Simple Summary
Current treatment options for advanced cervical cancer are limited, with a 5-year overall survival rate of 17%. New effective therapeutic approaches that can increase survival and reduce disparities are greatly needed. In this study, an approach using a multifunctional liquid immunogenic fiducial eluter (LIFE) that can provide image contrast and enhance therapeutic efficacy during hypo-fractionated radiotherapy is investigated. We found this technique provides both computed tomography (CT) and magnetic resonance imaging (MRI) image contrast to guide radiotherapy. Additionally, in a model of metastatic cervical cancer, local treatment with LIFE and radiotherapy resulted in distant disease control and improvement in survival. Successful development of this approach may provide a short therapeutic option that could enhance treatment outcomes and reduce disparities. LIFE Biomaterial may also significantly provide a cheaper and convenient alternative to prolonged systemic treatment with opportunities to increase access to care and reduce health outcome disparities.
Abstract
Globally, cervical cancer is the fourth leading cancer among women and is dominant in resource-poor settings in its occurrence and mortality. This study focuses on developing liquid immunogenic fiducial eluter (LIFE) Biomaterial with components that include biodegradable polymers, nanoparticles, and an immunoadjuvant. LIFE Biomaterial is designed to provide image guidance during radiotherapy similar to clinically used liquid fiducials while enhancing therapeutic efficacy for advanced cervical cancer. C57BL6 mice were used to grow subcutaneous tumors on bilateral flanks. The tumor on one flank was then treated using LIFE Biomaterial prepared with the immunoadjuvant anti-CD40, with/without radiotherapy at 6 Gy. Computed tomography (CT) and magnetic resonance (MR) imaging visibility were also evaluated in human cadavers. A pharmacodynamics study was also conducted to assess the safety of LIFE Biomaterial in healthy C57BL6 female mice. Results showed that LIFE Biomaterial could provide both CT and MR imaging contrast over time. Inhibition in tumor growth and prolonged significant survival (* p < 0.05) were consistently observed for groups treated with the combination of radiotherapy and LIFE Biomaterial, highlighting the potential for this strategy. Minimal toxicity was observed for healthy mice treated with LIFE Biomaterial with/without anti-CD40 in comparison to non-treated cohorts. The results demonstrate promise for the further development and clinical translation of this approach to enhance the survival and quality of life of patients with advanced cervical cancer.
1. Introduction
Cervical cancer is the fourth leading cancer among women worldwide [1,2]. Most cervical cancers are driven by the human papillomavirus (HPV). The three pillars for eliminating cervical cancer include HPV vaccination, screening, and treatment [3,4,5,6]. The highest burden of cervical cancer incidence and mortality is in low-resource settings in the USA and low- to middle-income countries [7,8,9].
Common treatment options for patients with cervical cancer include surgery if diagnosed in its early stages [10,11,12]. Radiation therapy and chemotherapy, separately or in combination, are used for patients with advanced stages of cervical cancer [13,14,15,16]. External beam and internally delivered radiation (brachytherapy) are often used in the radiotherapy of cervical cancer [17,18]. Newer treatments, such as targeted therapy and immunotherapy, have been used in the last decades, including current FDA approval of the combination of pembrolizumab and chemoradiotherapy for the treatment of newly diagnosed, high-risk, locally advanced cervical cancer [19,20,21,22,23,24]. However, limitations of these approaches include long treatment regimens, side effects, and high costs that drive persistent disparities and impact patient’s overall quality of life [25,26,27,28]. New effective treatment options that can also reduce disparities are needed, especially for patients with advanced cervical cancer.
This study investigates a novel therapeutic approach involving liquid immunogenic fiducial eluter (LIFE) Biomaterial loaded with immunotherapeutic drug anti-CD40 monoclonal antibody. Anti-CD40 has been chosen specifically in this study because it has been shown in previous studies to be an effective immunoadjuvant in enhancing in situ vaccination during radiotherapy [29,30]. One of the treatment cohorts explored if adding anti-PD1 would have a significant additional impact on the radiotherapy plus LIFE Biomaterial treatment. Therapeutic approaches involving drug-loaded systems have been investigated pre-clinically, including hydrogel delivery systems that are biocompatible, non-toxic, and can provide space–time control over the release, localization, or distribution of various drugs. This takes advantage of the ability to optimize or control their behavior, biodegradation, and capacity to protect labile drug payloads from degradation [31,32,33,34]. Meanwhile, previous work has investigated novel fiducials for use during cervical cancer radiotherapy. For example, a calcium phosphate cement (CPC) marker was investigated as an injectable non-metallic fiducial marker [35]. This study, conducted in six patients receiving 3–5 injections of the CPC markers, demonstrated that such fiducials injected into cervical tumors can be visualized on cone beam computed tomography (CT) and MRI with reductions in marker loss and artifacts [33]. In this study, LIFE Biomaterial is designed to accomplish more in serving as multifunctional technology with the capacity to not only load drugs but also provide CT and MRI image guidance during radiotherapy. This study explores LIFE biomaterial’s potential to (1) provide image guidance during radiotherapy similar to fiducial markers with investigations in both animals and cadavers, (2) enhance therapeutic efficacy in advanced cervical cancer animal models, and (3) ensure minimal toxicity as investigated in healthy mice.
2. Materials and Methods
2.1. Preparation of LIFE Biomaterial
LIFE Biomaterial was designed with a combination of natural polymers, namely 2% (w/v) of chitosan (Sigma Aldrich, St. Louis, MO, USA) and 4% (w/v) of sodium alginate (Sigma Aldrich, St. Louis, MO, USA) hydrogel, mixed in a 1:1 ratio. In addition, two solutions of nanoparticles were prepared separately. One of the prepared solutions was 0.85 g/mL of titanium dioxide (TiO2 Anatase, 99.5%, 5 nm, US Research Nanomaterials Inc., Houston, TX, USA). The other solution was 0.287 g/mL of Omniscan gadodiamide gadolinium-based (GdNPs) nanoparticles (GE Healthcare, Silver Spring, MD, USA). Both the TiO2 and the GdNPs were mixed in a 1:1 ratio and added to the collective LIFE Biomaterial hydrogel in a ¼:1 ratio, respectively. LIFE Biomaterial hydrogel was maintained at 4 °C until treatment. On treatment day, LIFE Biomaterial was partitioned based on the number of mice being treated, and 20 µg per mouse of anti-CD40 (Clone FGK4.5, BioXCell, Lebanon, NH, USA) antibody was added to the volume of hydrogel to constitute LIFE Biomaterial. Among the treatment groups, one group received an intraperitoneal injection of 200 µg of anti-PD1 (Clone RMP1-14, BioXCell, Lebanon, NH, USA), in addition to the intratumoral injection of LIFE Biomaterial loaded with 20 µg of anti-CD40 and exposed to one fraction of 6 Gy. In this study, LIFE Biomaterial was injected intratumorally in one subcutaneous cervical tumor of a mouse to assess both the image guidance during radiotherapy and the therapeutic potential. The same amount was injected subcutaneously in healthy non-tumor-bearing mice to assess any toxicity-related effects.
