Long-Term Survival in Patients with Oligometastatic Non-Small Cell Lung Cancer by a Multimodality Treatment—Comparison with Stage III Disease
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Statistical Analysis
3. Results
3.1. Baseline Patient Characteristics
3.2. OMD Patients
3.3. Stage III Patients
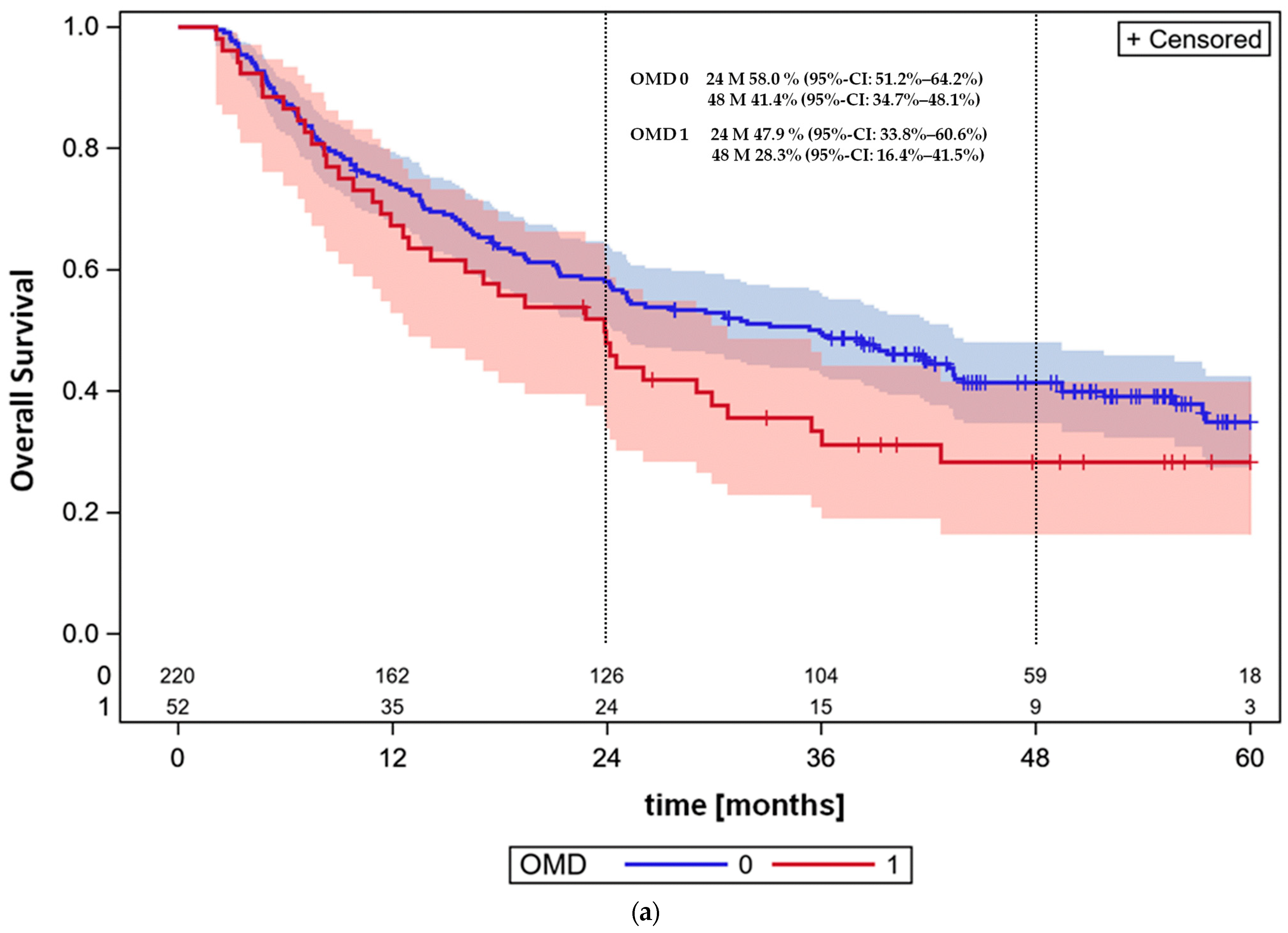
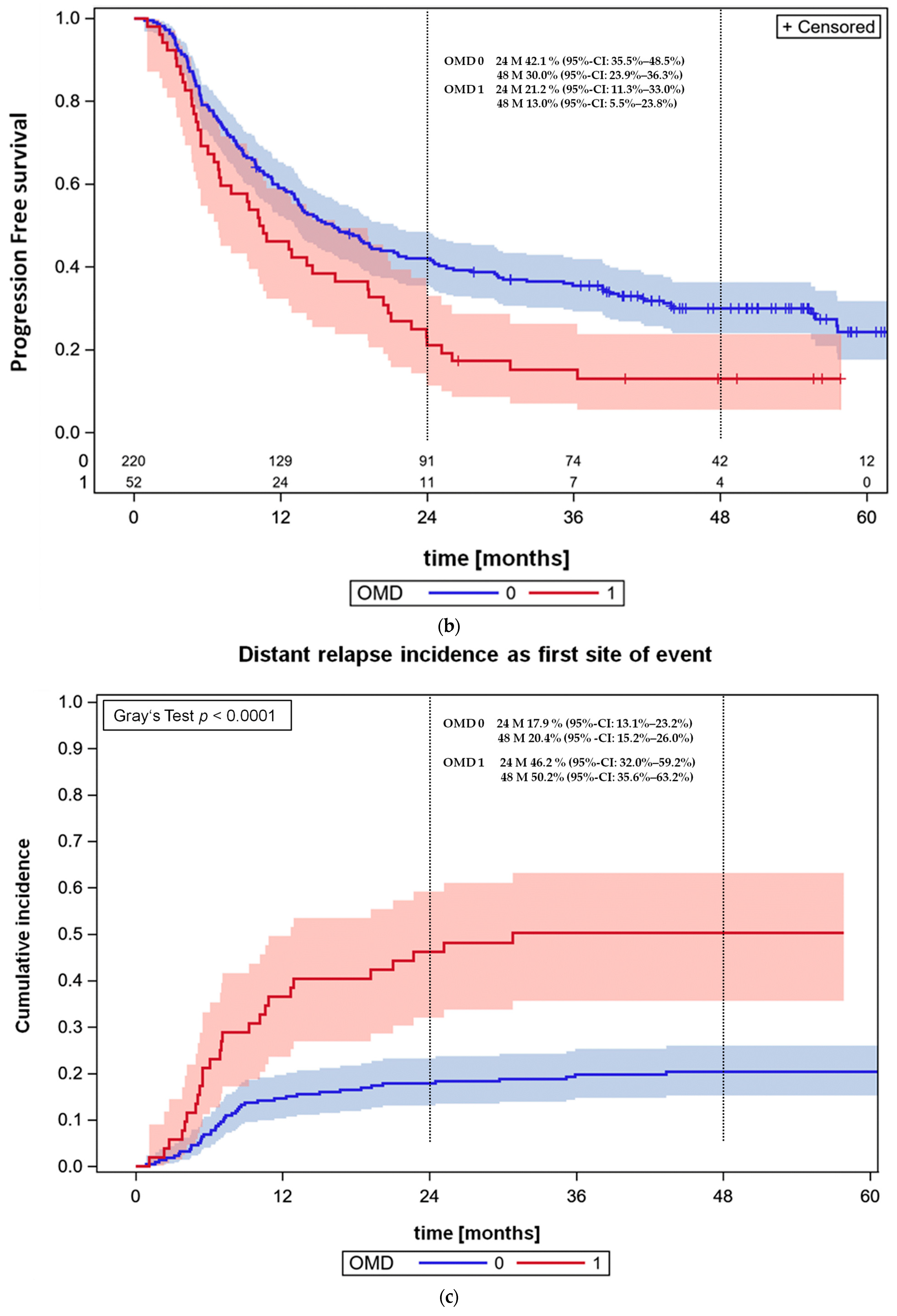
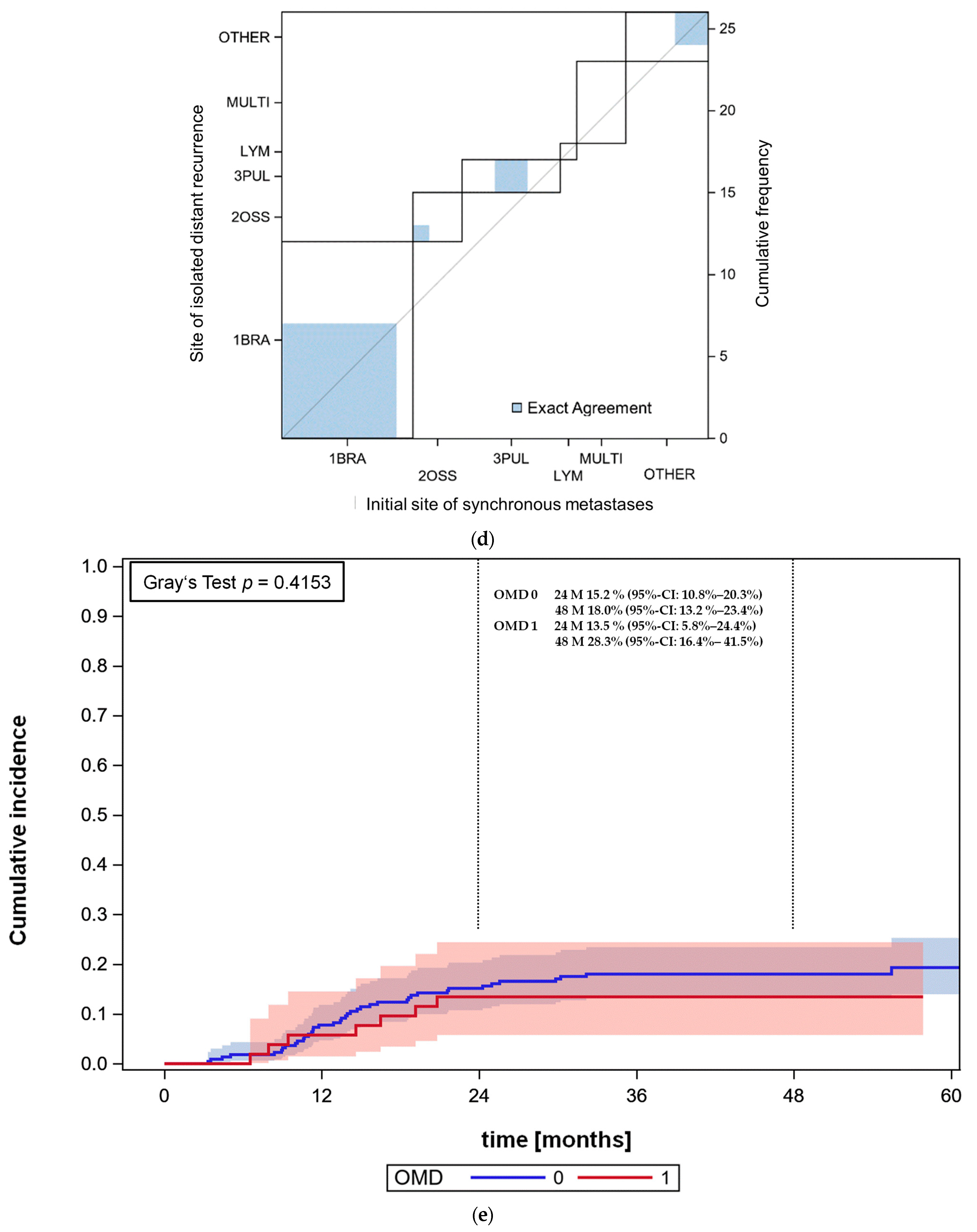
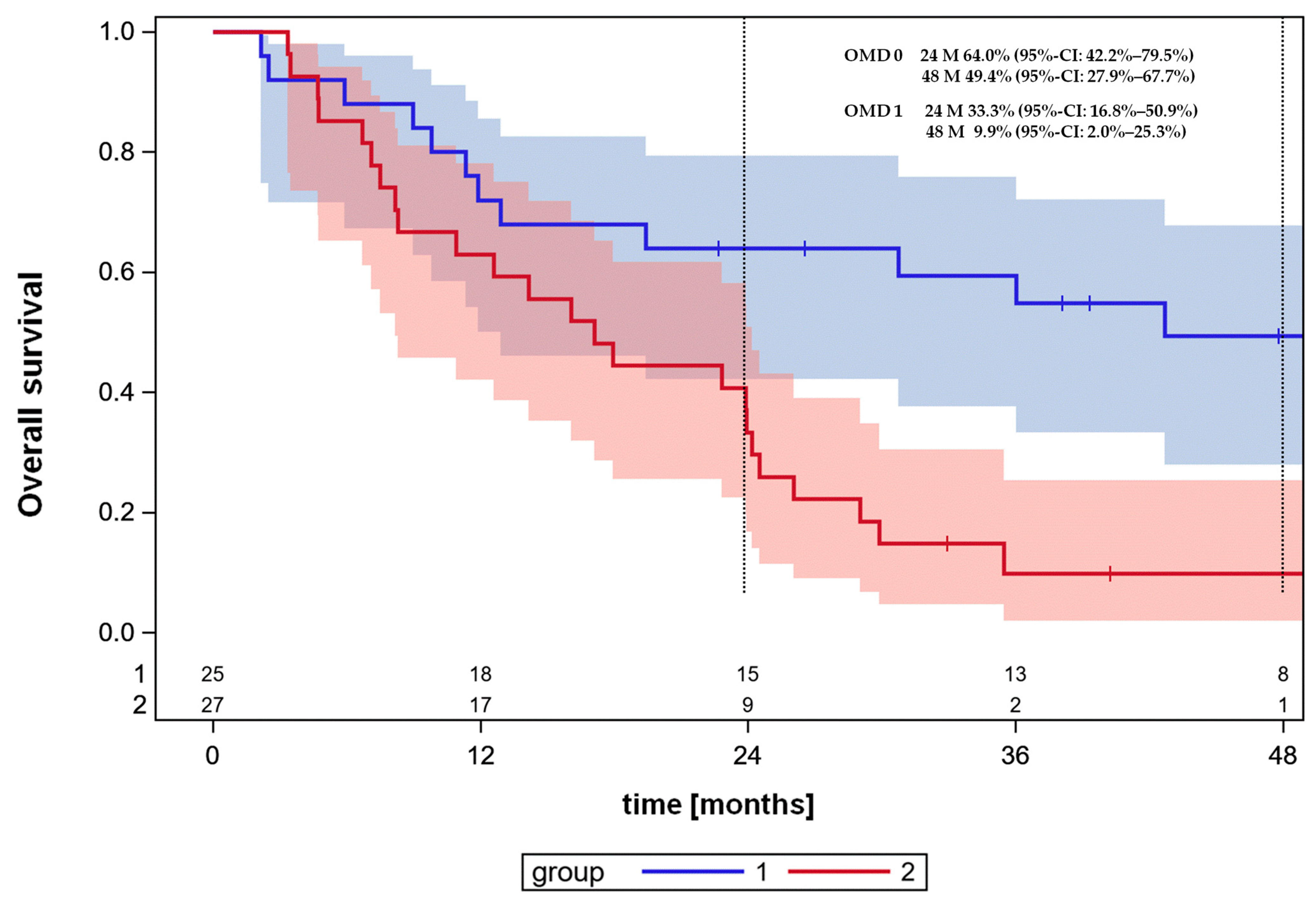
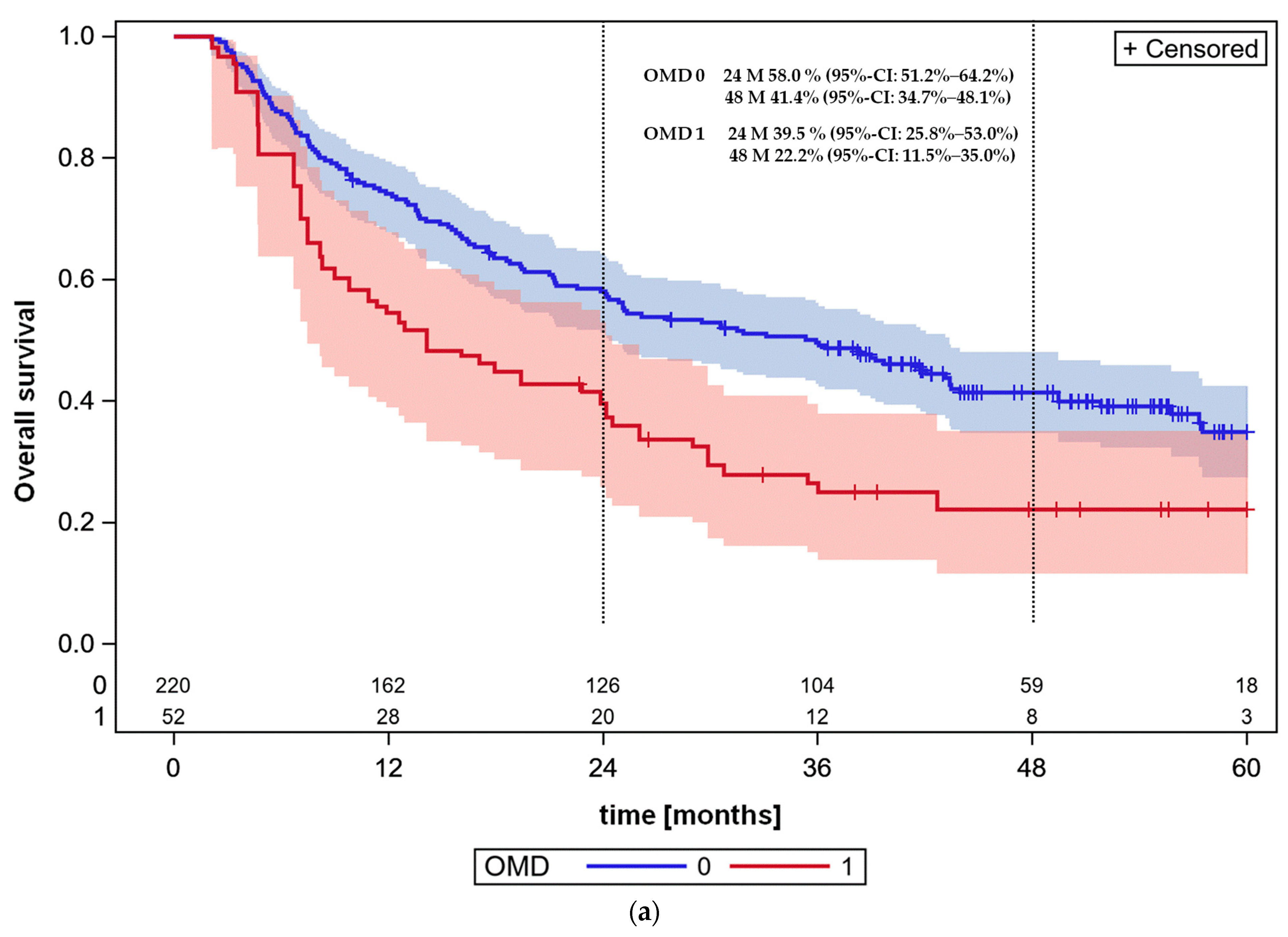
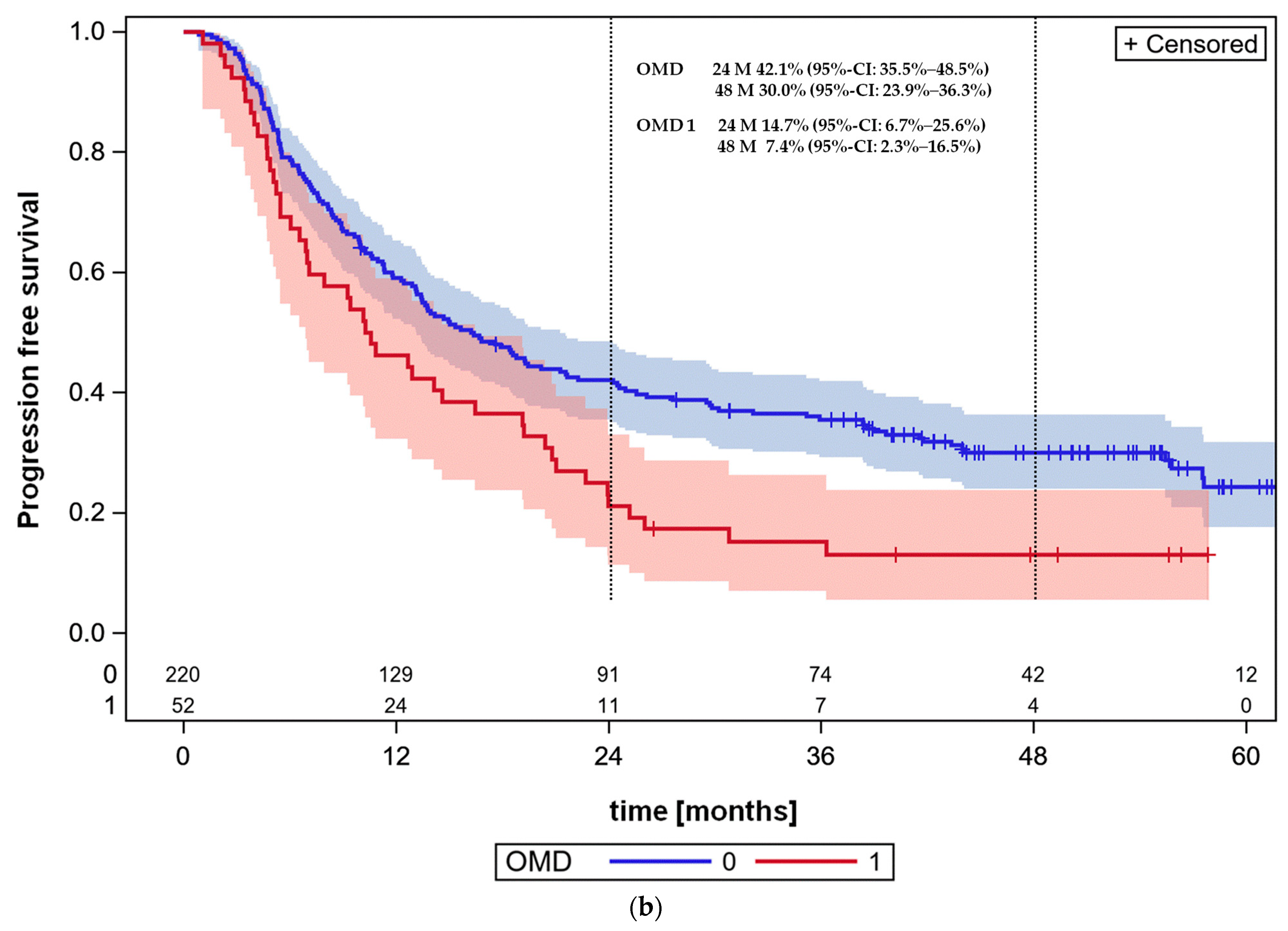
3.4. Overall Survival and Progression-Free Survival
3.5. Cross-Validated Prognostic Survival Classifier for the OMD Patient Cohort
3.6. Propensity Score Weighted Survival Analysis: Age, CRP at Diagnosis, Sex, Severe Comorbidity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Girard, N.; Bar, J.; Garrido, P.; Garassino, M.C.; McDonald, F.; Mornex, F.; Filippi, A.R.; Smit, H.J.M.; Peters, S.; Field, J.K.; et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: Findings from the pacific-r study. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2023, 18, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Guberina, M.; Guberina, N.; Pöttgen, C.; Gauler, T.; Richlitzki, C.; Metzenmacher, M.; Wiesweg, M.; Plönes, T.; Forsting, M.; Wetter, A.; et al. Effectiveness of durvalumab consolidation in stage III non-small-cell lung cancer: Focus on treatment selection and prognostic factors. Immunotherapy 2022, 14, 927–944. [Google Scholar] [CrossRef] [PubMed]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the eortc 22952–26001 study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Harrow, S.; Palma, D.A.; Olson, R.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic radiation for the comprehensive treatment of oligometastases (sabr-comet): Extended long-term outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.; Jiang, W.; Liu, M.; Bergman, A.; Schellenberg, D.; Mou, B.; Alexander, A.; Carolan, H.; Hsu, F.; Miller, S.; et al. Treatment with stereotactic ablative radiotherapy for up to 5 oligometastases in patients with cancer: Primary toxic effect results of the nonrandomized phase 2 sabr-5 clinical trial. JAMA Oncol. 2022, 8, 1644–1650. [Google Scholar] [CrossRef]
