Evaluation of a Population-Based Targeted Screening Approach for Skin Cancer with Long-Time Follow-Up in Austria including Potential Effects on Melanoma Mortality
Abstract
Simple Summary
Abstract
1. Introduction
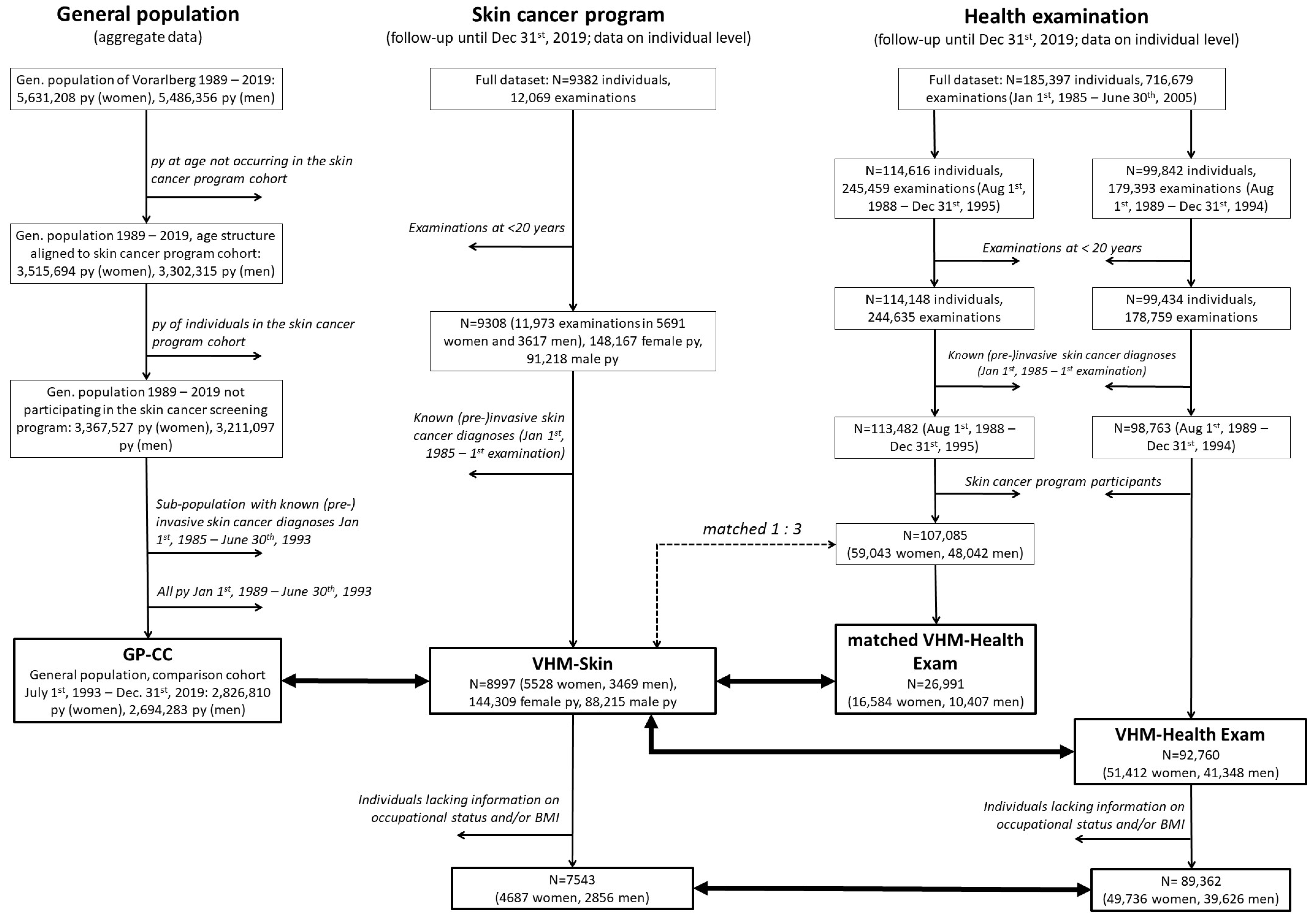
2. Patients and Methods
2.1. The Skin Cancer Screening Program as Part of the Vorarlberg Health Monitoring and Promotion Program (VHM&PP)
2.2. Study Design
2.3. Exposure and Covariates
2.4. Outcome
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zeng, W.; Jiang, A.; He, Z.; Shen, X.; Dong, X.; Feng, J.; Lu, H. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: An analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021, 10, 4905–4922. [Google Scholar] [CrossRef] [PubMed]
- Garbe, C.; Keim, U.; Gandini, S.; Amaral, T.; Katalinic, A.; Hollezcek, B.; Martus, P.; Flatz, L.; Leiter, U.; Whiteman, D. Epidemiology of cutaneous melanoma and keratinocyte cancer in white populations 1943–2036. Eur. J. Cancer 2021, 152, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Hackl, M.; Ihle, P. Krebserkrankungen in Österreich 2020; Statistik Austria: Wien, Austria, 2020; ISBN 978-3-903264-38-0. [Google Scholar]
- Rigel, D.S.; Russak, J.; Friedman, R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J. Clin. 2010, 60, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Rawlings Parker, E. The influence of climate change on skin cancer incidence—A review of the evidence. Int. J. Womens Dermatol. 2021, 7, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Oberaigner, W.; Geiger-Gritsch, S. Prediction of cancer incidence in Tyrol/Austria for year of diagnosis 2020. Wien. Klin. Wochenschr. 2014, 126, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.D.; Salciccioli, J.D.; Marshall, D.C.; Sheri, A.; Shalhoub, J. Trends in malignant melanoma mortality in 31 countries from 1985 to 2015. Br. J. Dermatol. 2020, 183, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer 2005, 41, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Zanetti, R.; Masini, C.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur. J. Cancer 2005, 41, 2040–2059. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef]
