Simple Summary
Although Warthin’s tumor is a well-known and frequent tumor in the salivary gland, its pathogenesis is not fully understood. Warthin’s tumor is composed of oncocytic epithelial cells lining papillary and cystic structures in a lymphoid stroma. Previously several hypotheses have been postulated. The risk factors for its development are known and they include aging, smoking, and radiation exposure. Recent findings have suggested that chronic inflammation and aging cells promote the growth of Warthin’s tumor. In this short review, we propose that DNA dame, metabolic dysfunction of mitochondria, senescence-associated secretory phenotype, human papillomavirus, and IgG-4 may be involved in the development of Warthin’s tumor.
Abstract
Warthin’s tumor is the second most frequent neoplasm next to pleomorphic adenoma in the salivary gland, mostly in the parotid gland. The epithelial cells constituting a tumor are characterized by the presence of mitochondria that undergo structural and functional changes, resulting in the development of oncocytes. In addition to containing epithelial cells, Warthin’s tumors contain abundant lymphocytes with lymph follicles (germinal centers) that are surrounded by epithelial cells. The pathogenesis of Warthin’s tumor is not fully understood, and several hypotheses have been proposed. The risk factors for the development of Warthin’s tumor, which predominantly occurs in males, include aging, smoking, and radiation exposure. Recently, it has been reported that chronic inflammation and aging cells promote the growth of Warthin’s tumor. Several reports regarding the origin of the tumor have suggested that (1) Warthin’s tumor is an IgG4-related disease, (2) epithelial cells that compose Warthin’s tumor accumulate mitochondria, and (3) Warthin’s tumor is a metaplastic lesion in the lymph nodes. It is possible that the pathogenesis of Warthin’s tumor includes mitochondrial metabolic abnormalities, accumulation of aged cells, chronic inflammation, and senescence-associated secretory phenotype (SASP). In this short review, we propose that DNA damage, metabolic dysfunction of mitochondria, senescent cells, SASP, human papillomavirus, and IgG4 may be involved in the development of Warthin’s tumor.
Keywords:
Warthin’s tumor; pathogenesis; neoplastic; SASP; senescent cells; DNA damage; inflammation 1. Introduction
Warthin’s tumor, also known as papillary cystadenoma lymphomatosum, monomorphic adenoma, or adenolymphoma, is the second most common tumor of the parotid gland after pleomorphic adenoma, accounting for approximately 15% of all parotid tumors, and is encountered relatively frequently in daily clinical practice [1,2]. An investigation by Franzen et al. [3] suggested that Warthin’s tumor was the most common histological type in the period from 1997 to 2017. The researchers also suggested a growing incidence in women and a decreasing age of patients [3]. The site of occurrence is restricted to the parotid gland and surrounding lymph nodes, with a high frequency of simultaneous or ectopic multiple or bilateral occurrence [4,5]. The tumor usually presents as a painless mass, but it may be painful when the lesion is associated with inflammation [6]. Clinically, an ultrasound examination reveals the tumor as an oval and well-defined mass with multiple anechoic areas, or an anechoic mass with posterior acoustic enhancement. Rapid growth is stimulated by infection. In some cases, multiple septa and intra-tumoral fluid thickness can cause non-uniform echo patterns [7,8,9]. Treatment is usually based on surgical resection; however, most patients have low malignancy rates. Therefore, conservative treatment may be an option if Warthin’s tumor is preoperatively diagnosed [10,11].
Although it has been more than 100 years since the discovery of Warthin’s tumor, the etiology remains unclear [12]. In this short review, we propose a possible association between DNA damage, metabolic dysfunction of mitochondria, senescent cells, senescence-associated secretory phenotype (SASP), human papillomavirus, and IgG4, which may be involved in the development of Warthin’s tumor. Our hypotheses as described support the results of the investigation by Kuzenko et al. [2].
2. Anatomy/Histology of the Salivary Glands
The salivary glands are exocrine glands that produce saliva. Humans have three major paired salivary glands (parotid, submandibular, and sublingual glands) as well as numerous minute salivary glands. The salivary glands can also be classified according to their secretions as serous, mucous, or seromucous (mixed). In serous secretions, the main type of secreted protein is α-amylase, an enzyme that breaks down starch into maltose and glucose, whereas in the mucous glands, mucin, which acts as a lubricant, is the main secreted protein. In humans, saliva is produced every day. The secretion of saliva (salivation) is mediated by parasympathetic stimulation. Acetylcholine is an active neurotransmitter that binds to muscarinic receptors in the glands, leading to increased salivation.
The working parts of salivary glandular tissue consist of secretory end pieces (acini) and a branched ductal system (Figure 1). In serous glands (e.g., parotid glands), the cells in the end piece are arranged in a roughly spherical shape. Mucous glands tend to be arranged in a tubular configuration with a larger central lumen. In both types of glands, the cells in the end piece surround the lumen, which is the start of the ductal system (Figure 1). Three types of ducts are present in most of the salivary glands. The fluid first passes through the intercalated ducts, which have a low cuboidal epithelium and a narrow lumen. From there, the secretions enter the striated ducts, which are lined with columnar cells with many mitochondria. Finally, the saliva passes through the excretory ducts, where the cell type is cuboidal, until the terminal part, which is lined with a stratified squamous epithelium. The end pieces may contain mucous cells, serous cells, or a mixture of both. A salivary gland can consist of a varied mixture of these types of end-pieces. In mixed glands, mucous acini are capped by a serous demilune. In addition, myoepithelial cells surround the end piece, and their function is to assist in propelling secretions into the ductal system. The gland and its specialized nerves and blood supply are supported by connective tissue stroma.
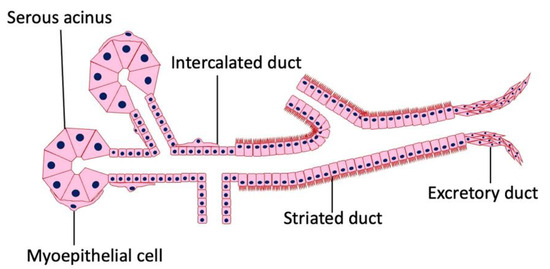
Figure 1.
Histology of the salivary gland. Salivary glands are composed of epithelial columnar cells, myoepithelial cells, intercalated duct cells, acinar cells, and connective tissue. There is no lymphatic tissue in the stroma of a normal salivary gland.
3. Histopathology of Warthin’s Tumor
The histopathology of Warthin’s tumor is defined by the tubular, cystic, and papillary proliferation of highly cylindrical oncocyte-like cells with eosinophilic granular sporulation. It is well demarcated from the surrounding normal salivary gland tissue [13,14]. The tumor is also characterized by a biphasic arrangement of similar oncocyte-like cuboidal cells with cylindrical cells on the basal side. The stroma is occupied by mature, non-atypical small lymphocytes with lymph follicles (germinal centers), although the number of stroma varies from case to case [2] (Figure 2a). This may be caused by an immune response to the tumor epithelium or by residual lymphoid tissue within the lymph nodes that is partially replaced by the tumor epithelium [15]. In addition, the cytoplasm of cells exhibiting oncocytes possesses an excessive accumulation of mitochondria [16]. This may be a result of the accumulation of senescent mitochondria due to the reduction in cellular mitophages and is associated with a deletion of 4977 bp in the mitochondrial genome [17,18,19,20]. It is not uncommon for intermingled goblet cell-type mucous cells, glandular hairy metaplastic cells, and squamous metaplastic cells to be observed. Basal cells do not usually differentiate into myoepithelial cells. The cystic structure, lined with epithelial cells, is filled with necrotic contents, including cholesterol crystals. When the cystic structures rupture, the fluid leaks into the lymphangitic stroma, causing epithelioid granulomas with neutrophil infiltration. In association with this, squamous epithelium and mucous cells sometimes appear due to inflammatory or chemogenic changes, and in rare cases, extensive necrosis may accompany the lesions. Neither the epithelial nor the lymphocytic component is usually atypical; however, secondary adenocarcinoma, mucoepidermoid carcinoma, squamous cell carcinoma, oncocytic carcinoma, or malignant lymphoma (follicular lymphoma) may occur [21,22,23,24,25,26,27,28]. The most common histologic type of carcinoma derived from Warthin’s tumor is squamous cell carcinoma, although mucoepidermoid carcinoma has also been reported [29,30].
