High Pretreatment DHEA Is Associated with Inferior Immunotherapy Response in Metastatic Non-Small Cell Lung Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Data Collection
2.3. Sample Collection
2.4. Quantification of Sex Hormones in Plasma
2.5. Statistical Analyses
3. Results
3.1. Patient Demographics and Clinical Characteristics of the Whole Cohort
3.2. Plasma Sex Hormone Levels in the Discovery Cohort
3.3. Patients with High DHEA Levels Had Poor Clinical Outcomes in the Whole Cohort
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cowley, L.A.; Liu, X.S. Sex Differences in Cancer Immunotherapy Efficacy, Biomarkers, and Therapeutic Strategy. Molecules 2019, 24, 3214. [Google Scholar] [CrossRef]
- Conforti, F.; Pala, L.; Bagnardi, V.; Viale, G.; De Pas, T.; Pagan, E.; Pennacchioli, E.; Cocorocchio, E.; Ferrucci, P.F.; De Marinis, F.; et al. Sex-Based Heterogeneity in Response to Lung Cancer Immunotherapy: A Systematic Review and Me-ta-Analysis. J. Natl. Cancer Inst. 2019, 111, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Jing, Y.; Li, L.; Mills, G.B.; Diao, L.; Liu, H.; Han, L. Sex-associated molecular differences for cancer immunotherapy. Nat. Commun. 2020, 11, 1779. [Google Scholar] [CrossRef] [PubMed]
- Ben-Batalla, I.; Vargas-Delgado, M.E.; Von Amsberg, G.; Janning, M.; Loges, S. Influence of Androgens on Immunity to Self and Foreign: Effects on Immunity and Cancer. Front. Immunol. 2020, 11, 1184. [Google Scholar] [CrossRef] [PubMed]
- Prall, S.P.; Muehlenbein, M.P. DHEA Modulates Immune Function: A Review of Evidence. Vitam. Horm. 2018, 108, 125–144. [Google Scholar] [PubMed]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; George, V.; Thomas Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef]