The LIFE Biomaterial schematic is highlighted in Figure 1 with nanoparticles and liquid/drug localized in a 3D network of cross-linked polymer chains that is easy to administer to tumors similar to fiducials. A physical depiction of the LIFE Biomaterial is displayed in Figure 2A.Scanning and transmission electron microscopy (SEM and TEM, respectively) images highlighting the morphology and surface texture of LIFE Biomaterial colloid are shown in Figure 2B,C.
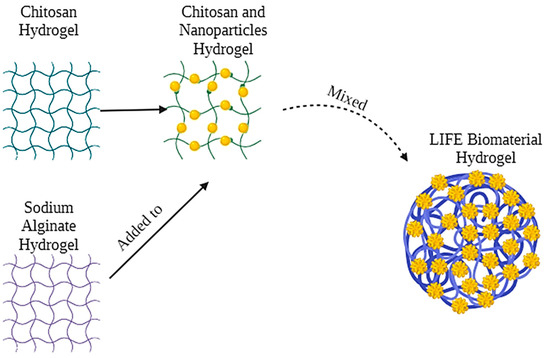
Figure 1.
LIFE Biomaterial depiction highlights the mixture of two natural polymers consisting of chitosan and sodium alginate and also incorporating nanoparticles that can provide both CT and MRI imaging contrasts to guide radiotherapy and enhance therapy outcomes.
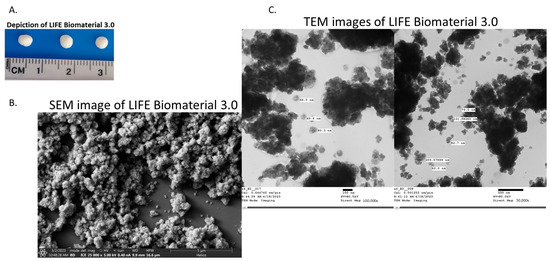
Figure 2.
Characterization of LIFE Biomaterial formulated with titanium dioxide and omniscan gadodiamide nanoparticles. (A) Photographs of LIFE Biomaterial colloid/gel. (B,C) SEM and TEM images of LIFE Biomaterial colloid. These images also visualize the micro-architectural properties of LIFE Biomaterial, such as its surface topology and organization of the polymeric networks.
2.2. TC1 Cell Culture Preparation and Tumor Growth in Mice
A cervical cancer cell line, TC1 (ATCC® CRL-2785, Manassas, VA, USA), was used to grow subcutaneous tumors in C57BL6 female mice following previously published protocols [34]. TC1 cells were cultured in RPMI 1640 medium supplemented with 2 Mm L-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, 1.0 mM sodium pyruvate, 0.1 mM non-essential amino acids, and 10% fetal bovine serum. All cells were cultured at 37 °C in a humidified incubator with 5% CO2. Immunocompetent wild-type C57BL/6 strain female mice were acquired from Charles River at 6–8 weeks old. They were inoculated subcutaneously with 3 × 105 cells mixed with Matrigel in the lateral flanks of mice and allowed to grow to a palpable tumor size of approximately 3 mm in diameter within ten days before the start of treatment. Mice tumor volume was determined using the formula V = 0.5 × length × (width2). A digital caliper was used to measure the longitude protrusion of the tumor designated as the tumor length and the latitude projection as the tumor width. Animal experiments followed the guidelines and regulations of the Johns Hopkins University Animal Care and Use Committee (ACUC) set under protocol# MO21M281. Mice maintenance in the Johns Hopkins University animal facility was according to the Institutional Animal Care and Use Committee approved guidelines.
2.3. Imaging and Efficacy Study
LIFE Biomaterial formulations prepared as described above were used to assess CT and MRI image contrasts in the C57BL6 mice bearing cervical subcutaneous tumors. Images were collected over different time points. The imaging was performed over several weeks to establish the potential for providing imaging contrast for hypo-fractionated radiotherapy. To further assess the feasibility of LIFE Biomaterial in providing imaging, contrast imaging was also conducted in a human cadaver approved by the Johns Hopkins Institutional Review Board (IRB) for protocol number NA_00070589 (PI KD). Refrigerated, unfixed cadaveric specimens were injected with LIFE Biomaterial following CT simulation (TOSHIBA Helical CT scan with 2 mm slice thickness, 120 kVp, and X-ray tube current of 100 mA) with the cadaveric specimen in the supine position. The specimens were then imaged again. For MRI, a Philips Achieva 3.0 T MRI System with BODY Transmit Coil was used with a repetition time of 5.31 ms, a flip angle of 100, a percent phase field of view of 70.833, and a slice thickness of 0.9 mm.
The potential of the formulations to enhance treatment outcomes was also investigated in the same mice bearing cervical tumors. The investigation with more than one tumor per mouse was representative of subjects with advanced cervical cancer burden with more than one tumor, as in the case of metastasis. A small animal radiation research platform was used to deliver a 6 Gy dose at 220 kVp locally at the tumor site using a 10 × 10 mm collimator size. Tumor volume and survival were assessed over time. Survival analysis using the log-rank (Mantel-Cox) test was performed using GraphPad Prism version 9.5.1 for Windows. (GraphPad Software, San Diego, CA USA). Analyzed data were deemed significant if their p-values were within the following ranges: (* p < 0.05) and (** p < 0.01).