- Siva, S.; Bressel, M.; Mai, T.; Le, H.; Vinod, S.; de Silva, H.; Macdonald, S.; Skala, M.; Hardcastle, N.; Rezo, A.; et al. Single-fraction vs multifraction stereotactic ablative body radiotherapy for pulmonary oligometastases (safron ii): The trans tasman radiation oncology group 13.01 phase 2 randomized clinical trial. JAMA Oncol. 2021, 7, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: Esmo clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Ghidini, M.; Bukovec, R.; Trevisan, F.; Turati, L.; Indini, A.; Seghezzi, S.; Lonati, V.; Moleri, G.; et al. Better survival of patients with oligo- compared with polymetastatic cancers: A systematic review and meta-analysis of 173 studies. F1000Research 2021, 10, 423. [Google Scholar] [CrossRef]
- Singh, N.; Jaiyesimi, I.A.; Ismaila, N. Therapy for stage IV non-small-cell lung cancer without driver alterations: Asco living guideline, version 2023.1. J. Clin. Oncol. 2023, 41, e51–e62. [Google Scholar] [CrossRef]
- Iyengar, P.; Wardak, Z.; Gerber, D.E.; Tumati, V.; Ahn, C.; Hughes, R.S.; Dowell, J.E.; Cheedella, N.; Nedzi, L.; Westover, K.D.; et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2018, 4, e173501. [Google Scholar] [CrossRef]
- Available online: https://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Lungenkrebs/lungenkrebs_node.html (accessed on 3 March 2024).
- Available online: https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2023/kid_2023_c33_c34_lunge.pdf?__blob=publicationFile (accessed on 3 March 2024).
- Available online: https://register.awmf.org/assets/guidelines/020-007OLl_S3_Praevention-Diagnostik-Therapie-Nachsorge-Lungenkarzinom_2023-07.pdf (accessed on 3 March 2024).
- Carbone, D.P.; Ciuleanu, T.E.; Schenker, M.; Cobo, M.; Bordenave, S.; Juan-Vidal, O.; Menezes, J.; Reinmuth, N.; Richardet, E.; Cheng, Y.; et al. Four-year clinical update and treatment switching-adjusted outcomes with first-line nivolumab plus ipilimumab with chemotherapy for metastatic non-small cell lung cancer in the checkmate 9la randomized trial. J. Immunother. Cancer 2024, 12, e008189. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lee, J.S.; Ciuleanu, T.E.; Bernabe Caro, R.; Nishio, M. