into patients with leukemia. Sci. Transl. Med. 2022, 14, eabm1375. [Google Scholar] [CrossRef]
- Bednarski, J.J.; Zimmerman, C.; Berrien-Elliott, M.M.; Foltz, J.A.; Becker-Hapak, M.; Neal, C.C.; Foster, M.; Schappe, T.; McClain, E.; Pence, P.P.; et al. Donor memory-like NK cells persist and induce remissions in pediatric patients with relapsed AML after transplant. Blood 2022, 139, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Berrien-Elliott, M.M.; Becker-Hapak, M.; Cashen, A.F.; Jacobs, M.; Wong, P.; Foster, M.; McClain, E.; Desai, S.; Pence, P.; Cooley, S.; et al. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood 2022, 139, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, N.; Coustan-Smith, E.; Kamiya, T.; Campana, D. Expanded and armed natural killer cells for cancer treatment. Cytotherapy 2016, 18, 1422–1434. [Google Scholar] [CrossRef]
- Chen, X.; Liang, R.; Zhu, X. Anti-EGFR therapies in nasopharyngeal carcinoma. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 131, 110649. [Google Scholar] [CrossRef]
- Tan, L.S.Y.; Wong, B.; Gangodu, N.R.; Lee, A.Z.E.; Kian Fong Liou, A.; Loh, K.S.; Li, H.; Yann Lim, M.; Salazar, A.M.; Lim, C.M. Enhancing the immune stimulatory effects of cetuximab therapy through TLR3 signalling in Epstein-Barr virus (EBV) positive nasopharyngeal carcinoma. Oncoimmunology 2018, 7, e1500109. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.M.; Liou, A.; Poon, M.; Koh, L.P.; Tan, L.K.; Loh, K.S.; Petersson, B.F.; Ting, E.; Campana, D.; Goh, B.C.; et al. Phase I study of expanded natural killer cells in combination with cetuximab for recurrent/metastatic nasopharyngeal carcinoma. Cancer Immunol. Immunother. CII 2022, 71, 2277–2286. [Google Scholar] [CrossRef]
- Prasad, M.; Ponnalagu, S.; Zeng, Q.; Luu, K.; Lang, S.M.; Wong, H.Y.; Cheng, M.S.; Wu, M.; Mallilankaraman, K.; Sobota, R.M.; et al. Epstein-Barr virus-induced ectopic CD137 expression helps nasopharyngeal carcinoma to escape immune surveillance and enables targeting by chimeric antigen receptors. Cancer Immunol. Immunother. 2022, 71, 2583–2596. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, Y.; Xu, Y.; Wang, Z.; Du, X.; Li, C.; Peng, J.; Gao, L.; Liang, X.; Ma, C. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 2017, 36, 6143–6153. [Google Scholar] [CrossRef] [PubMed]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vély, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbé, C.; et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 2016, 7, 72961–72977. [Google Scholar] [CrossRef]
- Hsu, J.; Hodgins, J.J.; Marathe, M.; Nicolai, C.J.; Bourgeois-Daigneault, M.C.; Trevino, T.N.; Azimi, C.S.; Scheer, A.K.; Randolph, H.E.; Thompson, T.W.; et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Investig. 2018, 128, 4654–4668. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Kumagai, Y.; Kato, H.; Guo, Z.; Matsushita, K.; Satoh, T.; Kawagoe, T.; Kumar, H.; Jang, M.H.; Kawai, T.; et al. Poly I:C-induced activation of NK cells by CD8 alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J. Immunol. 2009, 183, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- López-Albaitero, A.; Lee, S.C.; Morgan, S.; Grandis, J.R.; Gooding, W.E.; Ferrone, S.; Ferris, R.L. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol. Immunother. 2009, 58, 1853–1864. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Trivedi, P.P.; Ge, B.; Krzewski, K.; Strominger, J.L. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc. Natl. Acad. Sci. USA 2007, 104, 6329–6334. [Google Scholar] [CrossRef] [PubMed]
