Follicle-Stimulating Hormone Receptor Expression and Its Potential Application for Theranostics in Subtypes of Ovarian Tumors: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias in Studies
3.4. FSH Receptor Expression
3.4.1. Serous Carcinomas
3.4.2. Mucinous Carcinomas
3.4.3. Sex Cord–Stromal Tumors
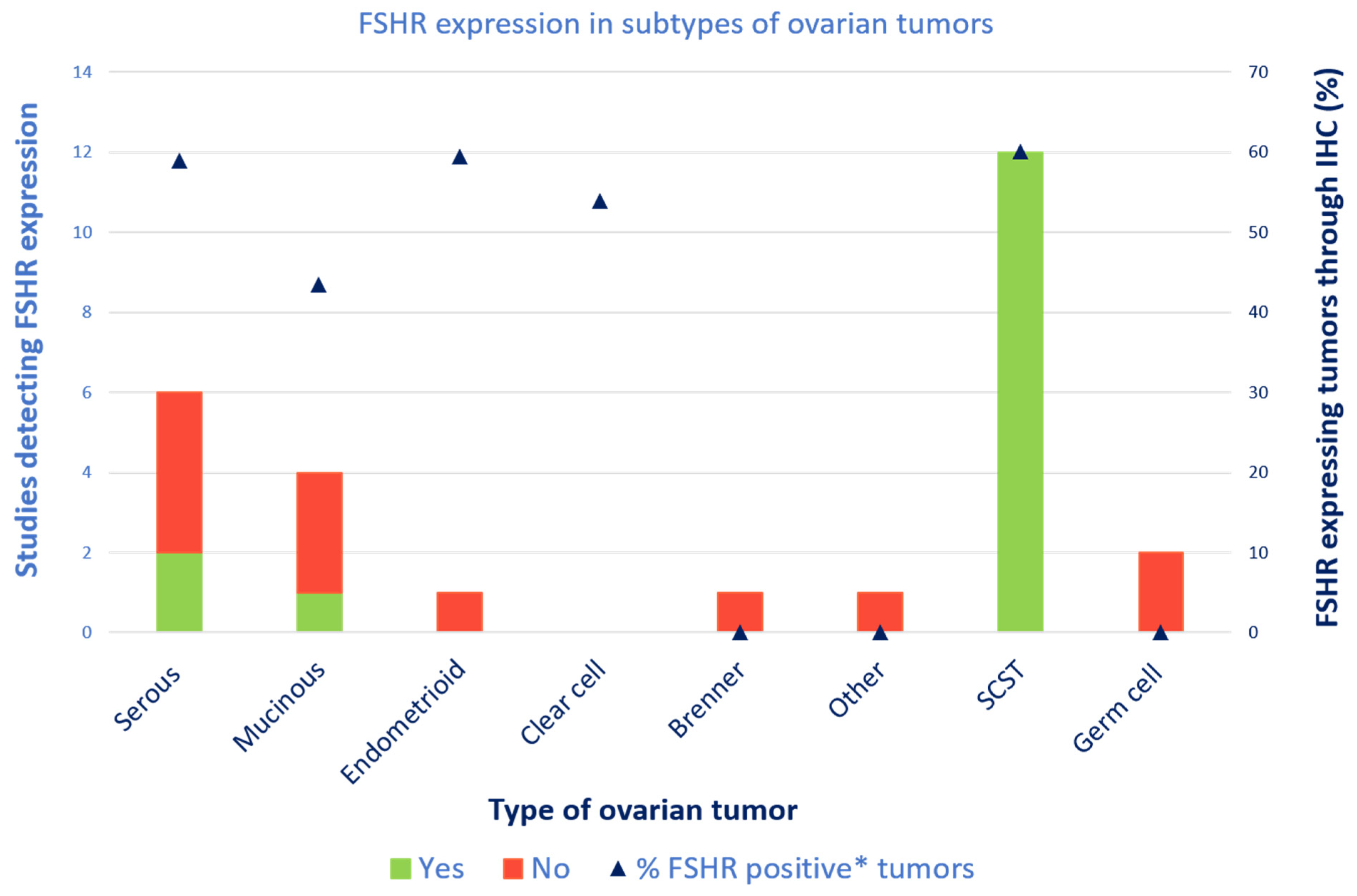
3.4.4. All Types of Ovarian Tumors
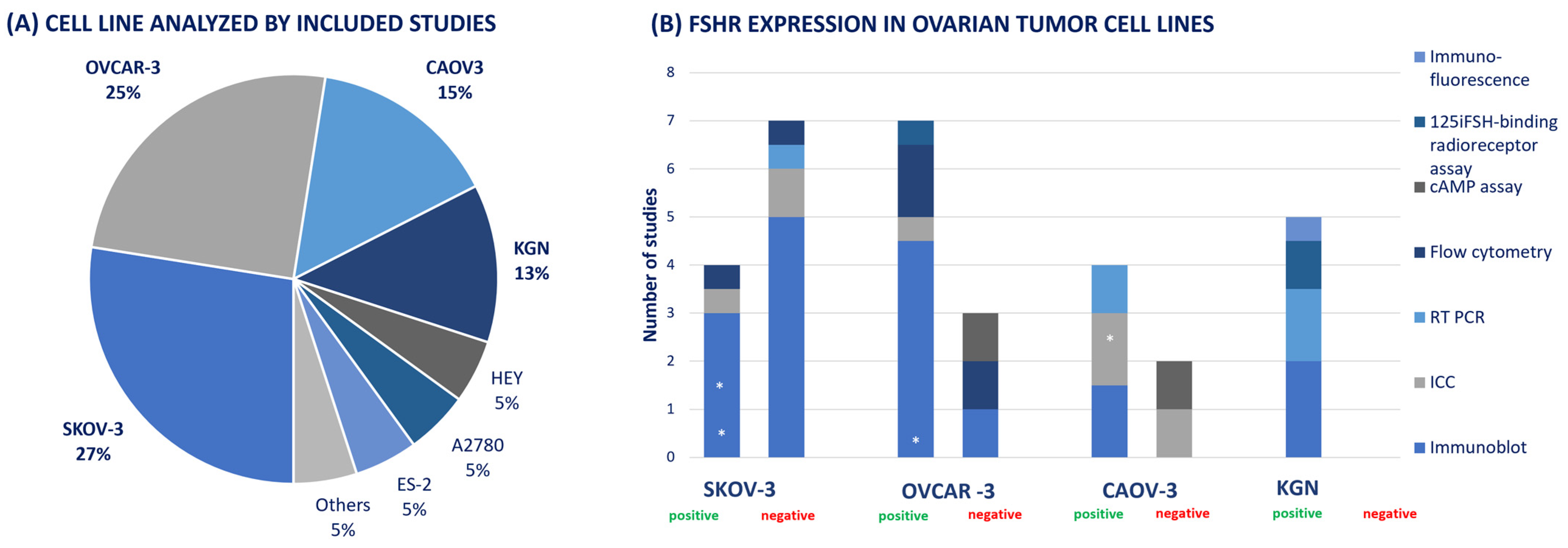
3.4.5. Cell Line Studies
4. Discussion
4.1. Summary of Main Results
4.2. Results in Context of Published Literature
4.3. Strengths and Weaknesses
4.4. Implications for Practice and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. Pathology of cancers of the female genital tract. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S2), S132–S145. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, L.; Hassell, L.A. WHO Classification. Available online: https://www.pathologyoutlines.com/topic/ovarytumorwhoclassif.html (accessed on 8 February 2023).