2.4. Pharmacodynamics of LIFE Biomaterial in Healthy Mice
Seven- to eight-week-old wild-type C57BL6 female mice were acquired from Charles River. Eleven- to thirteen-week-old healthy mice (n = 36) were allocated into several groups. Animals in one cluster received one inoculation of LIFE Biomaterial loaded with anti-CD40 (20 µg) (n = 12). A second set was administered with LIFE Biomaterial without anti-CD40 (n = 12). These noted groups were investigated for comparison against a healthy animal group with no treatment (n = 12). Mice were maintained, and all studies followed the Johns Hopkins University ACUC-approved standard of practice and protocol (#MO21M281).
Blood was drawn from the vena cava vein at several time points (day 1, 30, 60, and 90) post-treatment to evaluate hepatic and renal function parameters, including alkaline phosphatase, total protein, alanine transaminase (ALT), aspartate aminotransferase (AST), bilirubin direct, total bilirubin, gamma-glutamyl transferase (GGT), glucose, blood urea nitrogen (BUN), and creatinine, among other parameters. Results were compared to those of healthy mice with no treatment. Blood serum was separated within one hour after collection by centrifuging collected blood at 5000 rpm for 10 min at 4 °C following a standard protocol and sent for analysis. All collected sera were sent to VRL Laboratories (San Antonio, TX, USA) for analysis.
Complete blood count (CBC) hematology analysis was performed for a variety of parameters, highlighting red blood cells (RBCs), Hematocrit (HCT %), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), monocyte (MONO) count, and hemoglobin (HGB), among other CBC parameters at the following time points: days 1, 30, 60, and 90 post-treatment following vena cava blood sampling. Whole blood was collected into EDTA tubes and kept at 2–8 °C to send for analysis. Whole blood samples were sent for analysis to VRL Laboratories (San Antonio, TX, USA), within 24 h after collection.
Histopathological analysis of different organs (e.g., heart, lung, left and right kidneys, liver, and spleen) was achieved following vena cava blood sampling of treated and non-treated healthy mice at different time points post-treatment: days 1, 30, 60, and 90. Tissue samples were sent to VRL Laboratories (San Antonio, TX, USA) for histopathology assessments.
3. Results
3.1. Potential of LIFE Biomaterial for Image-Guided Radiotherapy
The first studies assessed the potential of LIFE Biomaterial to provide imaging contrast during radiotherapy similar to fiducials. Figure 3a shows both CT and MR imaging contrast over time from LIFE Biomaterial, as indicated by the red arrow. The region with the secondary tumor that was not administered with LIFE Biomaterial is highlighted within the yellow dotted circle. CT images are shown in Figure 3b starting a week post-treatment up to 23 days post-administration of LIFE Biomaterial. Figure 3c highlights both imaging modalities of LIFE Biomaterial in human cadavers.
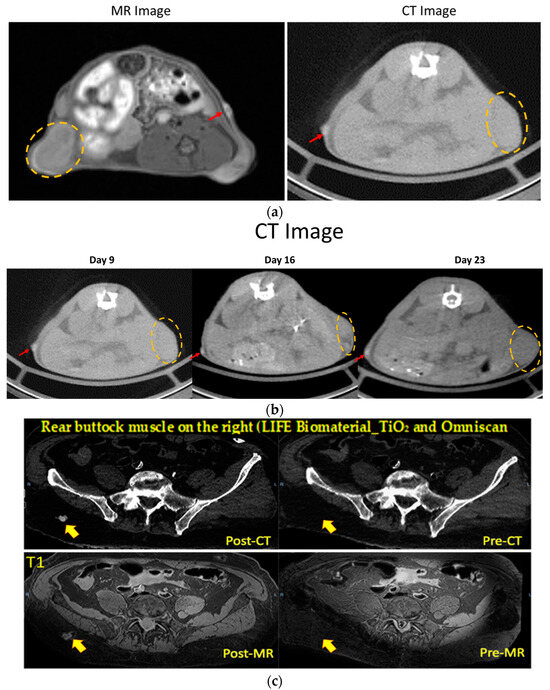
Figure 3.
The image contrast of LIFE Biomaterial in cervical tumors and a human cadaver. (a,b) LIFE Biomaterial provided CT and MR contrasts as indicated by the red arrows within the same cervical tumor mouse where the tumor is highlighted by the orange dotted circles. The CT contrast can be observed up to day 23. (c) LIFE Biomaterial seen in both CT and MR imaging modalities in a human cadaver denoted by the red/orange arrows.
3.2. Efficacy of the LIFE Biomaterial in Cervical Cancer
Two separate studies were conducted. In the first study, thirty mice bearing two subcutaneous tumors were used and divided into the following cohorts: (a) control (no treatment) (n =7); (b) one fraction of 6 Gy (n = 7); (c) LIFE Biomaterial loaded with 20 µg of anti-CD40 antibody (n = 8); and (d) LIFE Biomaterial loaded with 20 µg of anti-CD40 antibody and receiving one fraction of 6 Gy (n = 8). During the second study, the treatment parameters were varied with the following groups: (a) control (no treatment) (n = 6); (b) LIFE Biomaterial loaded with 20 µg of anti-CD40 antibody and 6 Gy (n = 6); (c) LIFE Biomaterial loaded with 20 µg of anti-CD40 antibody in addition to free injection of 20 µg of anti-CD40 and 6 Gy (n = 6); (d) LIFE Biomaterial loaded with 20 µg of anti-CD40 antibody in addition to intraperitoneal injection of 200 µg of anti-PD1 and 6 Gy (n = 6); and (e) free injection of 20 µg of anti-CD40 intratumorally. All treatments were administered in only one primary tumor per mouse, not the secondary tumor (Figure 4a). Both tumors were monitored for tumor volume and mice survival over time.