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in checkmate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Gadgeel, S. Pembrolizumab plus pemetrexed and platinum in nonsquamous non-small-cell lung cancer: 5-year outcomes from the phase 3 keynote-189 study. J. Clin. Oncol. 2023, 41, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Kowalski, D.M. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase iii keynote-407 study. J. Clin. Oncol. 2023, 41, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; et al. Impower150 final overall survival analyses for atezolizumab plus bevacizumab and chemotherapy in first-line metastatic nonsquamous nsclc. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C. The eighth edition tnm stage classification for lung cancer: What does it mean on main street? J. Thorac. Cardiovasc. Surg. 2018, 155, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Janning, M.; Süptitz, J.; Albers-Leischner, C.; Delpy, P.; Tufman, A.; Velthaus-Rusik, J.L.; Reck, M.; Jung, A.; Kauffmann-Guerrero, D.; Bonzheim, I.; et al. Treatment outcome of atypical egfr mutations in the german national network genomic medicine lung cancer (nngm). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A european society for radiotherapy and oncology and european organisation for research and treatment of cancer consensus recommendation. Lancet. Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef]
- Eberhardt, W.E.; Pöttgen, C.; Gauler, T.C.; Friedel, G.; Veit, S.; Heinrich, V.; Welter, S.; Budach, W.; Spengler, W.; Kimmich, M.; et al. Phase iii study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIa(n2) and selected iiib non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (espatue). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 4194–4201. [Google Scholar] [CrossRef]
- Rushing, C.; Bulusu, A.; Hurwitz, H.I.; Nixon, A.B.; Pang, H. A leave-one-out cross-validation sas macro for the identification of markers associated with survival. Comput. Biol. Med. 2015, 57, 123–129. [Google Scholar] [CrossRef]
- Hadley, J.; Yabroff, K.R.; Barrett, M.J.; Penson, D.F.; Saigal, C.S.; Potosky, A.L. Comparative effectiveness of prostate cancer treatments: Evaluating statistical adjustments for confounding in observational data. J. Natl. Cancer Inst. 2010, 102, 1780–1793. [Google Scholar] [CrossRef]