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjugate Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Langbein, T.; Weber, W.A.; Eiber, M. Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine. J. Nucl. Med. 2019, 60, 13s–19s. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Zariñán, T.; Jardón-Valadez, E.; Gutiérrez-Sagal, R.; Dias, J.A. Structure-Function Relationships of the Follicle-Stimulating Hormone Receptor. Front. Endocrinol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Zheng, W.; Magid, M.S.; Kramer, E.E.; Chen, Y.T. Follicle-stimulating hormone receptor is expressed in human ovarian surface epithelium and fallopian tube. Am. J. Pathol. 1996, 148, 47–53. [Google Scholar] [PubMed]
- Radu, A.; Pichon, C.; Camparo, P.; Antoine, M.; Allory, Y.; Couvelard, A.; Fromont, G.; Hai, M.T.; Ghinea, N. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N. Engl. J. Med. 2010, 363, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Kountourakis, P.; Kottorou, A.E.; Antonacopoulou, A.G.; Rolfo, C.; Peeters, M.; Kalofonos, H.P. Follicle-Stimulating Hormone Receptor (FSHR): A Promising Tool in Oncology? Mol. Diagn. Ther. 2016, 20, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Bonci, E.A.; Irimie, A.; Buiga, R.; Cosmin Lisencu, I.; Iuliana Maja, L.; Piciu, D. Follicle-stimulating hormone receptors: A new immunohistochemical marker in cancers? J. BUON 2017, 22, 1352–1359. [Google Scholar] [PubMed]
- Perales-Puchalt, A.; Svoronos, N.; Rutkowski, M.R.; Allegrezza, M.J.; Tesone, A.J.; Payne, K.K.; Wickramasinghe, J.; Nguyen, J.M.; O’Brien, S.W.; Gumireddy, K.; et al. Follicle-Stimulating Hormone Receptor Is Expressed by Most Ovarian Cancer Subtypes and Is a Safe and Effective Immunotherapeutic Target. Clin. Cancer Res. 2017, 23, 441–453. [Google Scholar] [CrossRef]
- Wei, S.; Lai, L.; Yang, J.; Zhuandi, G. Expression Levels of Follicle-Stimulating Hormone Receptor and Implication in Diagnostic and Therapeutic Strategy of Ovarian Cancer. Oncol. Res. Treat. 2018, 41, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Ghinea, N. Vascular Endothelial FSH Receptor, a Target of Interest for Cancer Therapy. Endocrinology 2018, 159, 3268–3274. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis. Available online: https://synthesismanual.jbi.global (accessed on 18 January 2023).
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid.-Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Cheung, J.; Lokman, N.A.; Abraham, R.D.; Macpherson, A.M.; Lee, E.; Grutzner, F.; Ghinea, N.; Oehler, M.K.; Ricciardelli, C. Reduced Gonadotrophin Receptor Expression Is Associated with a More Aggressive Ovarian Cancer Phenotype. Int. J. Mol. Sci. 2020, 22, 71. [Google Scholar] [CrossRef]
- Lenhard, M.; Lennerová, T.; Ditsch, N.; Kahlert, S.; Friese, K.; Mayr, D.; Jeschke, U. Opposed roles of follicle-stimulating hormone and luteinizing hormone receptors in ovarian cancer survival. Histopathology 2011, 58, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.P.; Bruneau, N.; Vega, M.; Selman, A.; Tapia, J.C.; Romero, C. Follicle-stimulating hormone promotes nerve growth factor and vascular endothelial growth factor expression in epithelial ovarian cells. Histol. Histopathol. 2020, 35, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Rushdi, S.; Zumpe, E.T.; Mamers, P.; Healy, D.L.; Jobling, T.; Burger, H.G.; Fuller, P.J. FSH-regulated gene expression profiles in ovarian tumours and normal ovaries. Mol. Hum. Reprod. 2002, 8, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Bashashati, A.; Anglesio, M.S.; Cochrane, D.R.; Grewal, D.S.; Ha, G.; McPherson, A.; Horlings, H.M.; Senz, J.; Prentice, L.M.; et al. Genomic consequences of aberrant DNA repair mechanisms stratify ovarian cancer histotypes. Nat. Genet. 2017, 49, 856–864. [Google Scholar] [CrossRef]
- Minegishi, T.; Kameda, T.; Hirakawa, T.; Abe, K.; Tano, M.; Ibuki, Y. Expression of gonadotropin and activin receptor messenger ribonucleic acid in human ovarian epithelial neoplasms. Clin. Cancer Res. 2000, 6, 2764–2770. [Google Scholar]