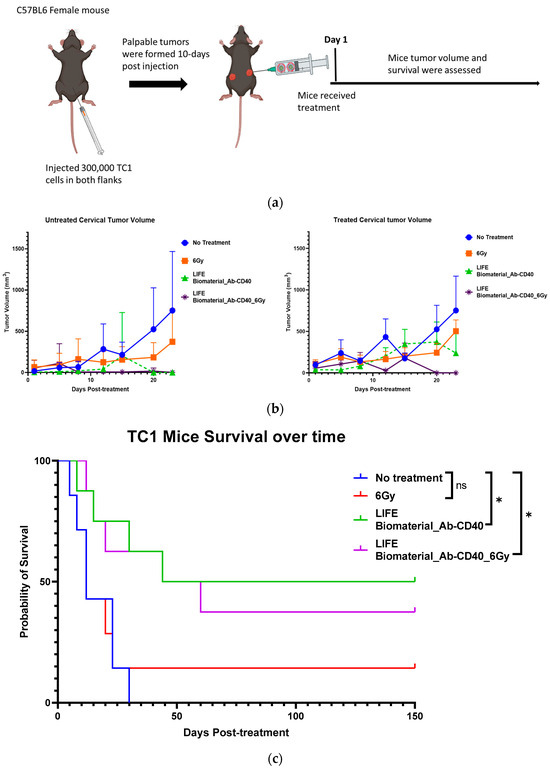
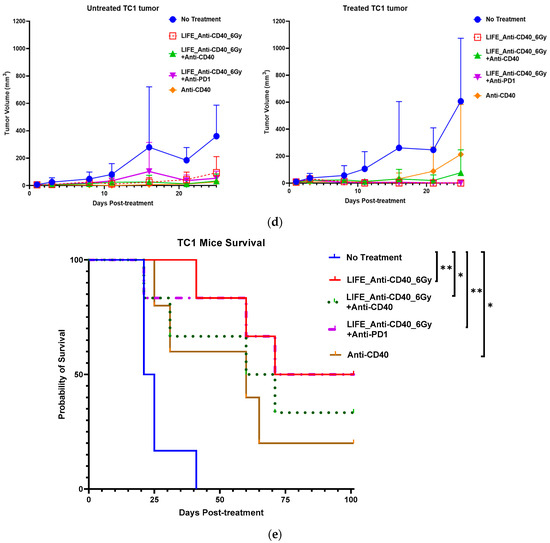
Figure 4.
Efficacy of LIFE Biomaterial loaded anti-CD40 antibody in mice cervical tumors. (a) Study design using C57BL6 female mice bearing subcutaneous tumors on lateral flanks with treatment according to the groups shown in the plots in (b,c). Slower tumor growth was observed for the groups treated with LIFE Biomaterial loaded anti-CD40 antibody, and significantly (* p < 0.05) longer mice survival was observed for up to 5 months post-treatment. The following study also showed similar trends relating to slowed tumor growth (d) and significantly (* p < 0.05 and ** p < 0.01)) longer survival post-treatment and ‘ns’ denotes not significant (e), highlighting the potential to further optimize the treatment approach.
Beyond LIFE Biomaterial providing image contrast/guidance (as in Figure 3), LIFE Biomaterial is designed to also augment the response to local therapy. Figure 4 highlights the inhibition observed for the groups locally treated with LIFE Biomaterial loaded with anti-CD40 monoclonal antibodies (mAbs) with/without one fraction of 6 Gy. Tumor growth was inhibited for both the treated/primary tumor and the untreated secondary tumor in both sets of studies shown in Figure 4b–e. Response in treatment groups without radiation highlights the fact, also seen in other studies, that anti-CD40 itself can cause immunogenic cell death and in situ vaccination [35,36].
Other groups that received either a free injection of anti-CD40 or one fraction of 6 Gy alone also showed some tumor growth inhibition but not as much as with LIFE Biomaterial treatment groups. Overall, from these two studies, a significant increase in survival is consistently observed for groups treated with LIFE Biomaterial. The results also show the potential for further optimization studies, e.g., with other doses of anti-CD40 or radiotherapy.
3.3. Pharmacodynamics of LIFE Biomaterial in Healthy Female Mice
It is important to show that LIFE Biomaterial itself is not toxic to healthy animals. Immunocompetent, wild-type C57BL/6 female mice were used for toxicity studies in healthy mice. Biochemical analysis evaluated several parameters related to renal and hepatic functions highlighted in Figure 5 and Supplementary Materials Table S1 following vena cava blood collection techniques comparing the no-treatment group against the treated groups (n = 3–4/time point). The time points were days 1, 30, 60, and 90 post-treatment.
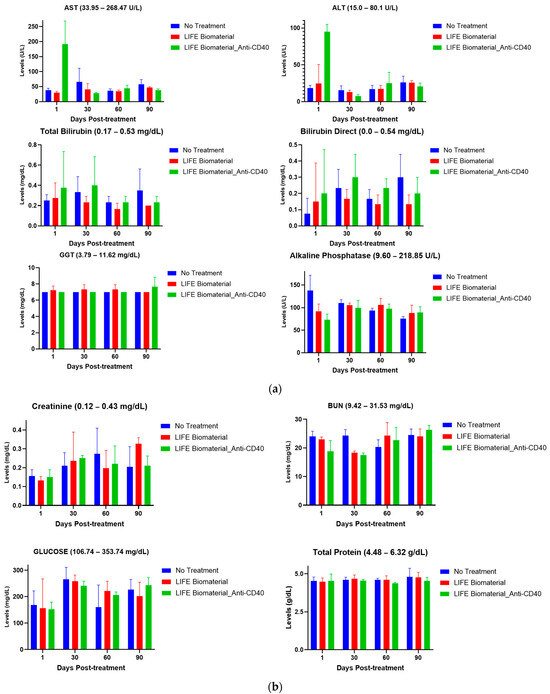
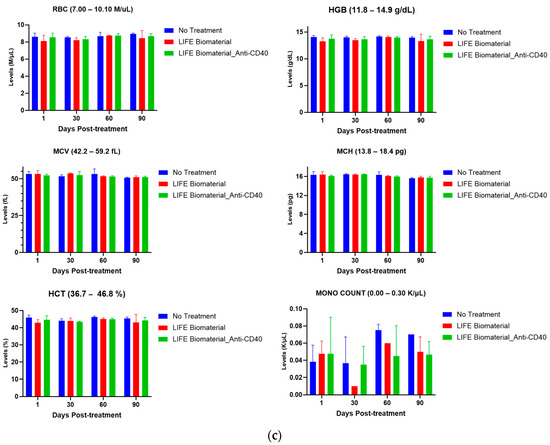
Figure 5.