- Robert, C.; Lebbé, C.; Lesimple, T.; Lundström, E.; Nicolas, V.; Gavillet, B.; Crompton, P.; Baroudjian, B.; Routier, E.; Lejeune, F.J. Phase I Study of Androgen Deprivation Therapy in Combination with Anti-PD-1 in Melanoma Patients Pretreated with Anti-PD-1. Clin. Cancer Res. 2023, 29, 858–865. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Hochmair, M.J.; Schwab, S.; Burghuber, O.C.; Krenbek, D.; Prosch, H. Symptomatic pseudo-progression followed by significant treatment response in two lung cancer pa-tients treated with immunotherapy. Lung Cancer 2017, 113, 4–6. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef]
- Kataoka, Y.; Hirano, K.; Narabayashi, T.; Hara, S.; Fujimoto, D.; Tanaka, T.; Yoshioka, H. Concordance between the response evaluation criteria in solid tumors version 1.1 and the im-mune-related response criteria in patients with non-small cell lung cancer treated with nivolumab: A multicenter retrospective cohort study. Cancer Chemother. Pharmacol. 2018, 81, 333–337. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Şenler, F.Ç.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Darville, L.N.; Cline, J.K.; Rozmeski, C.; Martinez, Y.C.; Rich, S.; Eschrich, S.A.; Egan, K.M.; Yaghjyan, L.; Koomen, J.M. LC-HRMS of derivatized urinary estrogens and estrogen metabolites in postmenopausal women. J. Chromatogr. B 2020, 1154, 122288. [Google Scholar] [CrossRef] [PubMed]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, F.; Habibzadeh, P.; Yadollahie, M. On determining the most appropriate test cut-off value: The case of tests with continuous results. Biochem. Med. 2016, 26, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Vasarhelyi, B.; Bencsik, P.; Treszl, A.; Bardóczy, Z.; Tulassay, T.L.; Szathmari, M. The effect of physiologic hyperinsulinemia during an oral glucose tolerance test on the levels of dehy-droepiandrosterone (DHEA) and its sulfate (DHEAS) in healthy young adults born with low and with normal birth weight. Endocr. J. 2003, 50, 689–695. [Google Scholar] [CrossRef][Green Version]