- Fears, T.R.; Guerry, D., 4th; Pfeiffer, R.M.; Sagebiel, R.W.; Elder, D.E.; Halpern, A.; Holly, E.A.; Hartge, P.; Tucker, M.A. Identifying individuals at high risk of melanoma: A practical predictor of absolute risk. J. Clin. Oncol. 2006, 24, 3590–3596. [Google Scholar] [CrossRef] [PubMed]
- Lasithiotakis, K.G.; Leiter, U.; Eigentler, T.; Breuninger, H.; Metzler, G.; Meier, F.; Garbe, C. Improvement of overall survival of patients with cutaneous melanoma in Germany, 1976-2001: Which factors contributed? Cancer 2007, 109, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Aitken, J.F.; Elwood, M.; Baade, P.D.; Youl, P.; English, D. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int. J. Cancer 2010, 126, 450–458. [Google Scholar] [CrossRef]
- Schneider, J.S.; Moore, D.H., 2nd; Mendelsohn, M.L. Screening program reduced melanoma mortality at the Lawrence Livermore National Laboratory, 1984 to 1996. J. Am. Acad. Dermatol. 2008, 58, 741–749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boniol, M.; Autier, P.; Gandini, S. Melanoma mortality following skin cancer screening in Germany. BMJ Open 2015, 5, e008158. [Google Scholar] [CrossRef]
- Stang, A.; Jöckel, K.-H.; Heidinger, O. Skin cancer rates in North Rhine-Westphalia, Germany before and after the introduction of the nationwide skin cancer screening program (2000–2015). Eur. J. Epidemiol. 2018, 33, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.; Augustin, M.; Hagenström, K.; Garbe, C.; Baltus, H.; Eisemann, N.; Hübner, J.; Katalinic, A.; Augustin, J. Evaluation of skin cancer screening in Germany—Spatiotemporal associations between skin cancer screening and skin cancer mortality based on ambulatory claims data. J. Dtsch. Dermatol. Ges. 2023, 21 (Suppl. S5), 22–31. [Google Scholar] [CrossRef]
- Schumann, L.; Eisemann, N.; Augustin, J.; Kieschke, J.; Meyer, M.; Kajüter, H.; Katalinic, A. Association of early-stage incidence and mortality in malignant melanoma—A population-based ecological study. J. Dtsch. Dermatol. Ges. 2023, 21 (Suppl. S5), 33–40. [Google Scholar] [CrossRef] [PubMed]
- U.S. Preventive Services Task Force, Final Recommendation Statement—Skin Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/skin-cancer-screening (accessed on 30 June 2023).