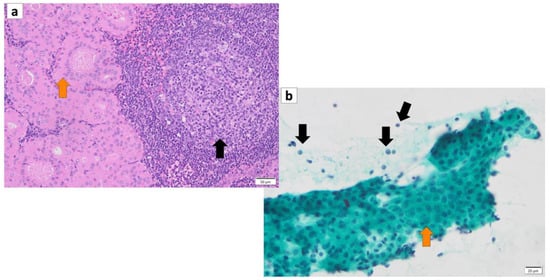
Figure 2.
(a) Histopathology of Warthin’s tumor. Note: varying proportions of papillary cystic structures are lined with bilayered oncocytic epithelial cells (orange arrow) and surrounded by a lymphoid stroma, including germinal centers (black arrow). (b) FNA cytology of Warthin’s tumor shows small cohesive sheets of oncocytes with abundant granular cytoplasm with a central round nucleus/prominent nucleolus (orange arrow). Lymphocytes (black arrows) with granular debris in the background. (a) Hematoxylin and eosin staining (Bar represents 50 μm) and (b) Papanicolaou staining (bar represents 20 μm).
4. Cytology of Warthin’s Tumor
A cytological diagnosis of Warthin’s tumor can be made by observing a two-cell pattern of lymphocytes and epithelial cells, with lymphocytes in the background and eosinophilic cells in clusters or sporadically isolated. The aggregates of oncocytes range in size from large to small sheets without abnormal overlapping [31]. On Papanicolaou staining smears, oncocytes appear with light green stained granular cytoplasm, often with eosinophilic changes that stain orange G. Their nuclei are small and slightly atypical, sometimes with a few small nucleoli, but they are often obscure. Occasional findings include cystic structures, including oncocytes floating in the lumen (Figure 2b). When cyst contents of punctured cells contain hypercylindrical nucleated oncocyte cells along with histiocytes, the diagnosis of Warthin’s tumor is inferred; however, in the absence of nucleated oncocyte cells, the diagnosis is more challenging [32]. In May–Giemsa-stained specimens, Warthin’s tumor cells do not possess a metachromatic component, and in some cases, there are various mature lymphocytes in the background.
The cellular presentation of squamous metaplastic Warthin’s tumor, which is a secondary alteration of Warthin’s tumor, is a mixed appearance of neutrophils and histiocytes with orange G-stained metaplastic squamous cells [33]. In addition, mucous cells often appear with metaplasticity, in which case mucous cells are interspersed with collections of oncocytes on a mucous background. For the diagnosis of Warthin’s tumor, fine needle aspiration cytology (FNAC) is useful in the preoperative diagnosis of salivary gland tumors because it is minimally invasive and has many typical cytological findings [34,35,36]. Data have been reported that FNAC has a sensitivity of 93%, a specificity of 94.8%, and an accuracy of 94.6% in the diagnosis of Warthin’s tumor [36].
5. Risk Factors for Warthin’s Tumor
Warthin’s tumors were first reported over 100 years ago, but their pathogenesis is not fully understood [12]. Various possible pathogeneses and risk factors have been described. (1) The majority of Warthin’s tumors show an obvious marginal sinus beneath the tumor capsule and have an intralymphatic origin or a metaplasia of normal salivary gland epithelial or ductal cell origin [37,38,39,40]. (2) Catalytically inactive glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was found to bind to damaged mitochondria and incorporate these mitochondria directly into lysosomes, exhibiting a characteristic immunohistochemical GAPDH staining pattern in Warthin’s tumor cells, suggesting either whole cell progressive loss of cytoplasmic GAPDH (Figure 3), likely due to loss or nuclear shift of the protein [18]. (3) The epithelium of Warthin’s tumor, whether hyperplastic, metaplastic, or neoplastic, interacts with lymphoid tissue [2]. (4) A high association with smoking, which causes chronic inflammation of the epithelium, has been reported [41,42,43]. (5) The incidence of Warthin’s tumor is reported to be high after radiation exposure [44]. (6) Patients with Warthin’s tumor have been reported to show an increased incidence of autoimmune or infectious diseases [12,45]. (7) It has been reported that angiogenesis and lymphangiogenesis are increased, reactive lymphocyte hyperplasia is induced, and that the two elements, epithelial cells and lymphocytes, are not simply present by chance but are interdependently related to tumor development [46]. (8) HPV infection is reportedly associated with the development of Warthin’s tumor [47]. (9) IgG4-reated disease (IgG4-RD) has been reported to be indirectly involved in the development of Warthin’s tumor [48]. To date, there is no consensus on the development of Warthin’s tumor. However, based on our previous studies, we believe that mitochondrial metabolic abnormalities, senescent cell accumulation, chronic inflammation, SASP, and HPV infection may be involved in the pathogenesis of Warthin’s tumor.
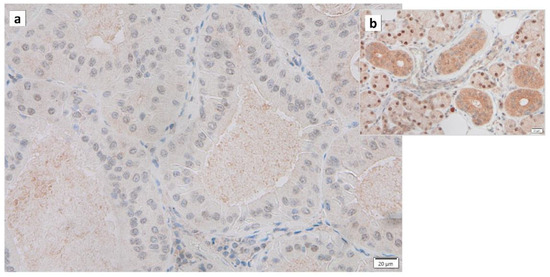
Figure 3.
Immunohistochemistry of GAPDH in Warthin’s tumor. Note the (a) negative reaction in the columnar epithelial cells and (b) positive reaction in the cytoplasm of intercalated ductal cells and some nuclei of acinar cells. Bars represent 20 μm.
6. Association between SASP and Warthin’s Tumor
Normal somatic cells irreversibly arrest the cell cycle after a certain number of divisions. This is caused by telomere shortening, also known as replicative senescence [49]. The same phenomenon occurs in normal epithelial cells with proliferative capacity when they receive carcinogenic stimuli such as DNA damage, oxidative stress, or excessive proliferative stimulation by oncogene (Ras) products. This is called “premature senescence”, which does not involve telomere shortening. This phenomenon is considered to be a mechanism of cancer suppression. It has been reported that the proportion of senescent cells increases in tissues and organs with aging, and cellular senescence is also involved in the pathogenesis of individual aging and age-associated diseases. The process of cellular senescence is as follows: telomere shortening, radiation, carcinogens, and oxidative stress trigger the DNA-damage response (DDR), which activates the p53 pathway and induces cellular senescence. Double-stranded DNA damage arrests the cell cycle and proceeds to the repair process, whereas irreparable DNA damage induces apoptosis and cellular senescence. DDR involves kinases such as ataxia telangiectasia mutated (ATM) and checkpoint-2 (CHK2), adapter proteins (e.g., 53BP1, mediator of DNA damage checkpoint protein-1 [MDC1]) and chromatin-modifying proteins (e.g., phosphorylation of histone H2AX [γ-H2AX]) (Figure 4), many of which are localized to sites of DNA damage [50]. When the p53 pathway is inhibited and the cell cycle continues to progress in senescent cells, the telomere length continues to decrease. Eventually, the loss of telomeric DNA causes severe genomic instability, leading to cell death, known as mitotic collapse. Cell cycle arrest activates the p53-p21 and p16-retinoblastoma (RB) pathways [51,52]. While senescent cells usually remain in the G1 phase of the cell cycle, their intracellular metabolism is active and characterized by protein secretion phenomena, including, for example, inflammatory cytokines (e.g., interleukin [IL1]β (Figure 5), IL6 (Figure 6), IL8, plasminogen activator inhibitor [PAI]-1, vascular endothelial growth factor receptor [VEGF], matrix metalloproteinase [MMP]3, etc.), which are called “senescence-associated secretory phenotypes” (SASPs) [53,54]. SASPs have been shown to promote the development of age-related diseases, induce oncogenesis/carcinogenesis, and increase tumor size while enhancing tissue repair and regeneration [55,56,57,58]. For example, senescent fibroblasts and many other SASP factors enhance cancer cells’ proliferation and invasion in culture systems [59,60]. Thus, cellular senescence has a dual nature: inhibiting tumors while promoting them [57,61,62,63].