- Gomez, D.R.; Blumenschein, G.R., Jr.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: A multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Bai, Y.F.; Verma, V.; Yu, R.L.; Tian, W.; Ao, R.; Deng, Y.; Zhu, X.Q.; Liu, H.; Pan, H.X.; et al. Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic egfr-mutated non-small cell lung cancer. J. Natl. Cancer Inst. 2023, 115, 742–748. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Wanders, R.; Hendriks, L.E.; van Baardwijk, A.; Reymen, B.; Houben, R.; Bootsma, G.; Pitz, C.; van Eijsden, L.; Dingemans, A.C. Progression-free survival and overall survival beyond 5 years of nsclc patients with synchronous oligometastases treated in a prospective phase ii trial (nct 01282450). J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1958–1961. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Barrón, F.; Maldonado, F.; Cabrera, L.; Corona-Cruz, J.F.; Blake, M.; Ramírez-Tirado, L.A.; Zatarain-Barrón, Z.L.; Cardona, A.F.; García, O.; et al. Radical consolidative treatment provides a clinical benefit and long-term survival in patients with synchronous oligometastatic non-small cell lung cancer: A phase ii study. Lung Cancer 2019, 130, 67–75. [Google Scholar] [CrossRef]
- Petty, W.J.; Urbanic, J.J.; Ahmed, T.; Hughes, R.; Levine, B.; Rusthoven, K.; Papagikos, M.; Ruiz, J.R.; Lally, B.E.; Chan, M.; et al. Long-term outcomes of a phase 2 trial of chemotherapy with consolidative radiation therapy for oligometastatic non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, R.; Li, D.; Li, N.; Zhu, X. Prognostic factors of oligometastatic non-small cell lung cancer: A meta-analysis. J. Thorac. Dis. 2018, 10, 3701–3713. [Google Scholar] [CrossRef]
- Ashworth, A.B.; Senan, S.; Palma, D.A.; Riquet, M.; Ahn, Y.C.; Ricardi, U.; Congedo, M.T.; Gomez, D.R.; Wright, G.M.; Melloni, G.; et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin. Lung Cancer 2014, 15, 346–355. [Google Scholar] [CrossRef]