- Henrikson, N.B.; Ivlev, I.; Blasi, P.R.; Nguyen, M.B.; Senger, C.A.; Perdue, L.A.; Lin, J.S. Skin cancer screening: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2023, 329, 1296–1307. [Google Scholar] [CrossRef]
- Cancer Council Australia, Position Statement—Early Detection of Skin Cancer. Available online: https://wiki.cancer.org.au/policy/Position_statement_-_Screening_and_early_detection_of_skin_cancer (accessed on 30 June 2023).
- Ulmer, H.; Kelleher, C.; Diem, G.; Concin, H. Long-term tracking of cardiovascular risk factors among men and women in a large population-based health system. The Vorarlberg Health Monitoring & Promotion Programme. Eur. Heart J. 2003, 24, 1004–1013. [Google Scholar] [CrossRef]
- Stocks, T.; Borena, W.; Strohmaier, S.; Bjørge, T.; Manjer, J.; Engeland, A.; Johansen, D.; Selmer, R.; Hallmans, G.; Rapp, K.; et al. Cohort Profile: The Metabolic syndrome and Cancer project (Me-Can). Int. J. Epidemiol. 2010, 39, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Längle, U. Drei Jahre Hautvorsorge-Untersuchung in Vorarlberg. Öst. Ärztezeitg. 1994, 49, 31–36. [Google Scholar]
- Psaty, E.L.; Scope, A.; Halpern, A.C.; Marghoob, A.A. Defining the patient at high risk for melanoma. Int. J. Dermatol. 2010, 49, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Girgis, A.; Campbell, E.M.; Redman, S.; Sanson-Fisher, R.W. Screening for melanoma: A community survey of prevalence and predictors. Med. J. Aust. 1991, 154, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Montella, M.; Crispo, A.; Grimaldi, M.; De Marco, M.R.; Ascierto, P.A.; Parasole, R.; Melucci, M.T.; Silvestro, P.; Fabbrocini, G. An assessment of factors related to tumor thickness and delay in diagnosis of melanoma in southern Italy. Prev. Med. 2002, 35, 271–277. [Google Scholar] [CrossRef]
- Sergentanis, T.N.; Antoniadis, A.G.; Gogas, H.J.; Antonopoulos, C.N.; Adami, H.-O.; Ekbom, A.; Petridou, E.T. Obesity and risk of malignant melanoma: A meta-analysis of cohort and case-control studies. Eur. J. Cancer 2013, 49, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Greene, F.L.; Page, D.L.; Fleming, I.D.; Fritz, A.G.; Balch, C.M.; Haller, D.G.; Morrow, M. (Eds.) AJCC Cancer Staging Manual, 6th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Statistik Austria. Available online: http://www.statistik.at (accessed on 30 June 2023).