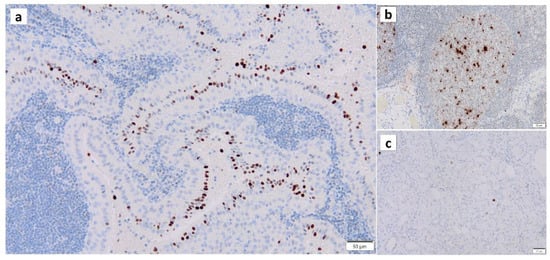
Figure 4.
Immunohistochemistry of γ-H2AX in Warthin’s tumor. γ-H2AX positivity is found (a) in the nuclei of the columnar epithelial cells and (b) the nuclei of lymphocytes in the germinal center. (c) The normal salivary gland is negative for γ-H2AX. Bars represent 50 μm.
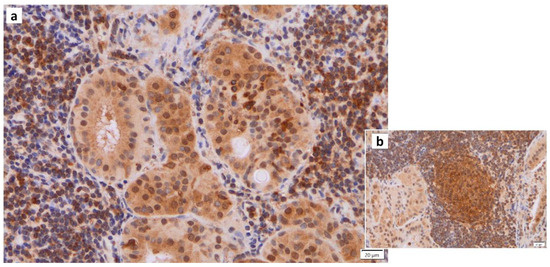
Figure 5.
IL1β immunohistochemistry of Warthin’s tumor. (a) IL1b positivity is found in some nuclei of the columnar epithelial cells and (b) the surrounding lymphocytes in and around the germinal center. Bars represent 20 μm.
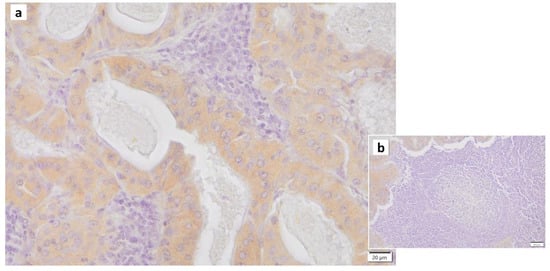
Figure 6.
IL6 immunohistochemistry of Warthin’s tumor. (a) Weak positivity for IL6 is found in the cytoplasm of the columnar epithelial cells. (b) The surrounding lymphocytes in and around the germinal center are negative. Bars represent 20 μm.
Chronic inflammation is closely associated with tumorigenesis [64,65]. In addition, age-associated cellular senescence is thought to act as a tumor promoter by initiating several inflammatory processes. Chronically activated leukocytes produce direct and indirect mitogenic growth factors (epidermal growth factor [EGF], tumor growth factor [TGF]β, tumor necrosis factor [TNA]α, fibroblast growth factor [FGA], interleukin [ILs], chemokines, histamine, and heparin), which stimulate tumor and stromal cell proliferation. In addition, inflammatory cells such as macrophages, granulocytes, monocytes, and mast cells secrete diverse classes of proteolytic enzymes that modify the structure and function of the extracellular matrix (ECM) and release mitogenic factors. Macrophages also produce vascular endothelial growth factor (VEGF) and EGF when exposed to T helper 2 (Th2)-type cytokines, such as IL4, which promote angiogenesis and metastasis. In addition, Th2 cells are well recognized as tumor promoters. Th2 cells are “driven” by OX40 ligand (L)-expressing dendritic cells in response to cancer-derived thymic stromal lymphopoietin (TSLP) [66]. Th2 CD4+ T lymphocytes secrete IL4 and IL13. Subsequently, macrophages release EGF, VEGF, and TGFβ to promote tumorigenesis [67,68].
7. Mitochondrial Dysfunction and Warthin’s Tumor
Warthin’s tumor is morphologically composed of oncocytic epithelial cells with abundant papillary and cystic mitochondrial structures in the lymphoid stroma. Mitochondria are intracellular organelles that synthesize ATP using high-energy electrons and oxygen molecules. In addition, mitochondria actively fuse and divide to stabilize their morphology. Recently, interesting findings showing that (1) “mitochondrial DNA mutations accumulate in human tissues with senescence” [69]; and (2) “mitochondrial dysfunction induces aging” have been reported [70]. With aging, mitochondria become highly susceptible to morphological changes, and these changes lead to reduced function due to oxygen radical damage, ultimately causing the organism to age [71]. Since mitochondria are the primary source of cellular ATP and are involved in the biosynthesis of deoxyribose nucleoside triphosphate (dNTP), mitochondrial dysfunction results in a reduction in ATP levels and alterations in ATP-dependent pathways that are involved in transcription, DNA replication, DNA repair, and DNA recombination. Additionally, mitochondrial defects may lead to mutagenesis of the nuclear genome [72]. Approximately 10% of mtDNA has a “common” 4977 bp deletion. One study, in which polymerase chain reaction (PCR) was used to further quantify 4977 bp deletion in normal parotid control tissue that was age-matched to Warthin’s tumor, revealed that deletions were present in all parotid tissues, but the changes were significantly greater in oncocytic tumors. Although there were a small number of controls, there was a tendency towards higher concentrations of deletions in smokers [73].
Recently, a group of hydrogen peroxides that function in mitochondrial fusion and fission was identified. Mitofusin (Mfn) 1, Mfn2, and OPA1 are involved in fusion, while dynamin-related protein 1 (Drp1) (as well as dynamin-like protein [DLP1]) is involved in fission [74]. Mitochondrial mitosis is associated with mechanisms that promote cell cycle arrest and apoptosis. In the cell cycle, Drp1 Ser 616 is mainly phosphorylated in the early S phase, which leads to the promotion of mitochondrial division and movement of cells into G2/M. Loss of Drp1 induces mitochondrial hyperfusion, leading to ATM-dependent G2/M arrest and apoptosis. The overexpression of Drp1 has been associated with malignant oncocytic thyroid tumors, and genetic and pharmacological blockade of Drp1 activity has been reported to affect the migration and invasion of thyroid cancer cells, which is a characteristic of malignant tumors [75]. In addition, it has been reported that Drp1 promotes KRas-driven metabolic changes to drive pancreatic tumor growth and that the expression of dynamin-related protein 1 (Drp1) in epithelial ovarian cancer has a poor prognosis, suggesting that Drp1 may be used as a biomarker for malignant tumors [76,77]. Mitochondrial dysfunction has recently been identified as a pathological factor related to cellular aging [78,79]. Therefore, we hypothesized that the development of Warthin’s tumor may be related to cellular senescence, including Drp1 (Figure 7). IL13 was recently shown to play a critical role in the induction of salivary gland epithelial cell senescence by increasing mitochondrial oxidative stress through a phosphorylated signal transducer and activator of transcription 6 (p-STAT6)-cAMP-response element binding protein (CREB)-binding protein (CBP)-superoxide dismutase 2 (SOD2)-dependent pathway in IgG4-related sialadenitis (IgG4-RS). In addition, a clear increase in SA-β-gal-positive cells in IgG4-RS in both acini tufts and ducts was observed, suggesting the possibility that epithelial cell senescence is present and may be related to salivary gland dysfunction in IgG4-RS [80].