Pharmacodynamics of healthy mice comparing treated mice with LIFE Biomaterial unloaded/loaded with anti-CD40 against a healthy non-treated cohort of mice at different time points. The reference range for each parameter is annotated in the title of each plot. (a) Hepatic function parameters, such as alanine transaminase (ALT), aspartate aminotransferase (AST), bilirubin direct, total bilirubin, gamma-glutamyl transferase (GGT), and alkaline phosphatase, were within the normal reference range for all of the cohorts investigated. (b) Renal function parameters, including blood urea nitrogen (BUN), creatinine, total protein, and glucose, were all within the noted reference ranges given for each particular parameter for all of the tested cohorts. (c) Blood cell count analysis of red blood cells (RBCs), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and monocyte (MONO) count were within the reference range given by VRL Laboratories and were comparable to the healthy group of mice at the different time points tested.
3.3.1. Hepatic Function
Hepatic function was evaluated in C57BL6 female mice comparing a healthy group of mice against the treated cohorts using LIFE Biomaterial unloaded/loaded with anti-CD40 monoclonal antibody. Various parameters (e.g., alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin, gamma-glutamyl transferase (GGT), bilirubin direct, and alkaline phosphatase) were tested to assess the effect of the drug product on the liver. The toxicity parameters for both treatment and control groups were found to be within the range that shows minimal toxicity at the different time points tested, as highlighted in Figure 5a. All the parameters measured for hepatic function are mentioned in Supplementary Materials Table S1.
3.3.2. Renal Function
Renal function was also gaged where different parameters (e.g., glucose, blood urea nitrogen (BUN), total protein, and creatinine) were tested to assess how safe the drug product is as it relates to impacting the kidney function of healthy mice. Based on the reference ranges provided by VRL Laboratories, there was no adverse effect on kidney function, as emphasized in Figure 5b. All the parameters measured for renal functions are displayed in Supplementary Materials Table S1.
3.3.3. Cell Blood Count Analysis
Cell blood count analysis was performed using whole blood collected from healthy C57BL6 female mice at different time points (days 1, 30, 60, and 90) to investigate the overall health and find a wide range of conditions, including anemia, infection, and other health conditions after administering LIFE Biomaterial treatment with/without loaded anti-CD40. Hematological analysis was performed for many parameters, such as red blood cells (RBCs), hemoglobin (HGB), monocyte (MONO) count, hematocrit (HCT %), mean corpuscular hemoglobin (MCH), and mean corpuscular volume (MCV), among other parameters. Figure 5c displays the normal blood cell count for the treated groups compared to the non-treated group of mice. Supplementary Materials Table S2 displays the complete blood cell count parameters for all the cohorts at all collection time points.
This study assessed biochemical parameters related to renal and hepatic functions of healthy female mice treated with LIFE Biomaterial unloaded/loaded with anti-CD40 mAbs and compared the results with a control (no treatment) healthy cohort of mice at different time points. Figure 5a,b and Supplementary Materials Table S1 highlight minimum to no damages incurred by the liver and/or kidneys among all the cohorts at days 1, 30, 60, and 90 post-treatment. The average values represented on the plots were within the reference range given for each tested parameter by VRL Laboratories or demonstrated minimal to no toxicities. Comparable cell blood count values for control and treated groups are shown in Figure 5c and Supplementary Materials Table S2, as observed for all the cohorts at the different collection times.
Histopathological assessments of the organs collected (heart, lung, liver, left and right kidneys, and spleen) at each collection time point (days 1, 30, 60, and 90) post-treatment are reported in Supplementary Materials Tables S3–S6, showing minimal to no lesions found in all the organs, except for the liver, which incurred minimal to mild lesions that were comparable across all the tested cohorts. A lesion is defined as any damage that occurred due to any treatments given to the mouse. Some common liver lesions that occurred in either the no-treatment or the treated cohorts consisted of (a) micro-granulomas—small aggregates of inflammatory cells, up to 100 cells, consisting of macrophages, lymphocytes, and neutrophils, which often contain one or more entrapped degenerating hepatocytes. In micro-granulomas, the mononuclear inflammatory cells predominate the cellular aggregate. (b) Micro-abscesses are small aggregates of inflammatory cells, up to 100 cells, consisting of macrophages, lymphocytes, and neutrophils, which often contain one or more entrapped degenerating hepatocytes. In micro-abscesses, the neutrophils are the predominant inflammatory cells in the cellular aggregate. Supplementary Materials Tables S3–S6 provide a detailed account of the pathology reports performed for each organ at each collection time point for all the cohorts using a scoring definitions system defined by VRL Laboratories as 0 = No finding; 1 = Minimal; 2 = Mild; 3 = Moderate; 4 = Marked; 5 = Severe; N = Normal; M = Missing; MF = Multifocal; F = Focal; D = Diffuse; U = Unilateral; and B = Bilateral. Minimum to no significant lesions were reported in Supplementary Materials Tables S3–S6 for all the organs collected for both the non-treated group and the treated cohorts.
4. Discussion
The results in this study demonstrate the potential for the use of LIFE Biomaterial technology for imaging contrast over time similar to that of fiducial markers. Development and clinical translation could provide a new option for fiducial markers. A limitation of this study is that the time point LIFE Biomaterial biodegrades and ceases to provide imaging contrast has not been established. However, ongoing studies will complete this characterization. The current studies clearly demonstrate image contrast that can be useful for image-guided radiotherapy accommodating hypo-fractionated radiotherapy schedules. Importantly, this provides contrast for both MRI and CT, as treatment planning for cervical cancer often incorporates both modalities. Notably, LIFE Biomaterial is composed of natural biodegradable polymers and biocompatible nanoparticles that can be cleared over time.