- Stang, A.; Schuldt, K.; Trocchi, P.; Neusser, S.; Speckemeier, C.; Pahmeier, K.; Wasem, J.; Lax, H.; Nonnemacher, M. The impossibility of mortality evaluation of skin cancer screening in Germany based on health insurance data: A case-control study. Eur. J. Cancer 2022, 173, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.; Agathos, M.; Balda, B.R.; Bauerdorf, R.; Beilner-Track, R.; Detmar, U.; Elsner, P.; Frühauf, W.; Galli, K.H.; von Hintzenstern, J.; et al. Verhalten und Einstellung gegenüber Hautkrebsvorsorge und –früherkennung in Abhängigkeit vom Kenntnisstand [Behavior and attitude regarding skin cancer prevention and early detection in relation to current knowledge—Studies of visitors to an industrial fair]. Gesundheitswesen 1992, 54, 325–330. [Google Scholar] [PubMed]
- Datzmann, T.; Schoffer, O.; Meier, F.; Seidler, A.; Schmitt, J. Are patients benefiting from participation in the German skin cancer screening programme? A large cohort study based on administrative data. Br. J. Dermatol. 2022, 186, 69–77. [Google Scholar] [CrossRef]
- Quéreux, G.; N’guyen, J.-M.; Cary, M.; Jumbou, O.; Lequeux, Y.; Dréno, B. Validation of the Self-Assessment of Melanoma Risk Score for a melanoma-targeted screening. Eur. J. Cancer Prev. 2012, 21, 588–595. [Google Scholar] [CrossRef]
- Rat, C.; Grimault, C.; Quereux, G.; Dagonne, M.; Gaultier, A.; Khammari, A.; Dreno, B.; Nguyen, J.-M. Proposal for an annual skin examination by a general practitioner for patients at high risk for melanoma: A French cohort study. BMJ Open 2015, 5, e007471. [Google Scholar] [CrossRef]
- Cuevas, L.M.; Daud, A.I. Immunotherapy for melanoma. Semin. Cutan. Med. Surg. 2018, 37, 127–131. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Hübner, J.; Baltus, H.; Eisemann, N.; Rohr, M.; Schumann, L.; Augustin, J.; Hagenström, K.; Wolf, S.; Garbe, C.; Augustin, M.; et al. Evaluation of early skin cancer detection in Germany with cancer registry data—Challenges, solutions and current trends. J. Dtsch. Dermatol. Ges. 2023, 21 (Suppl. S5), 13–20. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, A.; Waldmann, A.; Weinstock, M.A.; Geller, A.C.; Eisemann, N.; Greinert, R.; Volkmer, B.; Breitbart, E. Does skin cancer screening save lives? An observational study comparing trends in melanoma mortality in regions with and without screening. Cancer 2012, 118, 5395–5402. [Google Scholar] [CrossRef] [PubMed]
- Stang, A.; Jöckel, K.-H. Does skin cancer screening save lives? A detailed analysis of mortality time trends in Schleswig-Holstein and Germany. Cancer 2016, 122, 432–437. [Google Scholar] [CrossRef]
- Monshi, B.; Vujic, M.; Kivaranovic, D.; Sesti, A.; Oberaigner, W.; Vujic, I.; Ortiz-Urda, S.; Posch, C.; Feichtinger, H.; Hackl, M.; et al. The burden of malignant melanoma—Lessons to be learned from Austria. Eur. J. Cancer 2016, 56, 45–53. [Google Scholar] [CrossRef]