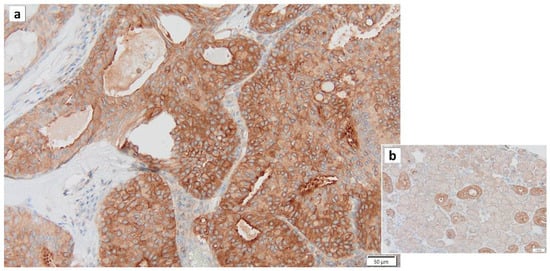
Figure 7.
Drp1 immunohistochemistry of Warthin’s tumor. (a) Most cytoplasm of the columnar epithelial cells is positive for Drp1. (b) In the normal salivary gland, the cytoplasm of intercalated duct cells is also positive for Drp1, while the cytoplasm of acinar cells is negative for Drp1. Bars represent 50 μm.
8. IgG4 and Warthin’s Tumor
IgG4-RD is a systemic inflammatory disease characterized by severe fibrosis, high serum IgG4 levels (>135 mg/dL), and marked IgG4-positive lymphoplasmacytic infiltrates. It was initially proposed as autoimmune pancreatitis and Mikulicz disease [81,82]. High IgE levels and eosinophilic infiltrates are often observed. However, the etiological mechanisms underlying this IgG4-related disease are largely unknown, and it is unclear whether IgG4-RD is caused by abnormal acquired immunity, such as autoimmune diseases, or whether increased IgG4 production has a direct impact [83]. IgG4 accounts for less than 5% of all IgGs in healthy individuals. IgG4 accounts for a lower percentage in comparison to IgG1 to IgG3, and the Fc region of IgG4 is thought to play a small role in immune activation due to its weak binding to C1q and Fcγ receptors. IgG4 differs from other IgGs after secretion from plasma cells in that the Fab region is exchanged for other Fab regions, allowing a single molecule to recognize different antigens. The resulting antibodies are thought to exhibit anti-inflammatory effects by reducing their ability to form immune complexes [84]. IgG4 production is primarily controlled by Th2 cells [85]. Under antigen stimulation, IgG4 production is induced by IL4 and IL13, which are Th2-type cytokines involved in allergic reactions. In the presence of IL10, IL12, and IL21, IgG4 production becomes predominant over IgE production. In Th2 cytokine-driven immune reactions, IgG4 production is preferentially induced by the activation of IL10 produced by regulatory T (Treg) cells [86]. The overexpression of IL10, TGFβ, and activation-induced cytidine deaminase (AID) has been reported in the labial salivary glands (LSGs) of IgG4-RD patients in comparison to SS patients, suggesting that Treg cytokines (IL10 and TGFβ) combined with AID, an IgG4-unrelated molecule in IgG4-RD (MD) patients, contributes to IgG4-specific class switch recombination and fibrosis [87]. Aga et al. recently suggested an association between Warthin’s tumor and IgG4-RD [48]. In addition, serum IgG4 levels showed an increasing trend in Warthin’s tumors in comparison to pleomorphic adenomas [88].
Our recent findings suggest that Warthin’s tumor tends to have more IgG4-positive cells upon immunohistochemical staining of histological sections, suggesting a certain but not causal relationship between IgG4-RD and Warthin’s tumor (Figure 8 and Figure 9). However, since salivary gland-like cystic carcinomas with IgG4-RD have also been reported recently [89], additional research is necessary to determine whether salivary gland tumors themselves, not just Warthin’s tumors, are prone to producing IgG4 or whether IgG4-positive plasma cell infiltration occurs via tumor-stimulated signals.
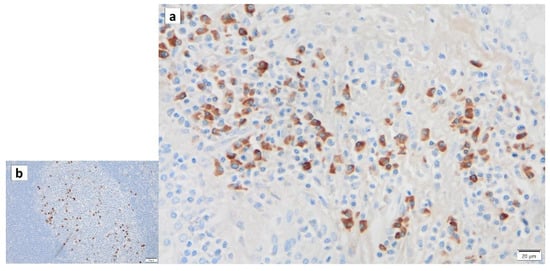
Figure 8.
IgG4 immunohistochemistry of Warthin’s tumor. IgG4 positivity is detected in the lymphocytes of (a) the surrounding tissue and (b) the germinal center. Bars represent 20 μm.
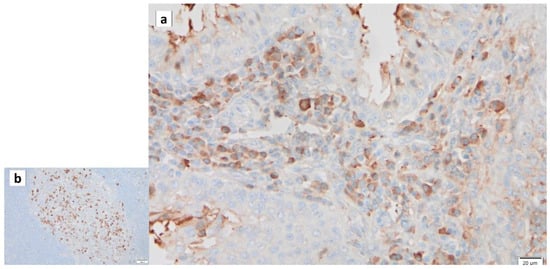
Figure 9.
IgG immunohistochemistry of Warthin’s tumor. IgG is detected in (a) some of lymphocytes in the surrounding stroma and (b) germinal center. Bars represent 20 μm.
9. The Role of GAPDH in Warthin’s Tumor Cells
Once considered a simple “housekeeping” protein, the glycolytic enzyme GAPDH has recently been shown to be involved in many cellular functions other than glycolysis [90,91]. In addition, studies pointing to its involvement in apoptosis-promoting functions and tumor progression have suggested that GAPDH depletion is associated with cellular senescence, such as accelerated senescence in tumor cells [92,93,94,95]. However, many aspects of the function of GAPDH remain unclear [96].
Regarding the association between Warthin’s tumor and GAPDH, on anti-GAPDH immunohistochemical staining, Warthin’s tumor cells had a significantly lower percentage of GAPDH-positive cells (p < 0.0001) in comparison to normal parotid duct cells [18]. The quantitative analysis of the expression of GAPDH mRNA by quantitative RT-PCR also showed that Warthin’s tumor cells had lower expression levels in comparison to normal parotid duct cells [18]. GAPDH was found to be associated with damaged mitochondria, resulting in direct incorporation of these mitochondria into lysosomes [97]. This suggests that Warthin’s tumor cells gradually lose cytoplasmic GAPDH due to cell-wide GAPDH loss or a nuclear shift [18], as shown in Figure 3.
10. The Role of p16 and p53 in Warthin’s Tumor
p16 is a tumor suppressor protein encoded by the cyclin-dependent kinase inhibition 2A (CDKN2A) gene. On immunostaining, p16 positivity is generally a biomarker for HPV infection-related malignancies, such as cervical and pharyngeal cancers. Although this seems contradictory, it is thought to be due to the interference of HPV E6 and E7 viral tumor protein expression with the tumor suppressor p53 and Rb pathways, causing upregulation of p16 expression through the inactivation of Rb by E7 [98,99].
p53 is a tumor suppressor gene that promotes or suppresses the transcription of various proteins, thereby conferring resistance to various cellular stresses. The inhibition of cell cycle progression by p53 is mediated by multiple mechanisms, including the upregulation of p21, which inhibits the cyclin-dependent kinase (CDK) family of kinases, and transcriptional regulation of Gadd45 and 14-3-3σ. p53 maintains genomic stability and is involved in DNA repair, apoptosis, and cellular senescence [100]. The inactivation of p53 tumor suppressor genes occurs frequently during tumorigenesis. In most cases, the p53 gene mutates to produce a stable mutant protein, the accumulation of which is considered a hallmark of cancer cells. Mutant p53 proteins not only lose their tumor suppressor activity, but often acquire additional oncogenic functions that confer growth and survival advantages to the cell [101].