- Zhao, D.; Guallar, E.; Ballantyne, C.M.; Post, W.S.; Ouyang, P.; Vaidya, D.; Jia, X.; Ying, W.; Subramanya, V.; Ndumele, C.E.; et al. Sex Hormones and Incident Heart Failure in Men and Postmenopausal Women: The Atherosclerosis Risk in Communities Study. J. Clin. Endocrinol. Metab. 2020, 105, e3798–e3807. [Google Scholar] [CrossRef]
- Wu, Y.; Hankinson, S.E.; Smith-Warner, S.A.; Wang, M.; Eliassen, A.H. Flavonoid Intake and Plasma Sex Steroid Hormones, Prolactin, and Sex Hormone-Binding Globulin in Premenopausal Women. Nutrients 2019, 11, 2669. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.L.; Archer-Chicko, C.; Barr, R.G.; Bluemke, D.A.; Foderaro, A.E.; Fritz, J.S.; Hill, N.S.; Kawut, S.M.; Klinger, J.R.; Lima, J.A.C.; et al. Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1800467. [Google Scholar] [CrossRef]
- Klinge, C.M.; Clark, B.J.; Prough, R.A. Dehydroepiandrosterone Research: Past, Current, and Future. Vitam. Horm. 2018, 108, 1–28. [Google Scholar] [PubMed]
- Sanchez, C.; Chan, R.; Bajgain, P.; Rambally, S.; Palapattu, G.; Mims, M.; Vera, J.F. Combining T-cell immunotherapy and anti-androgen therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2013, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ardiani, A.; Farsaci, B.; Rogers, C.J.; Protter, A.; Guo, Z.; King, T.H.; Apelian, D.; Hodge, J.W. Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin. Cancer Res. 2013, 19, 6205–6218. [Google Scholar] [CrossRef]
- Akins, E.J.; Moore, M.L.; Tang, S.; Willingham, M.C.; Tooze, J.A.; Dubey, P. In situ vaccination combined with androgen ablation and regulatory T-cell depletion reduces castra-tion-resistant tumor burden in prostate-specific pten knockout mice. Cancer Res. 2010, 70, 3473–3482. [Google Scholar] [CrossRef]
- Koh, Y.T.; Gray, A.; Higgins, S.A.; Hubby, B.; Kast, W.M. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate 2009, 69, 571–584. [Google Scholar] [CrossRef]