- Dietsch, G.N.; Lu, H.; Yang, Y.; Morishima, C.; Chow, L.Q.; Disis, M.L.; Hershberg, R.M. Coordinated Activation of Toll-Like Receptor8 (TLR8) and NLRP3 by the TLR8 Agonist, VTX-2337, Ignites Tumoricidal Natural Killer Cell Activity. PLoS ONE 2016, 11, e0148764. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Zhang, W.Y.; Tan, N.Y.; Khatoo, M.; Suter, M.A.; Tripathi, S.; Cheung, F.S.; Lim, W.K.; Tan, P.H.; Ngeow, J.; et al. The DNA Structure-Specific Endonuclease MUS81 Mediates DNA Sensor STING-Dependent Host Rejection of Prostate Cancer Cells. Immunity 2016, 44, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Schadt, L.; Sparano, C.; Schweiger, N.A.; Silina, K.; Cecconi, V.; Lucchiari, G.; Yagita, H.; Guggisberg, E.; Saba, S.; Nascakova, Z.; et al. Cancer-Cell-Intrinsic cGAS Expression Mediates Tumor Immunogenicity. Cell Rep. 2019, 29, 1236–1248.e1237. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, C.J.; Wolf, N.; Chang, I.C.; Kirn, G.; Marcus, A.; Ndubaku, C.O.; McWhirter, S.M.; Raulet, D.H. NK cells mediate clearance of CD8+ T cell-resistant tumors in response to STING agonists. Sci. Immunol. 2020, 5, eaaz2738. [Google Scholar] [CrossRef] [PubMed]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell 2018, 175, 1731–1743.e1713. [Google Scholar] [CrossRef]
- Vey, N.; Karlin, L.; Sadot-Lebouvier, S.; Broussais, F.; Berton-Rigaud, D.; Rey, J.; Charbonnier, A.; Marie, D.; André, P.; Paturel, C.; et al. A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget 2018, 9, 17675–17688. [Google Scholar] [CrossRef]
- Carlsten, M.; Korde, N.; Kotecha, R.; Reger, R.; Bor, S.; Kazandjian, D.; Landgren, O.; Childs, R.W. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK Cells in Patients with Myeloma. Clin. Cancer Res. 2016, 22, 5211–5222. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional Natural Killer Cell Engagers Targeting NKp46 Trigger Protective Tumor Immunity. Cell 2019, 177, 1701–1713.e1716. [Google Scholar] [CrossRef] [PubMed]
- Nikkhoi, S.K.; Li, G.; Eleya, S.; Yang, G.; Vandavasi, V.G.; Hatefi, A. Bispecific killer cell engager with high affinity and specificity toward CD16a on NK cells for cancer immunotherapy. Front. Immunol. 2022, 13, 1039969. [Google Scholar] [CrossRef] [PubMed]

| Molecules on NK Cells | Corresponding Receptors/Ligands | Functional Signal Reported in NPC | References |
|---|---|---|---|
| (1) Activating signaling | |||
| NKG2D | MICA/B ULBP | LMP2 induced MICA/B downregulation in NPC. | [66] |
| EBV-encoded mircoRNAs promoted MICA/B downregulation in NPC. | [67,68] | ||
| MICA gene deletion was found in NPC. | [69,70] | ||
| Free soluble ULBP protein secreted by NPC cells impaired NK function. | [71] | ||
| Over-expression of MACC1 on NPC cells reduced NKG2D expression on NK cells. | [61] | ||
| NKp30 | B7-H6 | A low percentage of NKp30+NK cells was found in NPC tissues. | [59] |
| NKG2C/CD94 | HLA-E | CD94 expression on NK cells was increased in NPC; NKG2C-HLA-E signaling pathway was not relevant to genetic susceptibility in NPC. | [44,72] |
| (2) Inhibitory signaling | |||
| KIR | MHC I | HLA-C (tumor cell) and KIR2DL4 (NK cell) interaction was reported in NPC. | [62,73] |
| Higher HLA-G expression was found in NPC when compared with normal tissue. | [74] | ||
| Higher soluble HLA-F was found in NPC plasma than normal controls. | [75] | ||
| miR-19 reduced MHC I molecule expression on NPC cell line. | [76] | ||
| Persistent exposure to CNE2 cell line increased KIR expression on NK. | [77] | ||
| NKG2A/CD94 | NKG2A expression of NK cells was not upregulated in NPC; HLA-E (tumor cell) and CD94 (NK cell) interaction was found in NPC. | [44,62] | |
| PD-1 | PD-L1 | B7-H3 knockdown in EBV+ NPC cell line increased PD-1 expression on cocultured NK cells. | [65] |
| Chemotherapy upregulated PD-1 on NK cells and PD-L1 on NPC cells via NF-κB pathway. | [78] | ||
| Radiotherapy for NPC increased PD-1 expression on NK cells and PD-L1 expression on tumor cells. | [79] | ||
| PD-1 expression on NK cells in NPC could be induced by IL-18. | [80] | ||
| Soluble PD-L1 was detected in NPC plasma, indicating poor prognosis. | [81] | ||
| TIGIT | PVR | TIGIT-PVR interaction was detected between NK and tumor cells and between NK and macrophages/dendritic cells in NPC; TIGIT expression was upregulated on exhausted NK cells in NPC. | [48,64] |
| CD96-PVR interaction was detected between NK and tumor cells in NPC | |||
| CD96 | TIGIT and CD96 were positively correlated with FCER2 and KHDRBS2 and negatively correlated with IGSF9 in NPC *. | [63] | |
| LAG3 | Gal-3 | LAG3 expression was upregulated on exhausted NK cells in NPC. | [64] |
| LAG3 was positively correlated with FCER2 and KHDRBS2 and negatively correlated with IGSF9 in NPC *. | [63] | ||