- Zhang, J.T.; Liu, S.Y.; Yan, H.H.; Wu, Y.L.; Nie, Q.; Zhong, W.Z. Recursive partitioning analysis of patients with oligometastatic non-small cell lung cancer: A retrospective study. BMC Cancer 2019, 19, 1051. [Google Scholar] [CrossRef]
- Johnson, K.K.; Rosen, J.E.; Salazar, M.C.; Boffa, D.J. Outcomes of a highly selective surgical approach to oligometastatic lung cancer. Ann. Thorac. Surg. 2016, 102, 1166–1171. [Google Scholar] [CrossRef]
- Spaggiari, L.; Bertolaccini, L.; Facciolo, F.; Gallina, F.T.; Rea, F.; Schiavon, M.; Margaritora, S.; Congedo, M.T.; Lucchi, M.; Ceccarelli, I.; et al. A risk stratification scheme for synchronous oligometastatic non-small cell lung cancer developed by a multicentre analysis. Lung Cancer 2021, 154, 29–35. [Google Scholar] [CrossRef]
- Mitchell, K.G.; Farooqi, A.; Ludmir, E.B.; Corsini, E.M.; Zhang, J.; Sepesi, B.; Vaporciyan, A.A.; Swisher, S.G.; Heymach, J.V.; Zhang, J.; et al. Improved overall survival with comprehensive local consolidative therapy in synchronous oligometastatic non-small-cell lung cancer. Clin. Lung Cancer 2020, 21, 37–46.e7. [Google Scholar] [CrossRef]
- Lopez Guerra, J.L.; Gomez, D.; Zhuang, Y.; Hong, D.S.; Heymach, J.V.; Swisher, S.G.; Lin, S.H.; Komaki, R.; Cox, J.D.; Liao, Z. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e61–e67. [Google Scholar] [CrossRef][Green Version]
- Lim, J.U.; Kang, H.S.; Shin, A.Y.; Yeo, C.D. Association between clinical outcomes and local treatment in stage iv non-small cell lung cancer patients with single extrathoracic metastasis. Thorac. Cancer 2022, 13, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, E.; van der Woude, L.; van Os, R.; van Bockel, L.; Coremans, I.; van Es, C.; De Jaeger, K.; Knol, H.P.; Kolff, W.; Koppe, F.; et al. The dutch lung cancer audit-radiotherapy (dlca-r): Real-world data on stage III non-small cell lung cancer patients treated with curative chemoradiation. Clin. Lung Cancer 2023, 24, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Huang, C.; Zhou, H.; Li, C.; Fan, L.; Chen, J.; Zhang, G.; Liu, Y.; Cui, Z.; Qi, D.; et al. Association between serum c-reactive protein value and prognosis of patients with non-small cell lung cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10633–10639. [Google Scholar] [PubMed]