In normal cells, the p53 protein has a very short half-life and is not present in sufficient amounts to be detected by immunostaining. However, abnormal p53 caused by mutations has a long half-life and accumulates in the nucleus. As a result, mutated p53 is easily detectable by IHC. There are interesting studies on the immunohistochemical staining of p16ink and p53 in Warthin’s tumors. Classic cytogenetic studies have identified clonal abnormalities in several Warthin’s tumors [102,103]. Recently, however, several molecular biological studies have begun to address the controversy over whether Warthin’s tumor is neoplastic or nonneoplastic and have shown conflicting results. For example, immunohistochemical staining of p16ink and p53 in 12 cases of Warthin’s tumor was negative in all cases, suggesting that there was no evidence of abnormal staining for tumor suppressor gene protein products (p16ink and p53) and no evidence of consistent clonal allelic deletions, indicating that Warthin’s tumor is non-neoplastic [104]. In another study that examined the clonality of the epithelial component of Warthin’s tumors using a PCR assay based on random inactivation of the gene by trinucleotide repeat polymorphisms and methylation of the X chromosome-related human androgen receptor gene (HUMARA), all cases showed a polyclonal X inactivation pattern, suggesting that Warthin’s tumors are non-neoplastic [105].
In our recent study, immunohistochemical staining of p16 (Figure 10) and p53 (Figure 11) revealed positive nuclei in the columnar cells of Warthin’s tumor, suggesting that Warthin’s tumor is neoplastic, although whether Warthin’s tumor is a true neoplasm that occurs as a clonal growth or a non-neoplastic developmental malformation is still a matter of debate. In addition, our recent unpublished study revealed positive immunohistochemical staining of Ki67/MIB-1 in columnar cells in Warthin’s tumors (Figure 12), indicating that columnar cells possess proliferative activity.
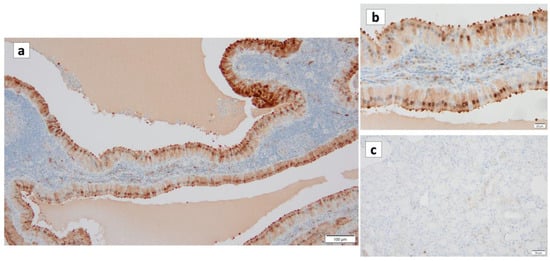
Figure 10.
p16 immunohistochemistry of Warthin’s tumor. (a,b) p16 immunoreactivity is noted in the nuclei of the columnar cells and some surrounding lymphocytes. (c) On the other hand, p16 is not detected in the normal salivary gland. (a) Bar represents 100 μm. (b) Bar represents 20 μm. (c) Bar represents 50 μm.
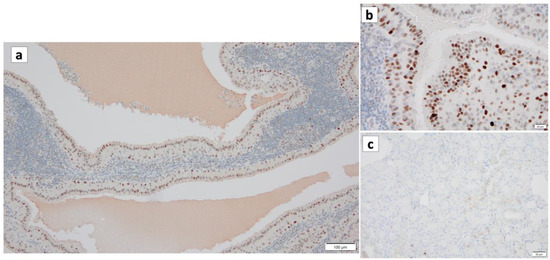
Figure 11.
p53 immunohistochemistry of Warthin’s tumor. (a,b) p53 immunoreactivity is noted in the nuclei of the columnar cells and some surrounding lymphocytes. (c) However, p53 is not detected in the normal salivary gland. (a) Bar represents 100 μm. (b) Bar represents 20 μm. (c) Bar represents 50 μm.
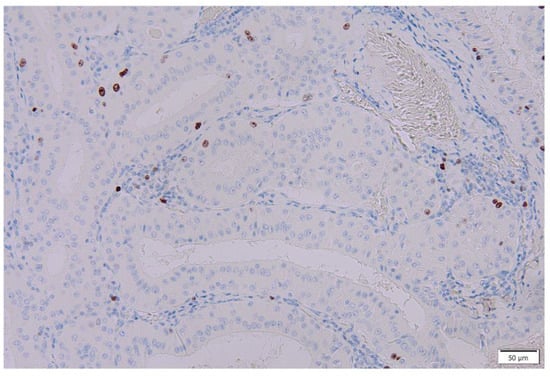
Figure 12.
Ki67/MIB-1 immunohistochemistry of columnar cells in Warthin’s tumors. Several nuclei of the columnar cells in the Warthin’s tumor are positive for Ki67/MIB-1. Bar represents 50 μm.
11. HPV Infection and Warthin’s Tumor
An interesting study showed that HPV-PCR was performed on 50 of 55 salivary gland tumors that tested positive for p16, and it was found that HPV was not involved in salivary gland pathogenesis [106]. However, in another study, HPV PCR was performed in 25 cases of Warthin’s tumor, and HPV was detected in 19 of 25 cases (76%). The remaining six cases were negative or had no amplified DNA; HPV type 16 was detected in all 19 positive cases that tested positive for HPV by PCR [47]. These results suggest a correlation between the nuclear overexpression of p16 and high-risk HPV infection in Warthin’s tumors. HPV type 16 has also been detected in other salivary gland neoplasms, such as adenoid cystic carcinoma; adenocarcinoma NOS; Warthin’s tumor; and to a lesser extent, acinus cell carcinoma, salivary duct carcinoma, and adenoid basal cell carcinoma. Thus, HPV appears to be involved in a significant proportion of salivary gland tumors, but its exact role remains controversial [47]. It is possible that salivary gland tumors may decrease as HPV vaccines become more widely available. We would propose additional research that can more definitively link HPV to Warthin’s tumor.
12. Conclusions
As illustrated in Figure 13, senescent cells and chronic inflammation (SASP) may be associated with the development and progression of Warthin’s tumors. Morphologically, the cytoplasm of Warthin’s tumor reflects a state of mitochondrial overaccumulation called oncocytosis, which is caused by abnormal mitochondrial metabolism and involves damage to the mitochondrial genome. Positive p53 immunohistochemical staining in Warthin’s tumors may reflect mutations in the p53 gene or p53 activity due to cellular senescence. Whether Warthin’s tumor is a true neoplasm that occurs as a clonal growth or nonneoplastic developmental malformation remains controversial. However, given the accumulation of aged mitochondria (senescent cells), HPV positivity, and p53 and p16 positivity in Warthin’s tumor, we believe that Warthin’s tumor is neoplastic.
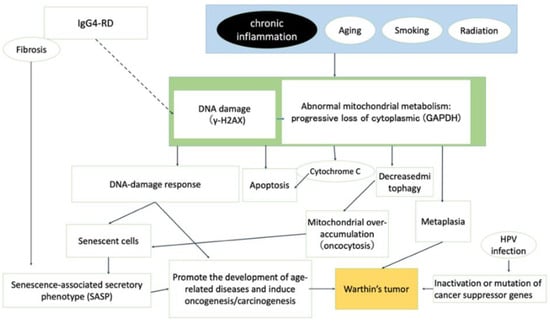
Figure 13.
Graphical summary of the different factors that contribute to the development of Warthin’s tumor.