The advantage of LIFE Biomaterial is that it is multifunctional and can also enhance therapeutic efficacy, as seen in this experimental model for metastatic cervical cancer. Limitations of this study in this respect include the need for the variation of additional parameters, such as the anti-CD40 dose or radiotherapy dose, including other hypo-fractionation schedules, to provide more insights and optimization outcomes. The studies demonstrate the potential for the optimization of therapy outcomes via this approach. Ongoing studies will explore these parameters and immune cell populations to elucidate the apparent response of the non-treated tumors, consistent with in situ vaccination reported in previous work. The results also motivate future studies that could explore the release of cytokines and any surface expression of different inhibitory/stimulatory molecules. Another limitation of this study in terms of therapy outcomes consists of using the subcutaneous model of cervical cancer for its simplicity. Studies involving orthotopic or genetic models would be valuable in providing additional rigor.
CD40, a receptor from the tumor necrosis factor (TNF) family, is heavily expressed on dendritic cells, which are vital for inciting an antigen-specific T-cell response [37,38]. In addition, cancer cells can undergo apoptosis induced by CD40 intracellular signaling, generating neo-antigens to help boost anti-cancer immune responses [39,40,41]. This ability is highlighted by the results showing that the use of anti-CD40/LIFE Biomaterial without radiotherapy can also lead to slowing both treated and untreated tumors in the same animal. In perspective, previous work [42,43] notes that the quality of CD40 stimulation may vary as the CD40 receptor has pleiotropic effects, and different residues on the CD40L/CD40 interaction may result in the differential production of pro-inflammatory cytokines. Our previous work [37,44] highlighted that when engaged by CD40L or by an agonistic antibody, CD40 signaling may lead to NF-κB upregulation and the expression of costimulatory ligands, the production of IL-12 and other cytokines, enhanced antigen presentation, and in the case of dendritic cells, the upregulation of CCR7 and trafficking to the draining lymph node. Overall, this previous work suggests the potential for synergies between radiotherapy and adjuvants like anti-CD40. One of the major limitations of anti-CD40 immunotherapy has been systemic toxicity. The use of LIFE Biomaterial presents advantages to minimize the off-target toxicities that can impact the quality of the treatment and life of subjects [45]. The addition of an anti-PD1 antibody did not seem to generate any significant additional effect on the tumor volume regression and mice survival over time. Further studies of varying parameters, such as dose, are needed to clearly establish any potential additional benefits.
The pharmacodynamics study was conducted at pre-determined time points for healthy cohorts of mice comparing a non-treated group to those treated with one single injection of either unloaded LIFE Biomaterial or LIFE Biomaterial loaded with anti-CD40 monoclonal antibody. Hepatic and renal parameter levels from mice treated with LIFE Biomaterial unloaded/loaded with anti-CD40 were found to be within the normal range and comparable with the levels from the untreated mice on days 1, 30, 60, and 90 post-intervention of the study. Similarly, the hematological analysis assessing the blood count levels remained within the normal range for the parameters tested with no substantial variations in the documented levels between the treatment groups and the control. The pathology report presented in the Supplementary Materials further corroborates the minimal lesions results found in the collected organs, such as the heart, spleen, liver, left and right kidneys, and the lungs of the non-treated and treated cohorts. In studies further optimizing therapeutic efficacy, the toxicity parameters examined here can also be further investigated.
5. Conclusions
Overall, this study highlights pre-clinical evidence for a novel multifunctional radiotherapy biomaterial approach that can be developed for advanced cervical cancer treatment. The imaging, therapy, and safety results demonstrate that this approach has significant potential for optimization and clinical translation to enhance the survival and quality of life of advanced cervical cancer patients. Moreover, the potential for reducing treatment time/costs via such an approach provides an exciting opportunity for reducing disparities.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers16061212/s1, Table S1: Hepatic and Renal function panels in female mice. Table S2: Hematology analysis from female mice. Table S3: Histopathology Report for Female mice Day 1. Table S4: Histopathology Report for Female mice Day 30. Table S5: Histopathology Report for Female mice Day 60. Table S6: Histopathology Report for Female mice Day 90.
Author Contributions
Conceptualization, M.M. and W.N.; methodology, M.M.; software, D.C. and H.H.; validation, M.M., S.A., D.C., L.S.K., S.M., G.S., K.F.C., E.S., J.W., P.O., E.B., K.D., A.V. and W.N.; formal analysis, M.M., D.C. and H.H.; investigation, M.M.; resources, M.M., S.A., D.C., L.S.K., S.M., G.S., K.F.C., E.S., J.W., P.O., E.B., K.D. and W.N.; data curation, M.M.; writing—M.M. and W.N.; writing—review and editing, M.M., S.A., D.C., L.S.K., S.M., G.S., K.F.C., E.S., J.W., P.O., E.B., K.D., A.V. and W.N.; visualization, M.M.; supervision, W.N.; project administration, W.N.; funding acquisition, W.N. All authors have read and agreed to the published version of the manuscript.
Funding
The National Institutes of Health, award numbers R41CA268623 and R01CA239042, funded this research. The content is solely the authors’ responsibility and does not necessarily represent the views of the National Institutes of Health.
Institutional Review Board Statement
The animal study was revised and permitted by the Johns Hopkins University Animal Care and Use Committee (ACUC) for protocol #MO21M281 approved on 6 October 2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials. Data are also available upon request from the corresponding author.
Acknowledgments
We would like to acknowledge VRL Laboratories for conducting the pharmacodynamics analyses from the tissues collected. We are grateful for their service.