- Hanlon, P.; Wightman, H.; Politis, M.; Kirkpatrick, S.; Jones, C.; Andrew, M.K.; Vetrano, D.L.; Dent, E.; Hoogendijk, E.O. The relationship between frailty and social vulnerability: A systematic review. Lancet Healthy Longev. 2024, 5, e214–e226. [Google Scholar] [CrossRef]
- Sheu, T.; Heymach, J.V.; Swisher, S.G.; Rao, G.; Weinberg, J.S.; Mehran, R.; McAleer, M.F.; Liao, Z.; Aloia, T.A.; Gomez, D.R. Propensity score-matched analysis of comprehensive local therapy for oligometastatic non-small cell lung cancer that did not progress after front-line chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 850–857. [Google Scholar] [CrossRef]
- Baydoun, A.; Lee, V.L.; Biswas, T. Oligometastatic non-small cell lung cancer: A practical review of prospective trials. Cancers 2022, 14, 5339. [Google Scholar] [CrossRef] [PubMed]
- Guberina, M.; Darwiche, K.; Hautzel, H.; Pöttgen, C.; Guberina, N.; Gauler, T.; Ploenes, T.; Umutlu, L.; Theegarten, D.; Aigner, C.; et al. Patterns of nodal spread in stage III NSCLC: Importance of ebus-tbna and (18)f-fdg pet/ct for radiotherapy target volume definition. Radiat. Oncol. 2021, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Guberina, M.; Eberhardt, W.; Stuschke, M.; Gauler, T.; Aigner, C.; Schuler, M.; Stamatis, G.; Theegarten, D.; Jentzen, W.; Herrmann, K.; et al. Pretreatment metabolic tumour volume in stage IIIa/b non-small-cell lung cancer uncovers differences in effectiveness of definitive radiochemotherapy schedules: Analysis of the espatue randomized phase 3 trial. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Guberina, M.; Darwiche, K.; Hautzel, H.; Ploenes, T.; Pöttgen, C.; Guberina, N.; Herrmann, K.; Umutlu, L.; Wetter, A.; Theegarten, D.; et al. Impact of ebus-tbna in addition to [(18)f]fdg-pet/ct imaging on target volume definition for radiochemotherapy in stage III NSCLC. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2894–2903. [Google Scholar] [CrossRef]

| Patient, Tumor and Treatment Characteristics | Stage III (N [%]) | OMD (N [%]) | p-Value |
|---|---|---|---|
| Age in years: median (range) Age groups: <40 y/40–≤60 y/60–≤70 y/>70 y | 64.9 (43.6–87.0) 0/65/85/70 [0.0/29.5/38.6/31.8] | 63.8 (38.7–87.8) 1/16/23/12 [1.9/30.8/44.2/23.1] | 0.1060 |