- Nakano, R.; Kitayama, S.; Yamoto, M.; Shima, K.; Ooshima, A. Localization of gonadotropin binding sites in human ovarian neoplasms. Am. J. Obstet. Gynecol. 1989, 161, 905–910. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, J.J.; Luo, F.; Zheng, Y.; Feng, Y.; Felix, J.C.; Lauchlan, S.C.; Pike, M.C. Ovarian epithelial tumor growth promotion by follicle-stimulating hormone and inhibition of the effect by luteinizing hormone. Gynecol. Oncol. 2000, 76, 80–88. [Google Scholar] [CrossRef]
- Stouffer, R.L.; Grodin, M.S.; Davis, J.R.; Surwit, E.A. Investigation of binding sites for follicle-stimulating hormone and chorionic gonadotropin in human ovarian cancers. J. Clin. Endocrinol. Metab. 1984, 59, 441–446. [Google Scholar] [CrossRef]
- Feng, Z.; Wen, H.; Bi, R.; Ju, X.; Chen, X.; Yang, W.; Wu, X. A clinically applicable molecular classification for high-grade serous ovarian cancer based on hormone receptor expression. Sci. Rep. 2016, 6, 25408. [Google Scholar] [CrossRef]
- Feng, Z.; Wen, H.; Ju, X.; Bi, R.; Chen, X.; Yang, W.; Wu, X. Hormone receptor expression profiles differ between primary and recurrent high-grade serous ovarian cancers. Oncotarget 2017, 8, 32848–32855. [Google Scholar] [CrossRef]
- Feng, Z.; Wen, H.; Ju, X.; Bi, R.; Chen, X.; Yang, W.; Wu, X. Expression of hypothalamic-pituitary-gonadal axis-related hormone receptors in low-grade serous ovarian cancer (LGSC). J. Ovarian Res. 2017, 10, 7. [Google Scholar] [CrossRef]
- Burger, H.G.; Baillie, A.; Drummond, A.E.; Healy, D.L.; Jobling, T.; Mamers, P.; Robertson, D.M.; Susil, B.; Cahir, N.; Shen, Y.; et al. Inhibin and ovarian cancer. J. Reprod. Immunol. 1998, 39, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Haltia, U.M.; Pihlajoki, M.; Andersson, N.; Mäkinen, L.; Tapper, J.; Cervera, A.; Horlings, H.M.; Turpeinen, U.; Anttonen, M.; Bützow, R.; et al. Functional Profiling of FSH and Estradiol in Ovarian Granulosa Cell Tumors. J. Endocr. Soc. 2020, 4, bvaa034. [Google Scholar] [CrossRef]
- Giacaglia, L.R.; Da Fonte Kohek, M.B.; Carvalho, F.M.; Fragoso, M.C.B.V.; Bilharinho Mendonca, B.; Latronico, A.C. No evidence of somatic activating mutations on gonadotropin receptor genes in sex cord stromal tumors. Fertil. Steril. 2000, 74, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Fuller, P.J.; Verity, K.; Shen, Y.; Mamers, P.; Jobling, T.; Burger, H.G. No evidence of a role for mutations or polymorphisms of the follicle-stimulating hormone receptor in ovarian granulosa cell tumors. J. Clin. Endocrinol. Metab. 1998, 83, 274–279. [Google Scholar] [CrossRef] [PubMed]
- McNeilage, J.; Alexiadis, M.; Susil, B.J.; Mamers, P.; Jobling, T.; Laslett, G.; Trajstman, A.; Fuller, P.J. Molecular characterization of sarcomatous change in a granulosa cell tumor. Int. J. Gynecol. Cancer 2007, 17, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, M.M.; Zschunke, M.A.; Roche, P.C. Loss of alternately spliced messenger RNA of the luteinizing hormone receptor and stability of the follicle-stimulating hormone receptor messenger RNA in granulosa cell tumors of the human ovary. Gynecol. Oncol. 2000, 79, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y.; Yanase, T.; Mu, Y.; Oba, K.; Ichino, I.; Saito, M.; Nomura, M.; Mukasa, C.; Okabe, T.; Goto, K.; et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001, 142, 437–445. [Google Scholar] [CrossRef]
- Choong, C.S.; Fuller, P.J.; Chu, S.; Jeske, Y.; Bowling, F.; Brown, R.; Borzi, P.; Balazs, N.D.; Suppiah, R.; Cotterill, A.M.; et al. Sertoli-Leydig cell tumor of the ovary, a rare cause of precocious puberty in a 12-month-old infant. J. Clin. Endocrinol. Metab. 2002, 87, 49–56. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Lin, L.; Parkash, V.; Schwartz, P.E.; Lauchlan, S.C.; Zheng, W. Quantitative analysis of follicle-stimulating hormone receptor in ovarian epithelial tumors: A novel approach to explain the field effect of ovarian cancer development in secondary mullerian systems. Int. J. Cancer 2003, 103, 328–334. [Google Scholar] [CrossRef]
- Cellosaurus SK-OV-3 (CVCL_0532). Available online: https://www.cellosaurus.org/CVCL_0532 (accessed on 3 February 2023).