Author Contributions
R.A. completed the collection and analysis of relevant literature and wrote the first draft of the manuscript; T.T. conceived this review and guided the writing and revision of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors extend sincere thanks to all the staff (Naoki Watanabe, Akane Onogi, Masashi Matsuyama, Riyoko Niwa, Asuka Ohashi, Risa Ito, Sachiko Oka, Fumimasa Etori, Toshie Naraki, and Junko Kondo) in the Department of Diagnostic Pathology, Gifu municipal Hospital for their support in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Teymoortash, A.; Krasnewicz, Y.; Werner, J.A. Clinical features of cystadenolymphoma (Warthin’s tumor) of the parotid gland: A retrospective comparative study of 96 cases. Oral Oncol. 2006, 42, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Kuzenko, Y.V.; Romanuk, A.M.; Dyachenko, O.O.; Hudymenko, O. Pathogenesis of Warthin’s tumors. Interv. Med. Appl. Sci. 2016, 8, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Franzen, A.M.; Kaup Franzen, C.; Guenzel, T.; Lieder, A. Increased incidence of Warthin tumours of the parotid gland: A 42-year evaluation. Eur. Arch. Otorhinolaryngol. 2018, 275, 2593–2598. [Google Scholar] [CrossRef] [PubMed]
- Schwalje, A.T.; Uzelac, A.; Ryan, W.R. Growth rate characteristics of Warthin’s tumours of the parotid gland. Int. J. Oral Maxillofac. Surg. 2015, 44, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Teymoortash, A.; Schrader, C.; Shimoda, H.; Kato, S.; Werner, J.A. Evidence of lymphangiogenesis in Warthin’s tumor of the parotid gland. Oral Oncol. 2007, 43, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Karthikeya, P.; Mahima, V.G.; Shalini, K. Papillary cystadenoma lymphomatosum: Case report and review of literature. Indian J. Dent. Res. 2005, 16, 153–158. [Google Scholar]
- Limaiem, F.; Jain, P. Warthin Tumor; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ning, C.; Koo, J.S.; Kim, E.K.; Lee, S. Clinical and sonographic characteristics of Warthin-like variant papillary thyroid carcinomas. Med. Ultrason. 2019, 21, 152–157. [Google Scholar] [CrossRef]
- Wu, S.; Liu, G.; Chen, R.; Guan, Y. Role of ultrasound in the assessment of benignity and malignancy of parotid masses. Dentomaxillofac Radiol. 2012, 41, 131–135. [Google Scholar] [CrossRef]
- Grandis, A.; El-Naggar, J.; Chan, J.; Takata, T.S.; Slootweg, P.J. WHO Classification of Head and Neck Tumours; IARC: Lyon, France, 2017; p. 85. [Google Scholar]
- Quer, M.; Hernandez-Prera, J.C.; Silver, C.E.; Casasayas, M.; Simo, R.; Vander Poorten, V.; Guntinas-Lichius, O.; Bradley, P.J.; Tong-Ng, W.; Rodrigo, J.P.; et al. Current Trends and Controversies in the Management of Warthin Tumor of the Parotid Gland. Diagnostics 2021, 11, 1467. [Google Scholar] [CrossRef]
- Orabona, G.D.; Abbate, V.; Piombino, P.; Romano, A.; Schonauer, F.; Iaconetta, G.; Salzano, G.; Farina, F.; Califano, L. Warthin’s tumour: Aetiopathogenesis dilemma, ten years of our experience. J. Craniomaxillofac Surg. 2015, 43, 427–431. [Google Scholar] [CrossRef]
- Daguci, L.; Simionescu, C.; Daguci, C.; Bataiosu, M.; Dragomir, L.P. Clinical and Morphological Aspects of Warthin’s Tumor. Curr. Health Sci. J. 2009, 35, 115–118. [Google Scholar]
- Bishop, J.A.; Thompson, L.D.R.; Paul EWakely, J.; Weinreb, I. Tumors of the Salivary Glands; AFIP Atlas of Tumor & Non-Tumor Pathology, 5th Series, Fascicle 5; American Registry of Pathology: Silver Spring, MD, USA, 2021. [Google Scholar]
- Barnes, L.; Eveson, J.; Reichart, P.; Sidransky, D. World Health Organization Classification of Tumours: Pathology and Genetics of Head and Neck Tumours; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Tandler, B.; Shipkey, F.H. Ultrastructure of Warthin’s Tumor. I. Mitochondria. J. Ultrastruct. Res. 1964, 11, 292–305. [Google Scholar] [CrossRef]
- Hartwick, R.W.; Batsakis, J.G. Non-Warthin’s tumor oncocytic lesions. Ann. Otol. Rhinol. Laryngol. 1990, 99, 674–677. [Google Scholar] [CrossRef]
- Mandic, R.; Agaimy, A.; Pinto-Quintero, D.; Roth, K.; Teymoortash, A.; Schwarzbach, H.; Stoehr, C.G.; Rodepeter, F.R.; Stuck, B.A.; Bette, M. Aberrant Expression of Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) in Warthin Tumors. Cancers 2020, 12, 1112. [Google Scholar] [CrossRef]
- Capone, R.B.; Ha, P.K.; Westra, W.H.; Pilkington, T.M.; Sciubba, J.J.; Koch, W.M.; Cummings, C.W. Oncocytic neoplasms of the parotid gland: A 16-year institutional review. Otolaryngol. Head. Neck Surg. 2002, 126, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Soares, P.; Maximo, V.; Samuels, D.C. Somatic mitochondrial DNA mutations in cancer escape purifying selection and high pathogenicity mutations lead to the oncocytic phenotype: Pathogenicity analysis of reported somatic mtDNA mutations in tumors. BMC Cancer 2012, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Bengoechea, O.; Sanchez, F.; Larrinaga, B.; Martinez-Penuela, J.M. Oncocytic adenocarcinoma arising in Warthin’s tumor. Pathol. Res. Pract. 1989, 185, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Alnoor, F.; Gandhi, J.S.; Stein, M.K.; Gradowski, J.F. Follicular Lymphoma Diagnosed in Warthin Tumor: A Case Report and Review of the Literature. Head. Neck Pathol. 2020, 14, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Gorai, S.; Numata, T.; Kawada, S.; Nakano, M.; Tamaru, J.; Kobayashi, T. Malignant lymphoma arising from heterotopic Warthin’s tumor in the neck: Case report and review of the literature. Tohoku J. Exp. Med. 2007, 212, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Smolka, W.; Markowski, J.; Piotrowska-Seweryn, A.; Palen, P.; Dobrosz, Z.; Dziubdziela, W. Mucoepidermoid carcinoma in Warthin tumor of the parotid gland. Arch. Med. Sci. 2015, 11, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.D.; Simmons, B.H.; el-Naggar, A.; Medeiros, L.J. Mucoepidermoid carcinoma involving Warthin tumor. A report of five cases and review of the literature. Am. J. Clin. Pathol. 2000, 114, 564–570. [Google Scholar] [CrossRef]
- Yu, C.; Song, Z.; Xiao, Z.; Lin, Q.; Dong, X. Mucoepidermoid carcinoma arising in Warthin’s tumor of the parotid gland: Clinicopathological characteristics and immunophenotypes. Sci. Rep. 2016, 6, 30149. [Google Scholar] [CrossRef]
- Yaranal, P.J.; Umashankar, T. Squamous Cell Carcinoma Arising in Warthin’s Tumour: A Case Report. J. Clin. Diagn. Res. 2013, 7, 163–165. [Google Scholar] [CrossRef]
- Banik, S.; Howell, J.S.; Wright, D.H. Non-Hodgkin’s lymphoma arising in adenolymphoma—A report of two cases. J. Pathol. 1985, 146, 167–177. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, T.G. Squamous cell carcinoma arising from Warthin’s tumor in the parotid gland. BJR Case Rep. 2019, 5, 20190032. [Google Scholar] [CrossRef]
- Mohapatra, M.; Satyanarayana, S. Low grade mucoepidermoid carcinoma in a setting of Warthin’s tumor. Indian J. Pathol. Microbiol. 2012, 55, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-C.; Li, C.-F.; Lin, C.-N. Unreported Cytologic Characteristics of Oncocytes in Warthin’s Tumors. Tzu Chi Med. J. 2010, 22, 137–140. [Google Scholar] [CrossRef]