| Sex: male/female | 150/70 [68.2/31.8] | 33/19 [63.5/36.5] | 0.6252 |
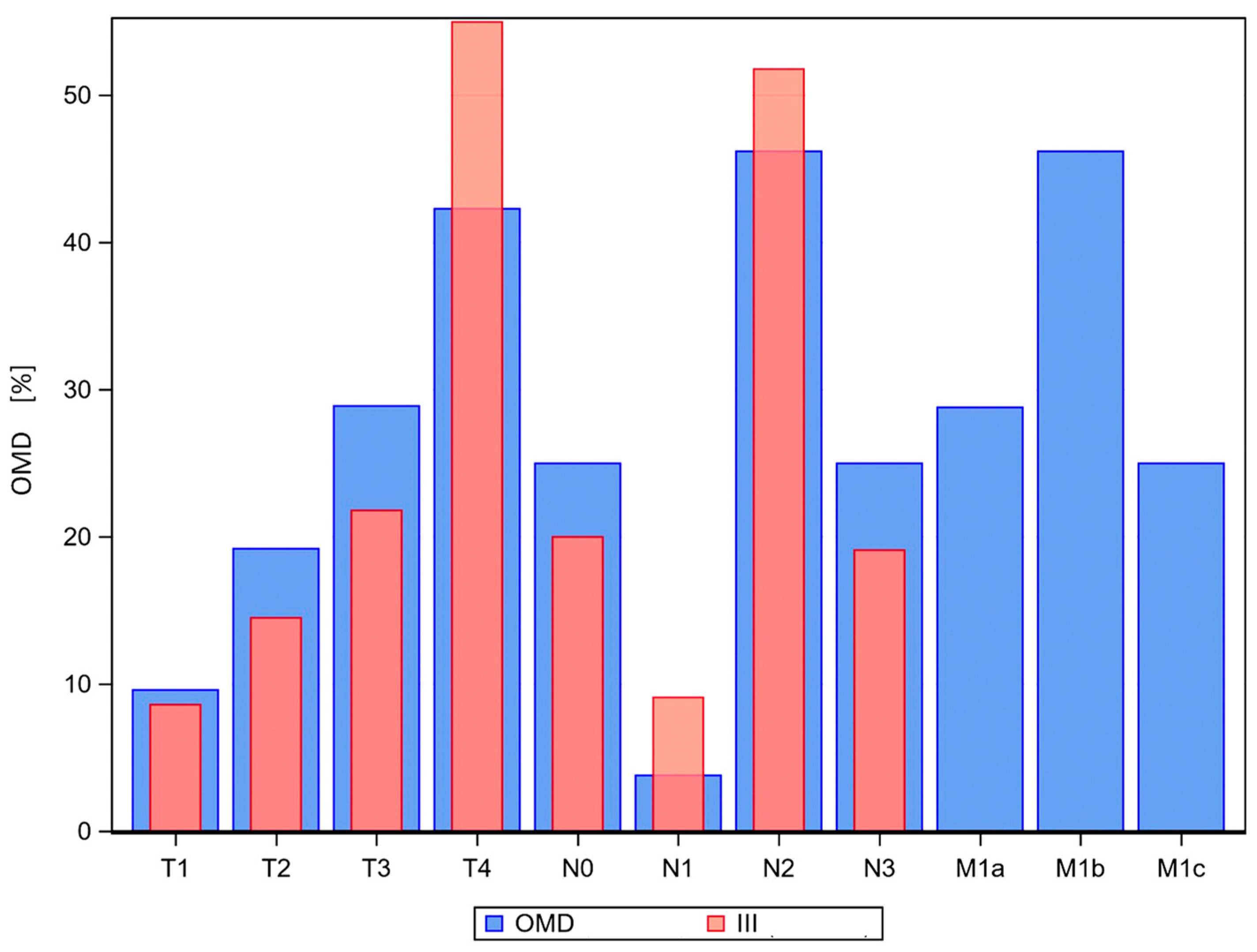
| T-category: T1/T2/T3/T4 | 19/32/48/121 [8.6/14.5/21.8/55.0] | 5/10/15/22 [9.6/19.2/28.9/42.3] | 0.3762 |
| N-category: N0/N1/N2/N3 | 44/20/114/42 [20.0/9.1/51.8/19.1] | 13/2/24/13 [25.0/3.8/46.2/25.0] | 0.4183 |
| M-category: M0/M1a/M1b/M1c | 220/0/0/0 [100/0/0/0] | 0/15/24/13 [0/28.8/46.2/25] | <0.0001 |
| UICC Stage: IIIA/IIIB/IIIC/IVA/IVB | 100/91/29/0/0 [45.4/41.4/13.2/0/0] | 0/0/0/44/8 [0/0/0/84.6/15.4] | <0.0001 |
| Thoracic stage, ignoring M-category: IIB/IIIA/IIIB/IIIC | 0/100/91/29 [0/45.4/41.4/13.2] | 3/21/21/7 [5.8/40.4/40.4/13.5] | 0.0249 |
| ECOG performance status: 0/1/2/3 | 99/92/29/0 [45/41.8/13.2/0] | 25/21/6/0 [48.1/40.4/11.5/0] | 0.9308 |
| Pre-existing comorbidity classified as grade > 2 according to CTC-AE-version 5: yes vs. no | 63/157 [28.6/71.4] | 9/43 [17.3/82.7] | 0.1161 |
| Histology: squamous/non-squamous | 113/107 [51.4/48.6] | 13/39 [25.0/75.0] | 0.0006 |
| COPD GOLD: 0–I/II/III/IV (%) | 85/101/27/7 [38.6/45.9/12.3/3.2] | 27/21/4/0 [51.9/40.4/7.7/0] | 0.2680 |
| PD-L1 expression: 0%/1%–<50%/≥50%/(n/d) | 69/63/27/61 [31.4/28.6/12.3/27.7] | 27/10/9/6 [51.9/19.2/17.3/11.5] | 0.0076 |
| 18F-FDG-PET/CT before therapy: no/yes | 18/202 [8.2/91.8] | 0/52 [0/100] | 0.0289 |
| EBUS-TBNA before therapy: no/yes | 17/203 [7.7/92.3] | 4/48 [7.7/92.3] | 1.0000 |
| Patient, Tumor and Treatment Characteristics | Stage III (N [%]) | OMD (N [%]) | p-Value |
|---|---|---|---|
| Hemoglobin at diagnosis in g/dL: median (range) | 13.5 (7.8–17.9) | 13.4 (9.4–16.2) | 0.9885 |
| LDH at diagnosis in U/L: median (range) | 218 (114–1116) | 217 (99–398) | 0.6869 |
| CRP at diagnosis in mg/dL: median (range) | 1.45 (0.0–25.0) | 1.50 (0.0–13.8) | 0.5041 |
| Patient, Tumor and Treatment Characteristics | Stage III (N [%]) | OMD (N [%]) | p-Value |
|---|---|---|---|
| Treatment modality: Definitive radiochemotherapy/Trimodality treatment (%) | 158/62 [71.8/21.2] | 45/7 [86.5/13.5] | 0.0328 |
| Total radiation dose in Gy: Median (interquartile range) | 61.0 (47.0–65.5) | 60.0 (60.0–64.0) | 0.5372 |
| Date of Start of radiotherapy: Days from 1 August 2016, Median (range) | 687 (17–1286) | 707 (158–1556) | 0.7555 |