- Cellosaurus OVCAR-3 (CVCL_0465). Available online: https://www.cellosaurus.org/CVCL_0465 (accessed on 3 February 2023).
- Cellosaurus Caov-3 (CVCL_0201). Available online: https://www.cellosaurus.org/CVCL_0201 (accessed on 3 February 2023).
- Gera, S.; Kumar, S.S.; Swamy, S.N.; Bhagat, R.; Vadaparty, A.; Gawari, R.; Bhat, R.; Dighe, R.R. Follicle-Stimulating Hormone Is an Autocrine Regulator of the Ovarian Cancer Metastatic Niche Through Notch Signaling. J. Endocr. Soc. 2019, 3, 340–357. [Google Scholar] [CrossRef]
- Choi, K.C.; Kang, S.K.; Tai, C.J.; Auersperg, N.; Leung, P.C. Follicle-stimulating hormone activates mitogen-activated protein kinase in preneoplastic and neoplastic ovarian surface epithelial cells. J. Clin. Endocrinol. Metab. 2002, 87, 2245–2253. [Google Scholar] [CrossRef]
- Hong, S.S.; Zhang, M.X.; Zhang, M.; Yu, Y.; Chen, J.; Zhang, X.Y.; Xu, C.J. Follicle-stimulating hormone peptide-conjugated nanoparticles for targeted shRNA delivery lead to effective gro-α silencing and antitumor activity against ovarian cancer. Drug Deliv. 2018, 25, 576–584. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, D.; Wang, Y. Detection of the DICER1 hotspot mutation alongside immunohistochemical analysis may provide a better diagnostic measure for ovarian Sertoli-Leydig cell tumors. Pathol. Res. Pract. 2018, 214, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Choi, K.C.; Auersperg, N.; Leung, P.C. Overexpression of follicle-stimulating hormone receptor activates oncogenic pathways in preneoplastic ovarian surface epithelial cells. J. Clin. Endocrinol. Metab. 2004, 89, 5508–5516. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Chen, J.; Zhang, X.; Liu, Y.; Xu, C. Follicle-stimulating hormone polypeptide modified nanoparticle drug delivery system in the treatment of lymphatic metastasis during ovarian carcinoma therapy. Gynecol. Oncol. 2014, 135, 125–132. [Google Scholar] [CrossRef]
- Heublein, S.; Mayr, D.; Vrekoussis, T.; Friese, K.; Hofmann, S.S.; Jeschke, U.; Lenhard, M. The G-protein coupled estrogen receptor (GPER/GPR30) is a gonadotropin receptor dependent positive prognosticator in ovarian carcinoma patients. PLoS ONE 2013, 8, e71791. [Google Scholar] [CrossRef] [PubMed]
- Modi, D.A.; Sunoqrot, S.; Bugno, J.; Lantvit, D.D.; Hong, S.; Burdette, J.E. Targeting of follicle stimulating hormone peptide-conjugated dendrimers to ovarian cancer cells. Nanoscale 2014, 6, 2812–2820. [Google Scholar] [CrossRef]
- Parrott, J.A.; Doraiswamy, V.; Kim, G.; Mosher, R.; Skinner, M.K. Expression and actions of both the follicle stimulating hormone receptor and the luteinizing hormone receptor in normal ovarian surface epithelium and ovarian cancer. Mol. Cell. Endocrinol. 2001, 172, 213–222. [Google Scholar] [CrossRef]
- Urbanska, K.; Stashwick, C.; Poussin, M.; Powell, D.J., Jr. Follicle-Stimulating Hormone Receptor as a Target in the Redirected T-cell Therapy for Cancer. Cancer Immunol. Res. 2015, 3, 1130–1137. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, J.; Zheng, Y.F.; Gao, X.L.; Kang, Y.; Liu, J.C.; Cheng, M.J.; Sun, H.; Xu, C.J. Follicle-stimulating hormone peptide can facilitate paclitaxel nanoparticles to target ovarian carcinoma in vivo. Cancer Re. 2009, 69, 6506–6514. [Google Scholar] [CrossRef]