| VHM-Skin | VHM-Health Exam | |||
|---|---|---|---|---|
| All | ||||
| n | 8997 | 92,760 | ||
| Age at baseline examination (years), mean ± SD | 40.0 ± 15.0 | 44.8 ± 15.3 | ||
| Follow-up * (years), median (IQR) | 26.8 (25.8–28.5) | 27.8 (25.4–29.3) | ||
| Overall deaths, n (%) | 1438 (16.0%) | 24,210 (26.1%) | ||
| Women | ||||
| n | 5528 | 51,412 | ||
| Age at baseline examination (years), mean ± SD | 39.4 ± 14.9 | 45.2 ± 15.8 | ||
| Follow-up * (years), median (IQR) | 26.9 (25.8–28.6) | 28.0 (25.6–29.4) | ||
| Overall deaths, n (%) | 771 (13.9%) | 12,602 (24.5%) | ||
| Men | ||||
| n | 3469 | 41,348 | ||
| Age at baseline examination (years), mean ± SD | 40.8 ± 15.2 | 44.3 ± 14.8 | ||
| Follow-up * (years), median (IQR) | 26.7 (25.6–28.4) | 27.6 (25.2–29.2) | ||
| Overall deaths, n (%) | 667 (19.2%) | 11,608 (28.1%) | ||
| 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 | 2015–2019 | ||
|---|---|---|---|---|---|---|---|---|
| All | ||||||||
| Invasive melanoma (C43) | 8.10 | 12.52 | 14.92 | 23.11 | 27.27 | 24.84 | 20.61 | |
| Melanoma in situ (D03) | 1.04 | 6.42 | 9.31 | 12.00 | 13.89 | 18.35 | 16.56 | |
| Melanoma mortality | 3.56 | 3.59 | 3.16 | 2.64 | 3.35 | 3.23 | 2.63 | |
| Women | ||||||||
| Invasive melanoma (C43) | 9.65 | 11.92 | 15.92 | 21.28 | 24.05 | 24.22 | 19.35 | |
| Melanoma in situ (D03) | 1.44 | 7.00 | 11.38 | 13.09 | 14.11 | 18.90 | 15.64 | |
| Melanoma mortality | 3.83 | 2.77 | 3.19 | 2.41 | 2.82 | 2.80 | 2.54 | |
| Men | ||||||||
| Invasive melanoma (C43) | 6.50 | 13.13 | 13.89 | 25.00 | 30.58 | 25.47 | 21.90 | |
| Melanoma in situ (D03) | 0.64 | 5.83 | 7.18 | 10.88 | 13.66 | 17.78 | 17.50 | |
| Melanoma mortality | 3.28 | 4.44 | 3.13 | 2.88 | 3.90 | 3.67 | 2.72 | |
| ΔIRR * per 5-Year Intervals (95%-CI) | |||||
|---|---|---|---|---|---|
| (1985–1990)– (2015–2019) | (2000–2004)– (2015–2019) | (1985–1990)– (2000–2004) | (2005–2009)– (2015–2019) | ||
| All | |||||
| Invasive melanoma (C43) | 1.18 (1.13–1.22) | 0.96 (0.90–1.03) | - | - | |
| Melanoma in situ (D03) | 1.32 (1.26–1.38) | 1.13 (1.07–1.20) | - | - | |
| Melanoma mortality | 0.96 (0.91–1.01) | 0.99 (0.88–1.11) | 0.90 (0.78–1.03) | 0.90 (0.76–1.06) | |
| Women | |||||
| Invasive melanoma (C43) | 1.13 (1.09–1.18) | 0.97 (0.91–1.03) | - | - | |
| Melanoma in situ (D03) | 1.24 (1.19–1.29) | 1.08 (1.01–1.16) | - | - | |
| Melanoma mortality | 0.95 (0.89–1.03) | 1.01 (0.85–1.20) | 0.88 (0.73–1.06) | 0.96 (0.74–1.24) | |
| Men | |||||
| Invasive melanoma (C43) | 1.19 (1.13–1.26) | 0.94 (0.89–1.01) | - | - | |
| Melanoma in situ (D03) | 1.35 (1.28–1.44) | 1.18 (1.08–1.27) | - | - | |
| Melanoma mortality | 0.96 (0.89–1.04) | 0.97 (0.83–1.12) | 0.92 (0.72–1.17) | 0.85 (0.68–1.07) | |
| VHM-Skin | GP-CC | IRR ** (95%-CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Person-Years | IR */MR * | n | Person-Years | IR */MR * | |||