- Kwon, H.; Schafer, J.M.; Song, N.J.; Kaneko, S.; Li, A.; Xiao, T.; Ma, A.; Allen, C.; Das, K.; Zhou, L.; et al. Androgen conspires with the CD8+ T cell exhaustion program and contributes to sex bias in cancer. Sci. Immunol. 2022, 7, eabq2630. [Google Scholar] [CrossRef]
- Roden, A.C.; Moser, M.T.; Tri, S.D.; Mercader, M.; Kuntz, S.M.; Dong, H.; Hurwitz, A.A.; McKean, D.J.; Celis, E.; Leibovich, B.C.; et al. Augmentation of T cell levels and responses induced by androgen deprivation. J. Immunol. 2004, 173, 6098–6108. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, Y.; Ran, S.; Segal, S. Sex-associated differences in the regulation of immune responses controlled by the MHC of the mouse. J. Immunol. 1984, 132, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Liva, S.M.; Voskuhl, R.R. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol. 2001, 167, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Huayllas, M.K.; Netzel, B.C.; Singh, R.J.; Kater, C.E. Serum Cortisol Levels via Radioimmunoassay vs Liquid Chromatography Mass Spectrophotome-try in Healthy Control Subjects and Patients with Adrenal Incidentalomas. Lab. Med. 2018, 49, 259–267. [Google Scholar] [PubMed]
- Jeon, D.S.; Kim, J.W.; Kim, S.G.; Kim, H.R.; Song, S.Y.; Lee, J.C.; Ji, W.; Choi, C.-M.; Kim, H.C. Sex differences in the characteristics and survival of patients with non-small-cell lung cancer: A retrospec-tive analytical study based on real-world clinical data of the Korean population. Thorac. Cancer 2022, 13, 2584–2591. [Google Scholar] [CrossRef]
- Löfling, L.; Karimi, A.; Sandin, F.; Bahmanyar, S.; Kieler, H.; Lambe, M.; Lamberg, K.; Wagenius, G. Clinical characteristics and survival in non-small cell lung cancer patients by smoking history: A popula-tion-based cohort study. Acta Oncol. 2019, 58, 1618–1627. [Google Scholar] [CrossRef]