- Koybasioglu, F.F.; Onal, B.; Han, U.; Adabag, A.; Sahpaz, A. Cytomorphological findings in diagnosis of Warthin tumor. Turk. J. Med. Sci. 2020, 50, 148–154. [Google Scholar]
- Ballo, M.S.; Shin, H.J.; Sneige, N. Sources of diagnostic error in the fine-needle aspiration diagnosis of Warthin’s tumor and clues to a correct diagnosis. Diagn. Cytopathol. 1997, 17, 230–234. [Google Scholar] [CrossRef]
- Das, D.K.; Petkar, M.A.; Al-Mane, N.M.; Sheikh, Z.A.; Mallik, M.K.; Anim, J.T. Role of fine needle aspiration cytology in the diagnosis of swellings in the salivary gland regions: A study of 712 cases. Med. Princ. Pract. 2004, 13, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.; Ronen, O. Cytologic diagnosis of parotid gland Warthin tumor: Systematic review and meta-analysis. Head Neck 2022, 44, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Zahran, M.; Alsedra, S.; Cope, D.; Youssef, A. The Role of FNAC in the Diagnosis and Management of Warthin Tumour: Analysis of 74 Cases. Int. Arch. Otorhinolaryngol. 2021, 25, e379–e382. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.T.; Sugihara, K.; Kawashima, K.; Yamashita, S. Scanning electron microscopic study of Warthin’s tumor. J. Oral. Pathol. 1987, 16, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, S.; Chen, F.; Aguirre, A. Oncocytoma of the Parotid Gland and its Mimickers: A Comprehensive Review. N. Am. J. Med. Sci. 2010, 3, 171. [Google Scholar] [CrossRef]
- el-Hossary, N.M.; Fathy, L.M. Luminal epithelium of Warthin’s tumours: A scanning electron microscopic study. Egypt Dent. J. 1994, 40, 791–794. [Google Scholar] [PubMed]
- Seifert, G. Primary salivary gland tumors in lymph nodes of the parotid gland. Report of 3 cases and review of the literature. Pathologe 1997, 18, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.M.; Hsu, P.L.; Nayak, R.N. Warthin’s tumor: An immunohistochemical study of its lymphoid stroma. Hum. Pathol. 1981, 12, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.M.; Raine, L. Warthin’s tumor—Epithelial cell differences. Am. J. Clin. Pathol. 1982, 77, 78–82. [Google Scholar] [CrossRef]
- Peter Klussmann, J.; Wittekindt, C.; Florian Preuss, S.; Al Attab, A.; Schroeder, U.; Guntinas-Lichius, O. High risk for bilateral Warthin tumor in heavy smokers—Review of 185 cases. Acta Otolaryngol. 2006, 126, 1213–1217. [Google Scholar] [CrossRef]
- Saku, T.; Hayashi, Y.; Takahara, O.; Matsuura, H.; Tokunaga, M.; Tokuoka, S.; Soda, M.; Mabuchi, K.; Land, C.E. Salivary gland tumors among atomic bomb survivors, 1950–1987. Cancer 1997, 79, 1465–1475. [Google Scholar] [CrossRef]
- Gallo, O.; Bocciolini, C. Warthin’s tumour associated with autoimmune diseases and tobacco use. Acta Otolaryngol. 1997, 117, 623–627. [Google Scholar] [CrossRef]
- Rabia, A.B.; Ebru, L.S.; Tuba, K.; Didar, G.; Gulhan, O.; Cengiz, O. Warthin’s tumor: An unknown pathogenesis: A neoplasm or a reactive hyperplasia? Indian J. Pathol. Microbiol. 2015, 58, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Huhns, M.; Simm, G.; Erbersdobler, A.; Zimpfer, A. HPV Infection, but Not EBV or HHV-8 Infection, Is Associated with Salivary Gland Tumours. Biomed. Res. Int. 2015, 2015, 829349. [Google Scholar] [CrossRef]
- Aga, M.; Kondo, S.; Yamada, K.; Sawada-Kitamura, S.; Yagi-Nakanishi, S.; Endo, K.; Wakisaka, N.; Murono, S.; Kawano, M.; Yoshizaki, T. Warthin’s tumor associated with IgG4-related disease. Auris Nasus Larynx. 2013, 40, 514–517. [Google Scholar] [CrossRef]
- Hayflick, L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.D.; Wagner, N. The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis. Cells 2022, 11, 1966. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kawamoto, S.; Ohtani, N.; Hara, E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017, 108, 563–569. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Terlecki-Zaniewicz, L.; Lammermann, I.; Latreille, J.; Bobbili, M.R.; Pils, V.; Schosserer, M.; Weinmüllner, R.; Dellago, H.; Skalicky, S.; Pum, D.; et al. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging 2018, 10, 1103–1132. [Google Scholar] [CrossRef] [PubMed]
- Lecot, P.; Alimirah, F.; Desprez, P.Y.; Campisi, J.; Wiley, C. Context-dependent effects of cellular senescence in cancer development. Br. J. Cancer 2016, 114, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Aoki, R.; Terasaki, M. Potential Chemopreventive Effects of Dietary Combination of Phytochemicals against Cancer Development. Pharmaceuticals 2023, 16, 1591. [Google Scholar] [CrossRef]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef]
- Yasuda, T.; Koiwa, M.; Yonemura, A.; Miyake, K.; Kariya, R.; Kubota, S.; Yokomizo-Nakano, T.; Yasuda-Yoshihara, N.; Uchihara, T.; Itoyama, R.; et al. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell Rep. 2021, 34, 108779. [Google Scholar] [CrossRef]
- Ou, H.L.; Hoffmann, R.; Gonzalez-Lopez, C.; Doherty, G.J.; Korkola, J.E.; Munoz-Espin, D. Cellular senescence in cancer: From mechanisms to detection. Mol. Oncol. 2021, 15, 2634–2671. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2020, 20, 89–106. [Google Scholar] [CrossRef]
- Kaur, A.; Webster, M.R.; Weeraratna, A.T. In the Wnt-er of life: Wnt signalling in melanoma and ageing. Br. J. Cancer 2016, 115, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ishikawa, H. Mast cells and inflammation-associated colorectal carcinogenesis. Semin. Immunopathol. 2013, 35, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Zhu, H.; Racila, E.; Khaja, S.; Hamlar, D.; Li, F. Xanthogranulomatous sialadenitis, an uncommon reactive change is often associated with Warthin’s tumor. Head Neck Pathol. 2020, 14, 525–553. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-Gonzalez, A.; Xu, K.; Wu, T.C.; Aspord, C.; Tindle, S.; Marches, F.; Gallegos, M.; Burton, E.C.; Savino, D.; Hori, T.; et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J. Exp. Med. 2011, 208, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Traub, B.; Shi, J.; Kornmann, M. Possible Roles of Interleukin-4 and -13 and Their Receptors in Gastric and Colon Cancer. Int. J. Mol. Sci. 2021, 22, 727. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.W.; Zhang, C.; Nagley, P. Mutations in mitochondrial DNA accumulate differentially in three different human tissues during ageing. Nucleic Acids Res. 1998, 26, 1268–1275. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.D.; Velarde, M.C.; Lecot, P.; Liu, S.; Sarnoski, E.A.; Freund, A.; Shirakawa, K.; Lim, H.W.; Davis, S.S.; Ramanathan, A.; et al. Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab. 2016, 23, 303–314. [Google Scholar] [CrossRef]
- Fakouri, N.B.; Hou, Y.; Demarest, T.G.; Christiansen, L.S.; Okur, M.N.; Mohanty, J.G.; Croteau, D.L.; Bohr, V.A. Toward understanding genomic instability, mitochondrial dysfunction and aging. FEBS J. 2019, 286, 1058–1073. [Google Scholar] [CrossRef]