| Invasive melanoma (C43) | ||||||||
| All | 207 | 232,524 | 89 | 1734 | 5,521,093 | 31.4 | 2.92 (2.49–3.41) | |
| Women | 94 | 144,309 | 65.1 | 789 | 2,826,810 | 27.9 | 2.40 (1.93–2.98) | |
| Men | 112 | 88,215 | 127 | 945 | 2,694,283 | 35.1 | 3.38 (2.77–4.12) | |
| Melanoma in situ (D03) | ||||||||
| All | 187 | 232,524 | 80.4 | 1106 | 5,521,093 | 20 | 4.13 (3.53–4.83) | |
| Women | 115 | 144,309 | 79.7 | 538 | 2,826,810 | 19 | 4.43 (3.61–5.43) | |
| Men | 71 | 88,215 | 80.5 | 568 | 2,694,283 | 21.1 | 3.67 (2.86–4.71) | |
| Melanoma deaths | ||||||||
| All | 16 | 232,524 | 6.9 | 250 | 5,521,093 | 4.5 | 1.66 (1.00–2.75) | |
| Women | 8 | 144,309 | 5.5 | 112 | 2,826,810 | 4 | 1.64 (0.80–3.37) | |
| Men | 8 | 88,215 | 9.1 | 138 | 2,694,283 | 5.1 | 1.64 (0.80–3.34) | |
| Full Follow-Up | Follow-Up until 31 December 1994 | 0–10 Years | >10–20 Years | 20+ Years | |||
|---|---|---|---|---|---|---|---|
| Incident invasive melanoma (C43) | |||||||
| All | |||||||
| (sub)cohort size, n/N | 8997/92,760 | 8997/92,760 | 8997/92,760 | 8644/87,072 | 8045/78,123 | ||
| cases, n/N | 207/790 | 30/27 | 61/165 | 99/338 | 47/287 | ||
| HR (95%-CI) * | 3.02 (2.59–3.52) | 18.50 (10.92–31.33) | 4.33 (3.22–5.82) | 3.27 (2.61–4.10) | 1.94 (1.42–2.65) | ||
| Women | |||||||
| (sub)cohort size, n/N | 5528/51,412 | 5528/51,412 | 5528/51,412 | 5367/48,754 | 5053/44,076 | ||
| cases, n/N | 95/371 | 14/12 | 29/91 | 45/152 | 21/128 | ||
| HR (95%-CI) ** | 2.55 (2.03–3.21) | 19.14 (8.75–41.87) | 3.44 (2.26–5.25) | 2.87 (2.05–4.02) | 1.59 (1.00–2.53) | ||
| Men | |||||||
| (sub)cohort size, n/N | 3469/41,348 | 3469/41,348 | 3469/41,348 | 3277/38,318 | 2992/34,047 | ||
| cases, n/N | 112/419 | 16/15 | 32/74 | 54/186 | 26/159 | ||
| HR (95%-CI) ** | 3.46 (2.81–4.27) | 18.80 (9.21–38.39) | 5.56 (3.67–8.43) | 3.61 (2.67–4.90) | 2.24 (1.48–3.40) | ||
| Incident melanoma in situ (D03) | |||||||
| All | |||||||
| (sub)cohort size, n/N | 8997/92,760 | 8997/92,760 | 8997/92,760 | 8653/87,123 | 8066/78,235 | ||
| cases, n/N | 187/562 | 17/23 | 50/98 | 80/231 | 57/233 | ||
| HR (95%-CI) * | 3.90 (3.30–4.61) | 12.76 (6.74–24.16) | 6.22 (4.41–8.77) | 3.78 (2.93–4.88) | 3.04 (2.27–4.07) | ||
| Women | |||||||
| (sub)cohort size, n/N | 5528/51,412 | 5528/51,412 | 5528/51,412 | 5360/48,782 | 5041/44,127 | ||
| cases, n/N | 115/276 | 9/13 | 33/56 | 49/110 | 33/110 | ||
| HR (95%-CI) ** | 4.37 (3.50–5.44) | 10.78 (4.52–25.74) | 6.77 (4.38–10.46) | 4.27 (3.04–6.01) | 3.26 (2.19–4.83) | ||
| Men | |||||||
| (sub)cohort size, n/N | 3469/41,348 | 3469/41,348 | 3469/41,348 | 3293/38,341 | 3025/34,108 | ||
| cases, n/N | 72/286 | 8/10 | 17/42 | 31/121 | 24/123 | ||
| HR (95%-CI) ** | 3.32 (2.56–4.30) | 15.35 (5.98–39.40) | 5.37 (3.06–9.45) | 3.16 (2.13–4.70) | 2.75 (1.77–4.26) | ||