Conflicts of Interest
The authors declare no conflicts of interest, excluding Jacques Walker, Philmo Oh, and Eric Broyles, who partake in consulting/advisory roles with Nanocan Therapeutics.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Vu, M.; Yu, J.; Awolude, O.A.; Chuang, L. Cervical Cancer Worldwide. Curr. Probl. Cancer 2018, 42, 457–465. [Google Scholar] [CrossRef]
- Luckett, R.; Feldman, S. Impact of 2-, 4- and 9-Valent HPV Vaccines on Morbidity and Mortality from Cervical Cancer. Hum. Vaccin. Immunother. 2016, 12, 1332–1342. [Google Scholar] [CrossRef]
- Choi, S.; Ismail, A.; Pappas-Gogos, G.; Boussios, S. HPV and Cervical Cancer: A Review of Epidemiology and Screening Uptake in the UK. Pathogens 2023, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Aarnio, R.; Östensson, E.; Olovsson, M.; Gustavsson, I.; Gyllensten, U. Cost-Effectiveness Analysis of Repeated Self-Sampling for HPV Testing in Primary Cervical Screening: A Randomized Study. BMC Cancer 2020, 20, 645. [Google Scholar] [CrossRef] [PubMed]
- Shojaeizadeh, D.; Hashemi, S.Z.; Moeini, B.; Poorolajal, J. The Effect of Educational Program on Increasing Cervical Cancer Screening Behavior among Women in Hamadan, Iran: Applying Health Belief Model. J. Res. Health Sci. 2011, 11, 20–25. [Google Scholar]
- Srinath, A.; van Merode, F.; Rao, S.V.; Pavlova, M. Barriers to Cervical Cancer and Breast Cancer Screening Uptake in Low- and Middle-Income Countries: A Systematic Review. Health Policy Plan. 2023, 38, 509–527. [Google Scholar] [CrossRef]
- Akinlotan, M.; Bolin, J.N.; Helduser, J.; Ojinnaka, C.; Lichorad, A.; McClellan, D. Cervical Cancer Screening Barriers and Risk Factor Knowledge Among Uninsured Women. J. Community Health 2017, 42, 770–778. [Google Scholar] [CrossRef]
- Al-amro, S.Q.; Gharaibeh, M.K.; Oweis, A.I. Factors Associated with Cervical Cancer Screening Uptake: Implications for the Health of Women in Jordan. Infect. Dis. Obstet. Gynecol. 2020, 2020, 9690473. [Google Scholar] [CrossRef] [PubMed]
- Fusegi, A.; Kanao, H.; Tsumura, S.; Murakami, A.; Abe, A.; Aoki, Y.; Nomura, H. Minimally Invasive Radical Hysterectomy and the Importance of Avoiding Cancer Cell Spillage for Early-Stage Cervical Cancer: A Narrative Review. J. Gynecol. Oncol. 2023, 34, e5. [Google Scholar] [CrossRef]
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef] [PubMed]
- Bansal, N.; Herzog, T.J.; Shaw, R.E.; Burke, W.M.; Deutsch, I.; Wright, J.D. Primary Therapy for Early-Stage Cervical Cancer: Radical Hysterectomy vs Radiation. Am. J. Obstet. Gynecol. 2009, 201, e1–e485. [Google Scholar] [CrossRef] [PubMed]
- Sagae, S.; Toita, T.; Matsuura, M.; Saito, M.; Matsuda, T.; Sato, N.; Shimizu, A.; Endo, T.; Fujii, M.; Gaffney, D.K.; et al. Improvement in Radiation Techniques for Locally Advanced Cervical Cancer during the Last Two Decades. Int. J. Gynecol. Cancer 2023, 33, 1295–1303. [Google Scholar] [CrossRef]
- Pötter, R.; Georg, P.; Dimopoulos, J.C.A.; Grimm, M.; Berger, D.; Nesvacil, N.; Georg, D.; Schmid, M.P.; Reinthaller, A.; Sturdza, A.; et al. Clinical Outcome of Protocol Based Image (MRI) Guided Adaptive Brachytherapy Combined with 3D Conformal Radiotherapy with or without Chemotherapy in Patients with Locally Advanced Cervical Cancer. Radiother. Oncol. 2011, 100, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Mileshkin, L.R.; Moore, K.N.; Barnes, E.H.; Gebski, V.; Narayan, K.; King, M.T.; Bradshaw, N.; Lee, Y.C.; Diamante, K.; Fyles, A.W.; et al. Adjuvant Chemotherapy Following Chemoradiotherapy as Primary Treatment for Locally Advanced Cervical Cancer versus Chemoradiotherapy Alone (OUTBACK): An International, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2023, 24, 468–482. [Google Scholar] [CrossRef]
- Vittrup, A.S.; Kirchheiner, K.; Pötter, R.; Fokdal, L.U.; Jensen, N.B.K.; Spampinato, S.; Haie-Meder, C.; Schmid, M.P.; Sturdza, A.E.; Mahantshetty, U.; et al. Overall Severe Morbidity After Chemo-Radiation Therapy and Magnetic Resonance Imaging-Guided Adaptive Brachytherapy in Locally Advanced Cervical Cancer: Results From the EMBRACE-I Study. Int. J. Radiat. Oncol. 2023, 116, 807–824. [Google Scholar] [CrossRef]
- Chargari, C.; Peignaux, K.; Escande, A.; Renard, S.; Lafond, C.; Petit, A.; Lam Cham Kee, D.; Durdux, C.; Haie-Méder, C. Radiotherapy of Cervical Cancer. Cancer/Radiothérapie 2022, 26, 298–308. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, H.; Mei, W.; Zhang, P.; Zeng, C. Combining Radiotherapy with Immunotherapy in Cervical Cancer: Where Do We Stand and Where Are We Going? Curr. Treat. Options Oncol. 2023, 24, 1378–1391. [Google Scholar] [CrossRef]
- Vora, C.; Gupta, S. Targeted Therapy in Cervical Cancer. ESMO Open 2018, 3, e000462. [Google Scholar] [CrossRef] [PubMed]
- Crafton, S.M.; Salani, R. Beyond Chemotherapy: An Overview and Review of Targeted Therapy in Cervical Cancer. Clin. Ther. 2016, 38, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Menderes, G.; Black, J.; Schwab, C.L.; Santin, A.D. Immunotherapy and Targeted Therapy for Cervical Cancer: An Update. Expert Rev. Anticancer Ther. 2016, 16, 83–98. [Google Scholar] [CrossRef]