- Lu, J.; Sharma, L.K.; Bai, Y. Implications of mitochondrial DNA mutations and mitochondrial dysfunction in tumorigenesis. Cell Res. 2009, 19, 802–815. [Google Scholar] [CrossRef]
- Lewis, P.D.; Baxter, P.; Paul Griffiths, A.; Parry, J.M.; Skibinski, D.O. Detection of damage to the mitochondrial genome in the oncocytic cells of Warthin’s tumour. J. Pathol. 2000, 191, 274–281. [Google Scholar] [CrossRef]
- Gao, S.; Hu, J. Mitochondrial Fusion: The Machineries In and Out. Trends Cell Biol. 2021, 31, 62–74. [Google Scholar] [CrossRef]
- Ferreira-da-Silva, A.; Valacca, C.; Rios, E.; Populo, H.; Soares, P.; Sobrinho-Simoes, M.; Scorrano, L.; Máximo, V.; Campello, S. Mitochondrial dynamics protein Drp1 is overexpressed in oncocytic thyroid tumors and regulates cancer cell migration. PLoS ONE 2015, 10, e0122308. [Google Scholar] [CrossRef]
- Nagdas, S.; Kashatus, J.A.; Nascimento, A.; Hussain, S.S.; Trainor, R.E.; Pollock, S.R.; Adair, S.J.; Michaels, A.D.; Sesaki, H.; Stelow, E.B.; et al. Drp1 Promotes KRas-Driven Metabolic Changes to Drive Pancreatic Tumor Growth. Cell Rep. 2019, 28, 1845–1859.e5. [Google Scholar] [CrossRef]
- Tsuyoshi, H.; Orisaka, M.; Fujita, Y.; Asare-Werehene, M.; Tsang, B.K.; Yoshida, Y. Prognostic impact of Dynamin related protein 1 (Drp1) in epithelial ovarian cancer. BMC Cancer 2020, 20, 467. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Chen, E.; Pan, Z. The role of mitochondrial dysfunction in mesenchymal stem cell senescence. Cell Tissue Res. 2020, 382, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Bu, W.; Lv, G.; Xu, L.; Hou, D.; Wang, J.; Liu, X.; Yang, T.; Zhang, X.; Liu, Q.; et al. Disrupted mitochondrial homeostasis coupled with mitotic arrest generates antineoplastic oxidative stress. Oncogene 2022, 41, 427–443. [Google Scholar] [CrossRef]
- Zhu, M.; Min, S.; Mao, X.; Zhou, Y.; Zhang, Y.; Li, W.; Li, L.; Wu, L.; Cong, X.; Yu, G. Interleukin-13 promotes cellular senescence through inducing mitochondrial dysfunction in IgG4-related sialadenitis. Int. J. Oral. Sci. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Kamisawa, T.; Zen, Y.; Pillai, S.; Stone, J.H. IgG4-related disease. Lancet 2015, 385, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.H.; Zen, Y.; Deshpande, V. IgG4-related disease. N. Engl. J. Med. 2012, 366, 539–551. [Google Scholar] [CrossRef]
- Floreani, A.; Okazaki, K.; Uchida, K.; Gershwin, M.E. IgG4-related disease: Changing epidemiology and new thoughts on a multisystem disease. J. Transl. Autoimmun. 2021, 4, 100074. [Google Scholar] [CrossRef] [PubMed]
- Aalberse, R.C.; Schuurman, J. IgG4 breaking the rules. Immunology 2002, 105, 9–19. [Google Scholar] [CrossRef]
- Nirula, A.; Glaser, S.M.; Kalled, S.L.; Taylor, F.R. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr. Opin. Rheumatol. 2011, 23, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Aalberse, R.C.; Stapel, S.O.; Schuurman, J.; Rispens, T. Immunoglobulin G4: An odd antibody. Clin. Exp. Allergy 2009, 39, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Koike, T. IgG4-related disease: Why high IgG4 and fibrosis? Arthritis Res. Ther. 2013, 15, 103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aga, M.; Kondo, S.; Yamada, K.; Wakisaka, N.; Yagi-Nakanishi, S.; Tsuji, A.; Endo, K.; Murono, S.; Ito, M.; Muramatsu, M.; et al. Immunoglobulin class switching to IgG4 in Warthin tumor and analysis of serum IgG4 levels and IgG4-positive plasma cells in the tumor. Hum. Pathol. 2014, 45, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Shimo, T.; Yao, M.; Takebe, Y.; Ono, Y.; Obata, K.; Kurio, N.; Ibaragi, S.; Yoshioka, N.; Kishimoto, K.; Yanagi, Y.; et al. A case of adenoid cystic carcinoma associated with IgG4-related disease. Int. J. Surg. Case Rep. 2015, 10, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The diverse functions of GAPDH: Views from different subcellular compartments. Cell Signal. 2011, 23, 317–323. [Google Scholar] [CrossRef]
- Sirover, M.A. New insights into an old protein: The functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta 1999, 1432, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, R.; Sunaga, K.; Hirano, A.; Saunders, P.; Katsube, N.; Chuang, D.M. Evidence that glyceraldehyde-3-phosphate dehydrogenase is involved in age-induced apoptosis in mature cerebellar neurons in culture. J. Neurochem. 1996, 66, 928–935. [Google Scholar] [CrossRef]
- Dastoor, Z.; Dreyer, J.L. Potential role of nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase in apoptosis and oxidative stress. J. Cell Sci. 2001, 114 Pt 9, 1643–1653. [Google Scholar] [CrossRef]
- Phadke, M.; Krynetskaia, N.; Mishra, A.; Krynetskiy, E. Accelerated cellular senescence phenotype of GAPDH-depleted human lung carcinoma cells. Biochem. Biophys. Res. Commun. 2011, 411, 409–415. [Google Scholar] [CrossRef]
- Phadke, M.S.; Krynetskaia, N.F.; Mishra, A.K.; Krynetskiy, E. Glyceraldehyde 3-phosphate dehydrogenase depletion induces cell cycle arrest and resistance to antimetabolites in human carcinoma cell lines. J. Pharmacol. Exp. Ther. 2009, 331, 77–86. [Google Scholar] [CrossRef]
- Colell, A.; Green, D.R.; Ricci, J.E. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009, 16, 1573–1581. [Google Scholar] [CrossRef]
- Hwang, S.; Disatnik, M.H.; Mochly-Rosen, D. Impaired GAPDH-induced mitophagy contributes to the pathology of Huntington’s disease. EMBO Mol. Med. 2015, 7, 1307–1326. [Google Scholar] [CrossRef]
- Teymoortash, A.; Bohne, F.; Jonsdottir, T.; Hoch, S.; Eivazi, B.; Roessler, M.; Werner, J.A.; Mandic, R. Human papilloma virus (HPV) is not implicated in the etiology of Warthin’s tumor of the parotid gland. Acta Otolaryngol. 2013, 133, 972–976. [Google Scholar] [CrossRef]
- Wiest, T.; Schwarz, E.; Enders, C.; Flechtenmacher, C.; Bosch, F.X. Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 2002, 21, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhamid, E.S.; Elshafei, M.M. Immunohistochemical localization of mdm-2, p27Kip1 and bcl-2 in Warthin’s tumor of the parotid gland. Diagn. Pathol. 2009, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Fonseca, I.; Roque, L.; Soares, J. Cytogenetic characterisation of Warthin’s tumour. Oral Oncol. 1997, 33, 344–347. [Google Scholar] [CrossRef]
- Bullerdiek, J.; Haubrich, J.; Meyer, K.; Bartnitzke, S. Translocation t(11;19)(q21;p13.1) as the sole chromosome abnormality in a cystadenolymphoma (Warthin’s tumor) of the parotid gland. Cancer Genet. Cytogenet. 1988, 35, 129–132. [Google Scholar] [CrossRef]
- Arida, M.; Barnes, E.L.; Hunt, J.L. Molecular assessment of allelic loss in Warthin tumors. Mod. Pathol. 2005, 18, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Kashima, K.; Daa, T.; Yokoyama, S.; Nakayama, I. Clonal analysis of the epithelial component of Warthin’s tumor. Hum. Pathol. 2000, 31, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Skalova, A.; Kaspirkova, J.; Andrle, P.; Hosticka, L.; Vanecek, T. Human papillomaviruses are not involved in the etiopathogenesis of salivary gland tumors. Cesk Patol. 2013, 49, 72–75. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).