| ICI Line: none (0)/1/2/3 | 124/38/54/4 [56.4/17.3/24.5/1.8] | 18/5/26/3 [34.6/9.6/50.0/5.8] | 0.0007 |
| Therapy of synchronous distant metastases: systemic therapy (ST) alone/ST + RT/ST + surgery | Not applicable | 7/29/16 [13.5/55.8/30.8] | Not applicable |
| HR (95%-CI (Range)) | p-Value | |
|---|---|---|
| Sex: female vs. male | 0.405 (0.190–0.866) | 0.0198 |
| Severe comorbidity | 2.552 (1.149–5.669) | 0.0214 |
| C-reactive protein * | 1.130 (1.035–1.234) | 0.0063 |
| ECOG status ** | 2.102 (1.057–4.179) | 0.0342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guberina, M.; Pöttgen, C.; Guberina, N.; Hoffmann, C.; Wiesweg, M.; Richlitzki, C.; Metzenmacher, M.; Aigner, C.; Bölükbas, S.; Gauler, T.; et al. Long-Term Survival in Patients with Oligometastatic Non-Small Cell Lung Cancer by a Multimodality Treatment—Comparison with Stage III Disease. Cancers 2024, 16, 1174. https://doi.org/10.3390/cancers16061174
Guberina M, Pöttgen C, Guberina N, Hoffmann C, Wiesweg M, Richlitzki C, Metzenmacher M, Aigner C, Bölükbas S, Gauler T, et al. Long-Term Survival in Patients with Oligometastatic Non-Small Cell Lung Cancer by a Multimodality Treatment—Comparison with Stage III Disease. Cancers. 2024; 16(6):1174. https://doi.org/10.3390/cancers16061174
Chicago/Turabian StyleGuberina, Maja, Christoph Pöttgen, Nika Guberina, Christian Hoffmann, Marcel Wiesweg, Cedric Richlitzki, Martin Metzenmacher, Clemens Aigner, Servet Bölükbas, Thomas Gauler, and et al. 2024. "Long-Term Survival in Patients with Oligometastatic Non-Small Cell Lung Cancer by a Multimodality Treatment—Comparison with Stage III Disease" Cancers 16, no. 6: 1174. https://doi.org/10.3390/cancers16061174
APA StyleGuberina, M., Pöttgen, C., Guberina, N., Hoffmann, C., Wiesweg, M., Richlitzki, C., Metzenmacher, M., Aigner, C., Bölükbas, S., Gauler, T., Eberhardt, W. E. E., Forsting, M., Herrmann, K., Theegarten, D., Darwiche, K., Jendrossek, V., Stuschke, M., & Schuler, M. (2024). Long-Term Survival in Patients with Oligometastatic Non-Small Cell Lung Cancer by a Multimodality Treatment—Comparison with Stage III Disease. Cancers, 16(6), 1174. https://doi.org/10.3390/cancers16061174