- Lee, C.W.; Guo, L.; Matei, D.; Stantz, K. Development of Follicle-Stimulating Hormone Receptor Binding Probes to Image Ovarian Xenografts. J. Biotechnol. Biomater. 2015, 5, 198. [Google Scholar]
- Möker, N.; Peters, S.; Rauchenberger, R.; Ghinea, N.; Kunz, C. Antibody Selection for Cancer Target Validation of FSH-Receptor in Immunohistochemical Settings. Antibodies 2017, 6, 15. [Google Scholar] [CrossRef]
- King, D.W.; Steinmetz, R.; Wagoner, H.A.; Hannon, T.S.; Chen, L.Y.; Eugster, E.A.; Pescovitz, O.H. Differential expression of GRK isoforms in nonmalignant and malignant human granulosa cells. Endocrine 2003, 22, 135–141. [Google Scholar] [CrossRef]
- Xie, M.; Li, M.; Zhou, J.; Ding, X.; Shao, Y.; Jing, J.; Liu, Y.; Yao, B. Brain-derived neurotrophic factor promotes human granulosa-like tumor cell steroidogenesis and proliferation by activating the FSH receptor-mediated signaling pathway. Sci. Rep. 2017, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Midgley, A.R., Jr. Protein hormone action: A key to understanding ovarian follicular and luteal cell development. Biol. Reprod. 1976, 14, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Zhang, X.; Chen, J.; Zhou, J.; Zheng, Y.; Xu, C. Targeted gene silencing using a follicle-stimulating hormone peptide-conjugated nanoparticle system improves its specificity and efficacy in ovarian clear cell carcinoma in vitro. J. Ovarian Res. 2013, 6, 80. [Google Scholar] [CrossRef]
- Ji, Q.; Liu, P.I.; Chen, P.K.; Aoyama, C. Follicle stimulating hormone-induced growth promotion and gene expression profiles on ovarian surface epithelial cells. Int. J. Cancer 2004, 112, 803–814. [Google Scholar] [CrossRef]
- Li, S.; Liu, H.; Wang, L.; Zhu, C. Effects of antisense oligodeoxynucleotide to follicle-stimulating hormone receptor on the expression of proliferating cell nuclear antigen and vascular endothelial growth factor in primary culture cells derived from human ovarian mucinous cystadenocarcinoma. J. Huazhong Univ. Sci. Technol. Med. Sci. 2006, 26, 111–115. [Google Scholar]
- Syed, V.; Ulinski, G.; Mok, S.C.; Yiu, G.K.; Ho, S.M. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001, 61, 6768–6776. [Google Scholar]
- Zhang, M.; Liu, Q.; Zhang, M.; Cao, C.; Liu, X.; Zhang, M.; Li, G.; Xu, C.; Zhang, X. Enhanced antitumor effects of follicle-stimulating hormone receptor-mediated hexokinase-2 depletion on ovarian cancer mediated by a shift in glucose metabolism. J. Nanobiotechnol. 2020, 18, 161. [Google Scholar] [CrossRef] [PubMed]


| Author (Year) | Patients (n) | FIGO Stage at Diagnosis | Analyzing Methods | FSH Receptor Expression (%) |
|---|---|---|---|---|
| (% of Total) | ||||
| Serous tumors | ||||
| Cheung (2020) [22] | 112 | II (1.8) | IHC | 65.2 |
| III (91.1) | ||||
| IV (7.1) | ||||
| Lenhard (2011) [23] | 110 | I (22.6) | IHC | 64.5 |