- Leblanc, M.; Labrie, C.; Bélanger, A.; Candas, B.; Labrie, F. Bioavailability and pharmacokinetics of dehydroepiandrosterone in the cynomolgus monkey. J. Clin. Endocrinol. Metab. 2003, 88, 4293–4302. [Google Scholar] [CrossRef][Green Version]
- Webb, S.J.; Geoghegan, T.E.; Prough, R.A.; Michael Miller, K.K. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab. Rev. 2006, 38, 89–116. [Google Scholar] [CrossRef]

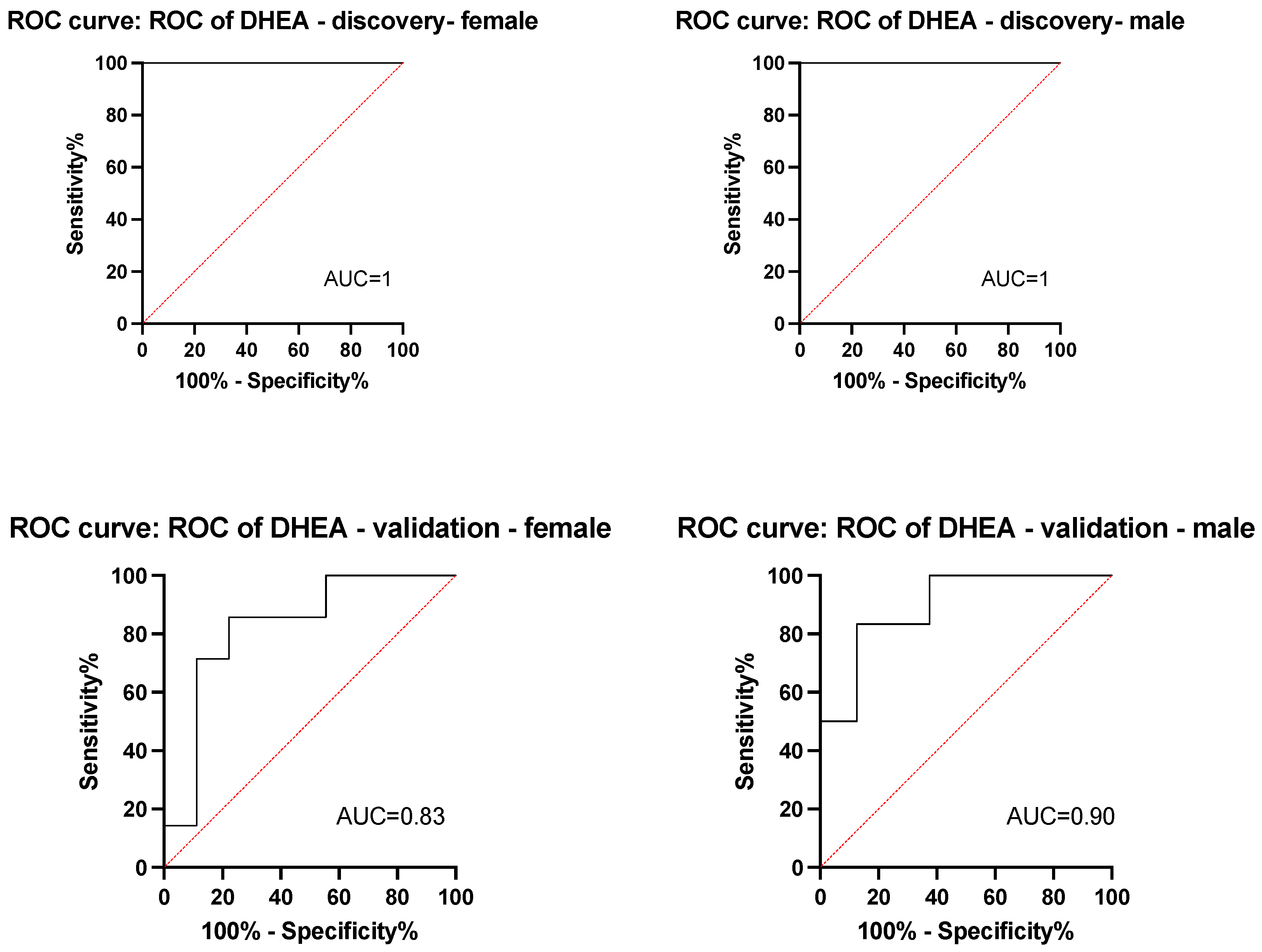
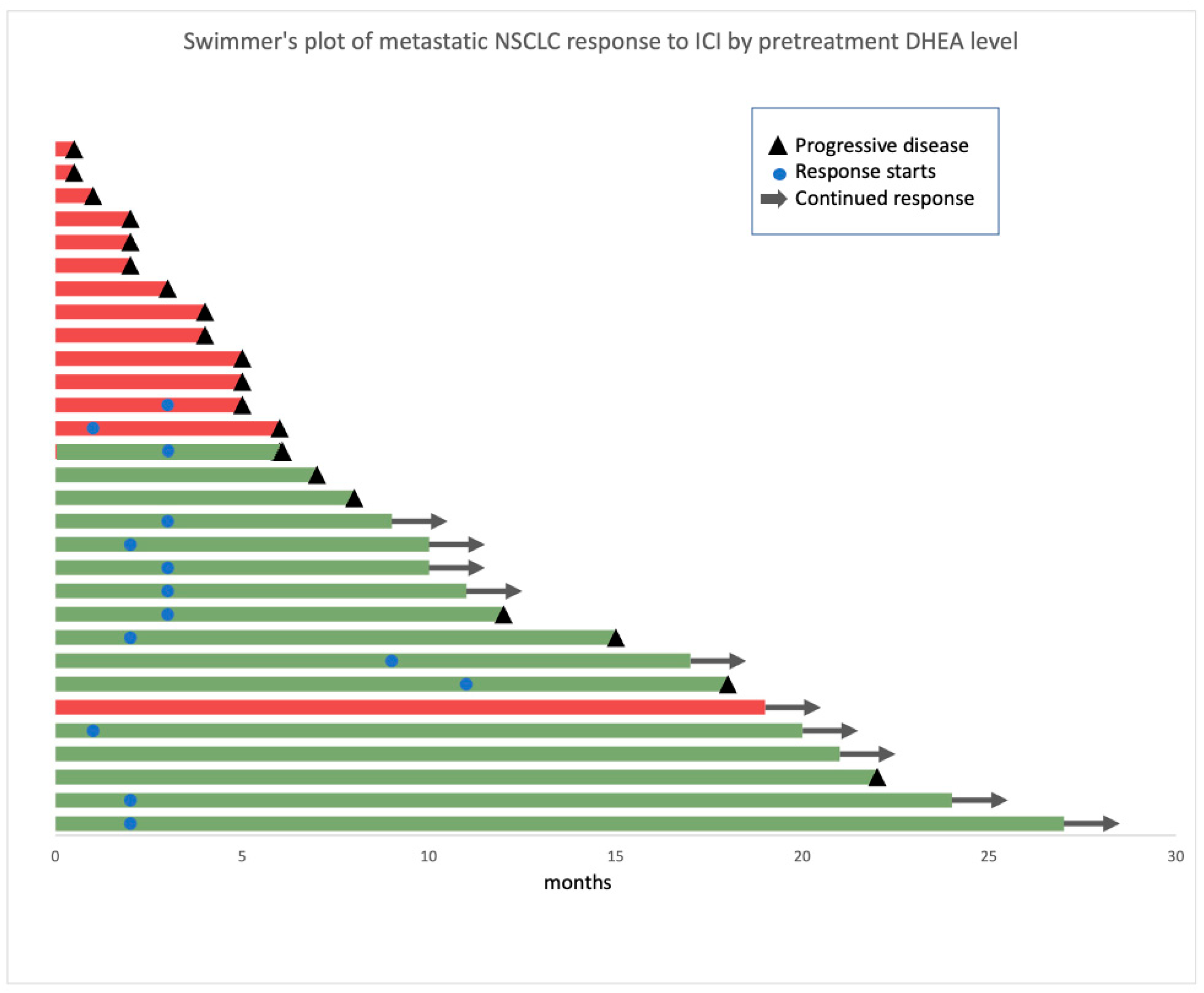
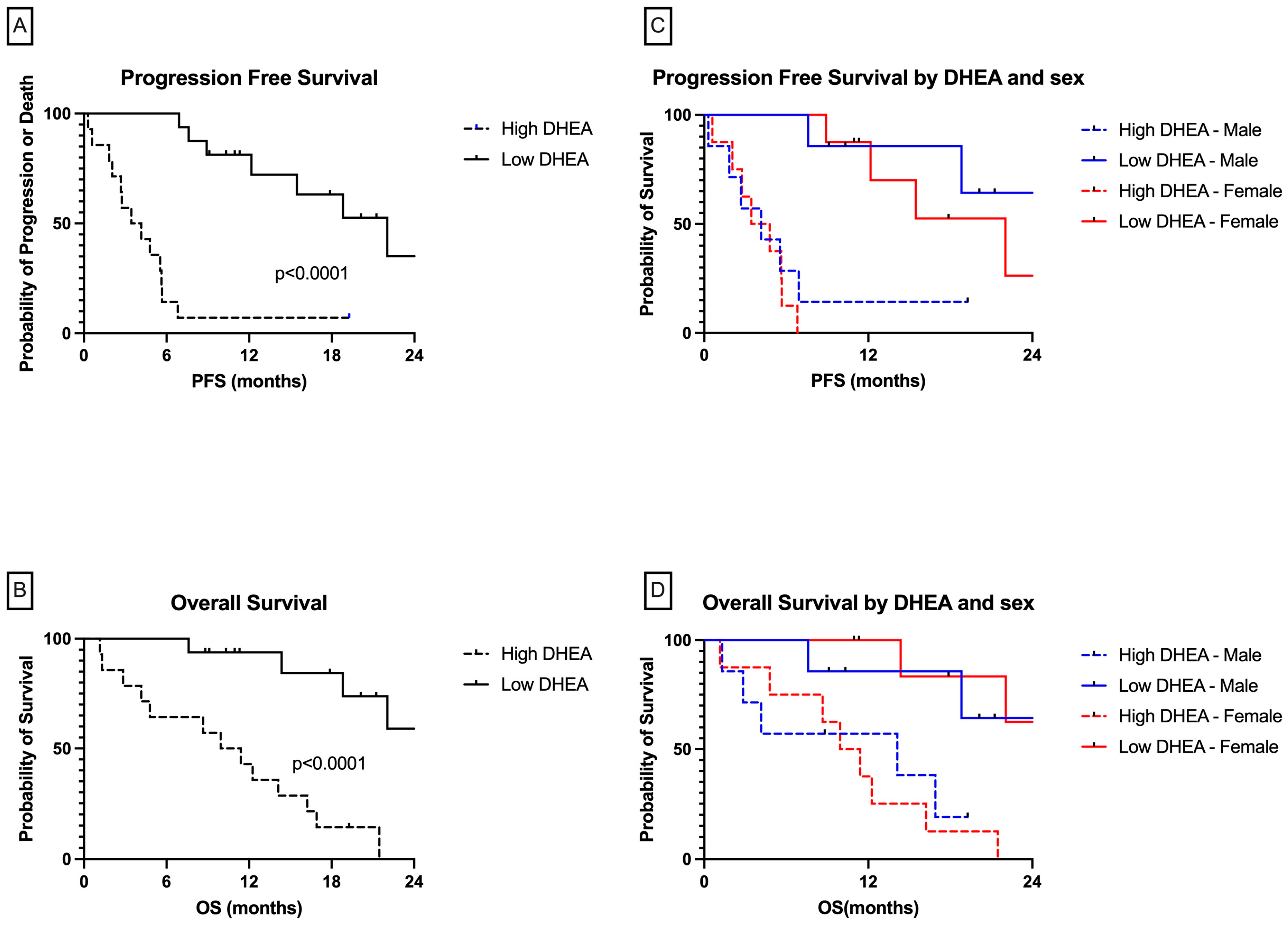
| Characteristic | Clinical Benefit Status | DHEA Level | ||||
|---|---|---|---|---|---|---|
| Clinical Benefit (N = 33) | No Clinical Benefit (N = 28) | p Value | Low DHEA (N = 16) | High DHEA (N = 14) | p Value | |
| Age at diagnosis, years, median [range] | 67 [44, 85] | 67.5 [51, 83] | 0.89 | 66 (54, 75) | 67 (48, 83) | 0.96 |
| Male sex, No. (%) | 15 (45.5) | 15 (53.6) | 0.527 | 8 (50) | 6 (42.9) | 0.73 |
| Race, No. (%) | 0.074 | 0.99 | ||||
| White | 31 (94) | 23 (82) | 14 (87.5) | 12 (85.7) | ||
| Black | 1 (3) | 3 (11) | 2 (12.5) | 2 (14.3) | ||
| BMI at time of treatment, median [95% CI] | 26.1 [18.7, 36.9] | 23.8 [16.9, 46.9] | 0.36 | 26 (20, 37) | 26 (20, 40) | 0.14 |
| Smoking status, No. (%) | 0.803 | 0.71 | ||||
| Current | 8 (24.2) | 9 (32.1) | 5 (31.3) | 5 (35.7) | ||
| Former | 21 (63.6) | 15 (53.6) | 9 (56.3) | 6 (42.9) | ||
| Never | 4 (12.1) | 4 (14.3) | 2 (12.5) | 3 (21.4) | ||
| ECOG performance status, No. (%) | 0.353 | 1.0 | ||||
| 0 | 5 (15.2) | 2 (7.1) | 2 (12.5) | 1 (7.1) | ||
| 1 | 28 (84.8) | 25 (89.3) | 14 (87.5) | 13 (92.9) | ||
| Histology, No. (%) | 0.529 | 0.31 | ||||
| Non-squamous | 29 (88) | 24 (86) | 14 (87.5) | 11 (21.4) | ||
| Squamous | 4 (12) | 4 (14) | 2 (12.5) | 3 (64.3) | ||
| Neutrophil-to-lymphocyte ratio, median [95% CI] | 4.2 [1.8, 14.8] | 4.9 [1.6, 13.7] | 0.85 | 4.2 (2.6, 10.1) | 6.4 (2.5, 13.7) | 0.38 |