| Melanoma deaths | |||||||
| All | |||||||
| (sub)cohort size, n/N | 8997/92,760 | 8997/92,760 | 8997/92,760 | 8701/87,214 | 8182/78,531 | ||
| cases, n/N | 16/97 | 2/4 | 4/20 | 8/37 | 4/40 | ||
| HR (95%-CI) * | 2.12 (1.25–3.61) | 10.40 (1.88–57.64) | 2.62 (0.89–7.67) | 2.61 (1.21–5.62) | 1.36 (0.49–3.82) | ||
| Women | |||||||
| (sub)cohort size, n/N | 5528/51,412 | - | - | - | - | ||
| cases, n/N | 8/38 | - | - | - | - | ||
| HR (95%-CI) ** | 2.49 (1.16–5.37) | - | - | - | - | ||
| Men | |||||||
| (sub)cohort size, n/N | 3469/41,348 | - | - | - | - | ||
| cases, n/N | 8/59 | - | - | - | - | ||
| HR (95%-CI) ** | 1.83 (0.87–3.83) | - | - | - | - | ||
| VHM-Skin | VHM-Health Exam | GP-CC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Breslow thickness (mm) | |||||||||
| n | median (IQR) | n | median (IQR) | p * | n | median (IQR) | p * | ||
| All | 185 | 0.5 (0.3–0.8) | 652 | 0.6 (0.4–1.3) | <0.05 | 1443 | 0.6 (0.4–1.4) | <0.01 | |
| Women | 84 | 0.5 (0.3–0.8) | 298 | 0.6 (0.4–1.2) | 0.29 | 631 | 0.6 (0.4–1.4) | 0.11 | |
| Men | 101 | 0.5 (0.3–0.8) | 354 | 0.6 (0.4–1.3) | <0.05 | 812 | 0.7 (0.4–1.5) | <0.05 | |
| Clark´s level (1–5) | |||||||||
| n | mean (±SD) | n | mean (±SD) | p * | n | mean (±SD) | p * | ||
| All | 176 | 3.0 ± 0.7 | 630 | 3.1 ± 0.8 | <0.05 | 1368 | 3.2 ± 0.9 | <0.01 | |
| Women | 81 | 3.0 ± 0.8 | 295 | 3.1 ± 0.8 | 0.36 | 744 | 3.1 ± 0.9 | 0.11 | |
| Men | 95 | 3.0 ± 0.6 | 335 | 3.1 ± 0.9 | <0.05 | 624 | 3.2 ± 0.8 | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brozek, W.; Clemens, P.; Ulmer, H.; Häring, N.; Concin, H.; Zitt, E.; Nagel, G. Evaluation of a Population-Based Targeted Screening Approach for Skin Cancer with Long-Time Follow-Up in Austria including Potential Effects on Melanoma Mortality. Cancers 2024, 16, 1283. https://doi.org/10.3390/cancers16071283
Brozek W, Clemens P, Ulmer H, Häring N, Concin H, Zitt E, Nagel G. Evaluation of a Population-Based Targeted Screening Approach for Skin Cancer with Long-Time Follow-Up in Austria including Potential Effects on Melanoma Mortality. Cancers. 2024; 16(7):1283. https://doi.org/10.3390/cancers16071283
Chicago/Turabian StyleBrozek, Wolfgang, Patrick Clemens, Hanno Ulmer, Nina Häring, Hans Concin, Emanuel Zitt, and Gabriele Nagel. 2024. "Evaluation of a Population-Based Targeted Screening Approach for Skin Cancer with Long-Time Follow-Up in Austria including Potential Effects on Melanoma Mortality" Cancers 16, no. 7: 1283. https://doi.org/10.3390/cancers16071283
APA StyleBrozek, W., Clemens, P., Ulmer, H., Häring, N., Concin, H., Zitt, E., & Nagel, G. (2024). Evaluation of a Population-Based Targeted Screening Approach for Skin Cancer with Long-Time Follow-Up in Austria including Potential Effects on Melanoma Mortality. Cancers, 16(7), 1283. https://doi.org/10.3390/cancers16071283