| II (5.8) | ||||
| III (70.3) | ||||
| IV (1.3) | ||||
| Garrido (2020) [24] | NA | NA | RT-PCR on RNA | Yes 1 |
| IHC | ||||
| Chu (2002) [25] | 9 | IIIC (88.9) | RT-PCR on RNA | Little—no 2 |
| NA (11.1) | ||||
| Wang (2003) [26] | 17 | I (35.3) | RT-PCR on RNA | Yes 1 |
| II (35.3) | ||||
| III (29.4) | ||||
| Minegishi (2000) [27] | 9 | Ia (16.6) 3 | Immunoblot | 0 |
| 2c (5.6) | RT-PCR on RNA | 22.2 | ||
| IIIa (11.1) | ||||
| IIIb (5.6) | ||||
| IIIc (38.9) | ||||
| IV (22.2) | ||||
| Perales (2017) [14] | 28 | III/IV | Immunoblot | 50 |
| IHC | ||||
| Nakano (1989) [28] | 7 | NA | 125IFSH-binding radioreceptor assay | No 2 |
| Zheng (2000) [29] | 17 | NA | RT-PCR on RNA | 59 |
| ISH | 65 | |||
| IHC | 53 | |||
| Stouffer (1984) [30] | 13 | NA | 125IFSH-binding radioreceptor assay | No 2 |
| High-grade serous tumors | ||||
| Cheung (2020) [22] | 29 | I (13.8) | RT-PCR on RNA | Little—no 2 |
| II (27.6) | ||||
| III (58.6) | ||||
| Feng (2016) [31] | 863 4 | I and II (9) | IHC | 55% |
| III and IV (91) | ||||
| Feng (2017) [32] | 214 | I and II (3.7) | IHC | 44.9 (P 5) |
| III and IV (96.3) | 55.3 (R 6) | |||
| Low-grade serous tumors | ||||
| Feng (2017) [33] | 25 | I (23.1) | IHC | 84 |
| III (73.1) | ||||
| IV (3.8) | ||||
| Mucinous tumors | ||||
| Lenhard (2011) [23] | 21 | I (22.6) 3 | IHC | 61.9 |
| II (5.8) | ||||
| III (70.3) | ||||
| IV (1.3) | ||||
| Burger (1998) [34] | 7 | NA | RT-PCR on RNA | Yes 1 |
| Chu (2002) [25] | 8 | NA (1) | RT-PCR on RNA | No 2 |
| Benigne (1) | ||||
| I (5) | ||||
| II (1) | ||||
| Zheng (2000) [29] | 4 | I (10) 3 | RT-PCR on RNA | 50 |
| II (23.3) | ISH | 50 | ||
| III (50) | IHC | 25 | ||
| IV (16.7) | ||||
| Perales (2017) [14] | 9 | NA | Immunoblot | 67 |
| Minegishi (2000) [27] | 3 | Ia (16.6) 7 | ||
| 2c (5.6) | ||||
| IIIa (11.1) | Immunoblot | 33.3 | ||
| IIIb (5.6) | RT-PCR on RNA | 100 | ||
| IIIc (38.9) | ||||
| IV (22.2) | ||||
| Nakano (1989) [28] | 2 | NA | 125IFSH-binding radioreceptor assay | No 2 |
| Stouffer (1984) [30] | 3 | NA | 125IFSH-binding radioreceptor assay | No 2 |
| Adult granulosa cell tumors | ||||
| Haltia (2020) [35] | 138 7 | I (91) | IHC | 60 |
| II (6) | ||||
| III (1) | ||||
| NA (2) | ||||
| 10 | NA | RT-PCR on RNA | Yes 1 | |
| Burger (1998) [34] | 3 | NA | RT-PCR on RNA | Yes 1 |
| Chu (2002) [25] | 7 | I (42.9) | RT-PCR on RNA | Yes 1 |
| IA (14.3) | ||||
| R 6 (28.6) | ||||
| NA (14.3) | ||||
| Giacigla (2000) [36] | 89 | NA | RT-PCR on RNA | Yes 1 |
| Fuller (1998) [37] | 3 | I (100) | RT-PCR on RNA | Yes 1 |
| 129 | Ia (16.7) | PCR op DNA | Yes 1 | |
| I (16.7) | ||||
| IIc (8.3) | ||||
| III (16.7) | ||||
| NA (16.6)) | ||||
| McNeilage (2007) [38] | 1 | NA 8 | RT-PCR on RNA | Yes 1 |
| Reinholtz (2000) [39] | 12 | I (58.3) | RT-PCR on RNA | Yes 1 |
| III (8.4) | ||||
| IV (33.3) | ||||
| Nishi (2001) [40] | 1 | III (8.4) | 125IFSH-binding radioreceptor assay | Yes 1 |
| Nakano (1989) [28] | 1 | NA | 125IFSH-binding radioreceptor assay | Yes 1 |
| Sertoli–Leydig cell tumors | ||||