| Platelet-to-lymphocyte ratio, median [95% CI] | 245.6 [62.2, 1104.2] | 229.9 [105.7, 796.2] | 0.81 | 239 (137, 523) | 262 (146, 1068) | 0.46 |
| Comorbidities, No. (%) | ||||||
| COPD | 12 (36) | 16 (57) | 0.11 | 4 (25) | 8 (57.1) | 0.14 |
| HLD | 22 (66.7) | 17 (60.7) | 0.63 | 12 (75) | 7 (50) | 0.26 |
| MI/heart failure | 3 (9.1) | 6 (21.4) | 0.18 | 1 (6.3) | 4 (28.6) | 0.16 |
| DM | 4 (12.1) | 10 (36) | 0.038 | 4 (25) | 5 (35.7) | 0.69 |
| Prior chemotherapy, No. (%) | 12 (36) | 15 (54) | 0.406 | 9 (56.3) | 7 (50) | 1.0 |
| PD-L1 positive (>1%), No. positive/No. evaluable (%) | 20/27 (74) | 13/24 (54) | 0.84 | 9/13 (0.69) | 10/13 (0.77) | 0.58 |
| Other mutations, No. positive/No. evaluable (%) | ||||||
| ALK fusion | 1/19 | 1/20 | 0.93 | 1/11 | 0/8 | 0.4 |
| EGFR | 5/24 | 2/31 | 0.16 | 0/15 | 1/12 | 0.4 |
| KRAS | 6/19 | 7/31 | 0.07 | 5/14 | 2/10 | 0.39 |
| NRAS | 0/14 | 0/15 | 0.72 | 0/7 | 0/6 | 0.96 |
| TP53 | 6/11 | 6/15 | 0.95 | 2/7 | 3/5 | 0.51 |
| Clinical Factors | Progression-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| No. Progression/No. Cases | Univariable HR (95% CI) | Multivariable HR (95% CI) | No. Death/No. Cases | Univariable HR (95% CI) | Multivariable HR (95% CI) | |
| Age | 45/61 | 1.01 (0.98–1.05) | 1.03 (1.97, 1.08) | 34/61 | 0.98 (0.94, 1.02) | 0.97 (0.92, 1.04) |
| Sex | ||||||
| Male | 21/30 | 1 | 1 | 17/30 | 1 | 1 |
| Female | 24/31 | 0.85 (0.47, 1.53) | 0.68 (0.23, 2.02) | 17/31 | 1.39 (0.70, 2.73) | 1.14 (0.33, 3.83) |
| p value for trend | 0.58 | 0.48 | 0.35 | 0.36 | ||
| BMI | 45/61 | 1.00 (0.95, 1.06) | 1.02 (0.92, 1.11) | 34/61 | 0.98 (0.93, 1.05) | 0.96 (0.88, 1.05) |
| Smoking status | ||||||
| Current | 13/17 | 1 | 1 | 12/17 | 1 | 1 |
| Former | 27/36 | 0.92 (0.47, 1.79) | 0.25 (0.037, 1.72) | 18/36 | 0.58 (0.28, 1.20) | 0.52 (0.047, 5.62) |