- Doghish, A.S.; Ali, M.A.; Elyan, S.S.; Elrebehy, M.A.; Mohamed, H.H.; Mansour, R.M.; Elgohary, A.; Ghanem, A.; Faraag, A.H.I.; Abdelmaksoud, N.M.; et al. MiRNAs Role in Cervical Cancer Pathogenesis and Targeted Therapy: Signaling Pathways Interplay. Pathol. Res. Pract. 2023, 244, 154386. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Cui, H.; Qin, S.; Yu, S. Manipulating TGF-β Signaling to Optimize Immunotherapy for Cervical Cancer. Biomed. Pharmacother. 2023, 166, 115355. [Google Scholar] [CrossRef] [PubMed]
- Grau, J.-F.; Farinas-Madrid, L.; Garcia-Duran, C.; Garcia-Illescas, D.; Oaknin, A. Advances in Immunotherapy in Cervical Cancer. Int. J. Gynecol. Cancer 2023, 33, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Gottschlich, A.; Payne, B.A.; Trawin, J.; Albert, A.; Jeronimo, J.; Mitchell-Foster, S.; Mithani, N.; Namugosa, R.; Naguti, P.; Orem, J.; et al. Experiences with Thermal Ablation for Cervical Precancer Treatment after Self-collection HPV-based Screening in the ASPIRE Mayuge Randomized Trial. Int. J. Cancer 2023, 152, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, K.; Yu, Z.; Zhang, B.; Chen, C.; Lin, L.; Li, Z.; Li, Z.; Zheng, Y.; Yu, Z. Targeting Self-Enhanced ROS-Responsive Artesunatum Prodrug Nanoassembly Potentiates Gemcitabine Activity by down-Regulating CDA Expression in Cervical Cancer. Chin. Chem. Lett. 2023, 34, 108184. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Jiang, P.; Wang, X.; Zhang, L.; Zhang, Y. Clinical Research of the Value of High-Risk CTV Setting on Intensity-Modulated Radiotherapy for Stage IIB-IVA Cervical Cancer. BMC Cancer 2023, 23, 481. [Google Scholar] [CrossRef] [PubMed]
- Babakanrad, E.; Mohammadian, T.; Esmaeili, D.; Behzadi, P. Cervical Cancer: A Review of Epidemiology, Treatments and Anticancer Drugs. Curr. Cancer Ther. Rev. 2023, 19, 198–212. [Google Scholar] [CrossRef]
- Moreau, M.; Yasmin-Karim, S.; Kunjachan, S.; Sinha, N.; Gremse, F.; Kumar, R.; Chow, K.F.; Ngwa, W. Priming the Abscopal Effect Using Multifunctional Smart Radiotherapy Biomaterials Loaded with Immunoadjuvants. Front. Oncol. 2018, 8, 56. [Google Scholar] [CrossRef]
- Wood, J.; Yasmin-Karim, S.; Mueller, R.; Viswanathan, A.N.; Ngwa, W. Single Radiotherapy Fraction with Local Anti-CD40 Therapy Generates Effective Abscopal Responses in Mouse Models of Cervical Cancer. Cancers 2020, 12, 1026. [Google Scholar] [CrossRef]
- Bishnoi, A.; Chanda, S.; Bonde, G.V.; Tiwari, R.K. Advanced Drug Delivery System for Treating Inflammation. In Recent Developments in Anti-Inflammatory Therapy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 155–161. [Google Scholar]
- Charman, W.N.; Chan, H.-K.; Finnin, B.C.; Charman, S.A. Drug Delivery: A Key Factor in Realising the Full Therapeutic Potential of Drugs. Drug Dev. Res. 1999, 46, 316–327. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Ogino, I.; Kitagawa, M.; Watanabe, S.; Yoshida, H.; Hata, M. Calcium Phosphate Cement Paste Injection as a Fiducial Marker of Cervical Cancer. In Vivo 2018, 32, 1609–1615. [Google Scholar] [CrossRef]
- Sadraeian, M.; Khoshnood Mansoorkhani, M.J.; Mohkam, M.; Rasoul-Amini, S.; Hesaraki, M.; Ghasemi, Y. Prevention and Inhibition of TC-1 Cell Growth in Tumor Bearing Mice by HPV16 E7 Protein in Fusion with Shiga Toxin B-Subunit from Shigella Dysenteriae. Cell J. 2013, 15, 176–181. [Google Scholar]
- Yasmin-Karim, S.; Bruck, P.T.; Moreau, M.; Kunjachan, S.; Chen, G.Z.; Kumar, R.; Grabow, S.; Dougan, S.K.; Ngwa, W. Radiation and Local Anti-CD40 Generate an Effective in Situ Vaccine in Preclinical Models of Pancreatic Cancer. Front. Immunol. 2018, 9, 2030. [Google Scholar] [CrossRef] [PubMed]
- Lanzavecchia, A. Licence to Kill. Nature 1998, 393, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Ziebold, J.L.; Hixon, J.; Boyd, A.; Murphy, W.J. Differential Effects of CD40 Stimulation on Normal and Neoplastic Cell Growth. Arch. Immunol. Ther. Exp. 2000, 48, 225–233. [Google Scholar]
- Costello, R.T.; Gastaut, J.-A.; Olive, D. What Is the Real Role of CD40 in Cancer Immunotherapy? Immunol. Today 1999, 20, 488–493. [Google Scholar] [CrossRef]
- Wu, R.C.; Luke, J.J. Uncovering the Potential of CD40 Agonism to Enhance Immune Checkpoint Blockade. Clin. Cancer Res. 2024, 30, 9–11. [Google Scholar] [CrossRef]
- Sarode, A.Y.; Jha, M.K.; Zutshi, S.; Ghosh, S.K.; Mahor, H.; Sarma, U.; Saha, B. Residue-Specific Message Encoding in CD40-Ligand. iScience 2020, 23, 101441. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Gurjar, D.; Saha, B.; Bodhale, N. Decoding the Contextual Duality of CD40 Functions. Hum. Immunol. 2023, 84, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Yasmin-Karim, S.; Ziberi, B.; Wirtz, J.; Bih, N.; Moreau, M.; Guthier, R.; Ainsworth, V.; Hesser, J.; Makrigiorgos, G.M.; Chuong, M.D.; et al. Boosting the Abscopal Effect Using Immunogenic Biomaterials With Varying Radiation Therapy Field Sizes. Int. J. Radiat. Oncol. 2022, 112, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Acter, S.; Ngema, L.M.; Bih, N.; Sy, G.; Keno, L.S.; Chow, K.F.; Sajo, E.; Nebangwa, O.; Walker, J.; et al. Pre-Clinical Investigations of the Pharmacodynamics of Immunogenic Smart Radiotherapy Biomaterials (ISRB). Pharmaceutics 2023, 15, 2778. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).