| Choong (2002) [41] | 1 | I (100) | RT-PCR on RNA | Yes 1 |
| Granulosa theca cell tumor | ||||
| Stouffer (1984) [30] | 1 | NA | 125IFSH-binding radioreceptor assay | Yes 1 |
| Endometrioid tumors | ||||
| Lenhard (2011) [23] | 12 | I (22.6) 3 | IHC | 58.3 |
| II (5.8) | ||||
| III (70.3) | ||||
| IV (1.3) | ||||
| Zheng (2000) [29] | 2 | I (10) 3 | IHC | 50 |
| II (23.3) | RT-PCR on RNA | 86 | ||
| III (50) | ISH | 50 | ||
| IV (16.7) | ||||
| Minegishi (2000) [27] | 4 | Ia (16.6) 3 | ||
| 2c (5.6) | ||||
| IIIa (11.1) | RT-PCR on RNA | 50 | ||
| IIIb (5.6) | immunoblot | 0 | ||
| IIIc (38.9) | ||||
| IV (22.2) | ||||
| Perales (2017) [14] | 23 | NA | IHC | 70 |
| immunoblot | 70 | |||
| Stouffer (1984) [30] | 1 | NA | 125IFSH-binding radioreceptor assay | No 2 |
| Clear cell tumors | ||||
| Lenhard (2011) [23] | 13 | NA | IHC | 61.5 |
| Zheng (2000) [29] | 3 | I (10) 3 | IHC | 67 |
| II (23.3) | RT-PCR on RNA | 67 | ||
| III (50) | ISH | 67 | ||
| IV (16.7) | ||||
| Minegishi (2000) [27] | 2 | Ia (16.6) 3 | ||
| 2c (5.6) | ||||
| IIIa (11.1) | RT-PCR on RNA | 50 | ||
| IIIb (5.6) | immunoblot | 0 | ||
| IIIc (38.9) | ||||
| IV (22.2) | ||||
| Perales (2017) [14] | 12 | NA | IHC | 33 |
| immunoblot | 33 | |||
| Germ cell tumors | ||||
| Nakano (1989) [28] | 4 | NA | 125IFSH-binding radioreceptor assay | No 2 |
| Stouffer (1984) [30] | 1 | NA | 125IFSH-binding radioreceptor assay | No 2 |
| Brenner tumors | ||||
| Nakano (1989) [28] | 2 | NA | 125IFSH-binding radioreceptor assay | No 2 |
| Carcinosarcoma NOS | ||||
| Perales (2017) [14] | 4 | NA | Western blotting | 0 |
| Carcinoma, undifferentiated, NOS | ||||
| Stouffer (1984) [30] | 3 | NA | 125IFSH-binding radioreceptor assay | No 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakker, M.-C.E.; Brink, G.J.; Poot, A.J.; Braat, A.J.A.T.; Jonges, G.N.; Zweemer, R.P. Follicle-Stimulating Hormone Receptor Expression and Its Potential Application for Theranostics in Subtypes of Ovarian Tumors: A Systematic Review. Cancers 2024, 16, 1140. https://doi.org/10.3390/cancers16061140
Bakker M-CE, Brink GJ, Poot AJ, Braat AJAT, Jonges GN, Zweemer RP. Follicle-Stimulating Hormone Receptor Expression and Its Potential Application for Theranostics in Subtypes of Ovarian Tumors: A Systematic Review. Cancers. 2024; 16(6):1140. https://doi.org/10.3390/cancers16061140
Chicago/Turabian StyleBakker, Marie-Christine E., Geertruid J. Brink, Alex J. Poot, Arthur J. A. T. Braat, Geertruida N. Jonges, and Ronald P. Zweemer. 2024. "Follicle-Stimulating Hormone Receptor Expression and Its Potential Application for Theranostics in Subtypes of Ovarian Tumors: A Systematic Review" Cancers 16, no. 6: 1140. https://doi.org/10.3390/cancers16061140
APA StyleBakker, M.-C. E., Brink, G. J., Poot, A. J., Braat, A. J. A. T., Jonges, G. N., & Zweemer, R. P. (2024). Follicle-Stimulating Hormone Receptor Expression and Its Potential Application for Theranostics in Subtypes of Ovarian Tumors: A Systematic Review. Cancers, 16(6), 1140. https://doi.org/10.3390/cancers16061140