| Never | 5/8 | 0.84 (0.30, 2.36) | 0.20 (0.018, 2.12) | 4/8 | 0.67 (0.21, 2.07) | 0.26 (0.017, 4.09) |
| p value for trend | 0.94 | 0.58 | 0.33 | 0.43 | ||
| Medical comorbidities | ||||||
| DM present | 10/14 | 1.74 (0.85, 3.59) | 1.3 (0.38, 4.25) | 10/14 | 2.63 (1.21, 5.71) | 1.9 (0.49, 7.47) |
| DM absent | 35/47 | 1 | 1 | 24/47 | 1 | 1 |
| p value for trend | 0.13 | 0.70 | 0.015 | 0.31 | ||
| DHEA | ||||||
| High | 13/14 | 9.22 (3.23–26.31) | 10.63 (3.4, 32.96) | 13/14 | 8.75 (2.45,30.3) | 11.59 (2.99, 44.9) |
| Low | 7/16 | 1 | 1 | 4/16 | 1 | 1 |
| p value for trend | <0.001 | <0.001 | <0.001 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Darville, L.; Hogue, S.; Hallanger Johnson, J.E.; Rose, T.; Kim, Y.; Bailey, A.; Gray, J.E.; Robinson, L.A. High Pretreatment DHEA Is Associated with Inferior Immunotherapy Response in Metastatic Non-Small Cell Lung Cancer. Cancers 2024, 16, 1152. https://doi.org/10.3390/cancers16061152
Zhang Y, Darville L, Hogue S, Hallanger Johnson JE, Rose T, Kim Y, Bailey A, Gray JE, Robinson LA. High Pretreatment DHEA Is Associated with Inferior Immunotherapy Response in Metastatic Non-Small Cell Lung Cancer. Cancers. 2024; 16(6):1152. https://doi.org/10.3390/cancers16061152
Chicago/Turabian StyleZhang, Yumeng, Lancia Darville, Stephanie Hogue, Julie E. Hallanger Johnson, Trevor Rose, Youngchul Kim, Alexis Bailey, Jhanelle E. Gray, and Lary A. Robinson. 2024. "High Pretreatment DHEA Is Associated with Inferior Immunotherapy Response in Metastatic Non-Small Cell Lung Cancer" Cancers 16, no. 6: 1152. https://doi.org/10.3390/cancers16061152
APA StyleZhang, Y., Darville, L., Hogue, S., Hallanger Johnson, J. E., Rose, T., Kim, Y., Bailey, A., Gray, J. E., & Robinson, L. A. (2024). High Pretreatment DHEA Is Associated with Inferior Immunotherapy Response in Metastatic Non-Small Cell Lung Cancer. Cancers, 16(6), 1152. https://doi.org/10.3390/cancers16061152