| (3) NK-cell-related cytokines/chemokines | |||
| TGF-β | TGF-β inhibited NK cell function by promoting Tregs differentiation; TGF-β promoted the transmission of EBV infection in NPC. | [82] | |
| Type I Interferon | Type I Interferon induced granzyme B directly in NK cells; IFN-β induced NK-cell-mediated cytotoxicity against NPC targets in vitro. | [83,84] | |
| IL-2 | IL-2 promoted strong NK cytotoxicity. | [50] | |
| IL-12 | IL-12 and IL-18 stimulated IFN-γ production by NK cells synergistically; IL-12 secretion was related to EBV viral stimuli; the anti-EBV NK subset could be activated by IL-12. | [85,86,87] | |
| IL-15 | IL-15 promoted strong NK cytotoxicity and ADCC and induced the expression of NKp30. | [88,89] | |
| IL-18 | A negative correlation between IL-18 and NK cytotoxicity in NPC was reported; IL-18 level increased during EBV infection. IL-12, IL-15, and IL-18 cocktail cytokines converted NK cells into long-lived, activated NK cells called “memory-like” NK cells. | [80,90] | |
| IL-21 | IL-21 promoted the expansion of NK cells in vitro; IL-21 expression elevated on follicular TLSs in NPC. | [91,92] | |
| XCL1/2 | Interaction between XCR1 and XCL1/2 was involved in DCs recruited by NK cells; XCL1/2 were overexpressed by NK cells in NPC. | [18,44,48] | |
| CCL5 | NK-cell-derived CCL5 played a vital role in recruiting DCs; CCL5 was identified as an EBV-regulated molecule driver promoting NPC angiogenesis. | [93] | |
| (4) Functional signal inducing apoptosis in target cells | |||
| TRAIL | TRAILR | Binding of TRAIL and TRAILR activated downstream death signal; TRAIL sensitivity was redox-dependent in NPC cells; NK-cell-dependent NPC cell killing was predominately mediated via TRAIL pathway. | [94] |
| FASL | FAS | FAS/FASL signal triggered apoptosis in target cells; FAS expression was suppressed in radioresistant NPC patients. | [95] |
| CD16 | Fc region | CD16 was considered as an activated biomarker for NK cells; CD16/CD32 binding with Fc region of IgG antibody triggered ADCC effect; ADCC participated in anti-EBV infection in lytic phase. | [96] |
| Phase | Treatment | Condition | Estimated Enrollment | Completion Date | Status | NCT No. |
|---|---|---|---|---|---|---|
| Phase I | IL-2 and NK cells | Metastatic NPC | Not mentioned | Not mentioned | Completed | NCT00717184 |
| Phase I/II | Cetuximab with NK cells | NPC | 31 | 1 August 2019 | Unknown | NCT02507154 [116] |
| Phase I/II | High-activity natural killer immunotherapies | Small metastatic NPC | 20 | 1 June 2019 | Completed | NCT03007836 |
| Phase I | Haplo/Allogeneic NKG2DL-targeting CAR-grafted γδ T Cells | Relapsed or refractory solid tumors including NPC | 10 | 1 March 2021 | Unknown | NCT04107142 |
| Phase I | VTX-2337 with cetuximab | Locally advanced, recurrent, or metastatic SCCHN including NPC | 13 | Not mentioned | Completed | NCT01334177 [125] |
| Phase I/II | TAK-500 with or without pembrolizumab | Locally advanced or metastatic solid tumors including NPC | 313 | 11 August 2026 | Recruiting | NCT05070247 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Dai, W.; Kam, N.-W.; Zhang, J.; Lee, V.H.F.; Ren, X.; Kwong, D.L.-W. The Role of Natural Killer Cells in the Tumor Immune Microenvironment of EBV-Associated Nasopharyngeal Carcinoma. Cancers 2024, 16, 1312. https://doi.org/10.3390/cancers16071312
Li S, Dai W, Kam N-W, Zhang J, Lee VHF, Ren X, Kwong DL-W. The Role of Natural Killer Cells in the Tumor Immune Microenvironment of EBV-Associated Nasopharyngeal Carcinoma. Cancers. 2024; 16(7):1312. https://doi.org/10.3390/cancers16071312
Chicago/Turabian StyleLi, Shuzhan, Wei Dai, Ngar-Woon Kam, Jiali Zhang, Victor H. F. Lee, Xiubao Ren, and Dora Lai-Wan Kwong. 2024. "The Role of Natural Killer Cells in the Tumor Immune Microenvironment of EBV-Associated Nasopharyngeal Carcinoma" Cancers 16, no. 7: 1312. https://doi.org/10.3390/cancers16071312
APA StyleLi, S., Dai, W., Kam, N.-W., Zhang, J., Lee, V. H. F., Ren, X., & Kwong, D. L.-W. (2024). The Role of Natural Killer Cells in the Tumor Immune Microenvironment of EBV-Associated Nasopharyngeal Carcinoma. Cancers, 16(7), 1312. https://doi.org/10.3390/cancers16071312






