Focus on RAS Codon 61 Mutations in Metastatic Colorectal Cancer: A Retrospective Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
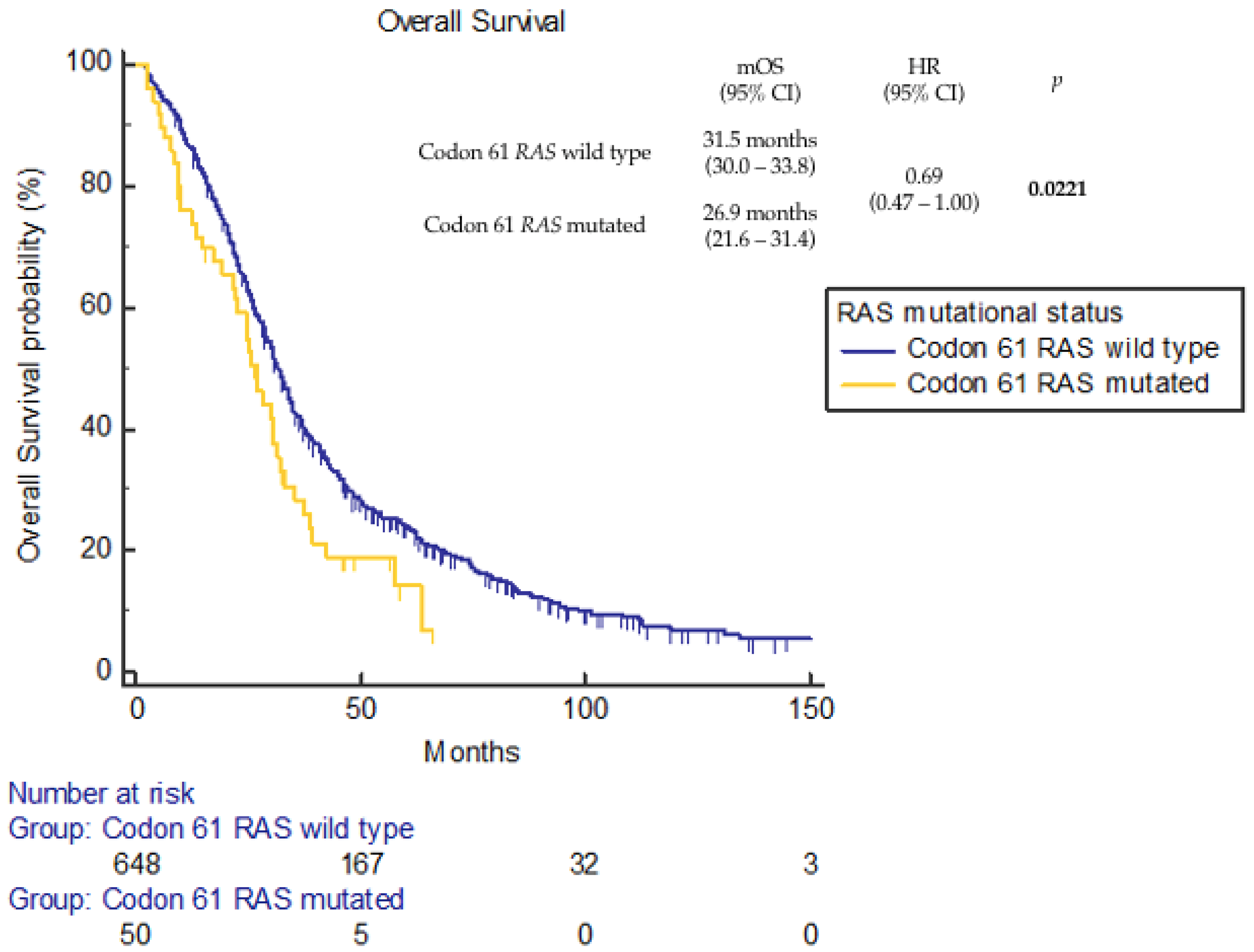
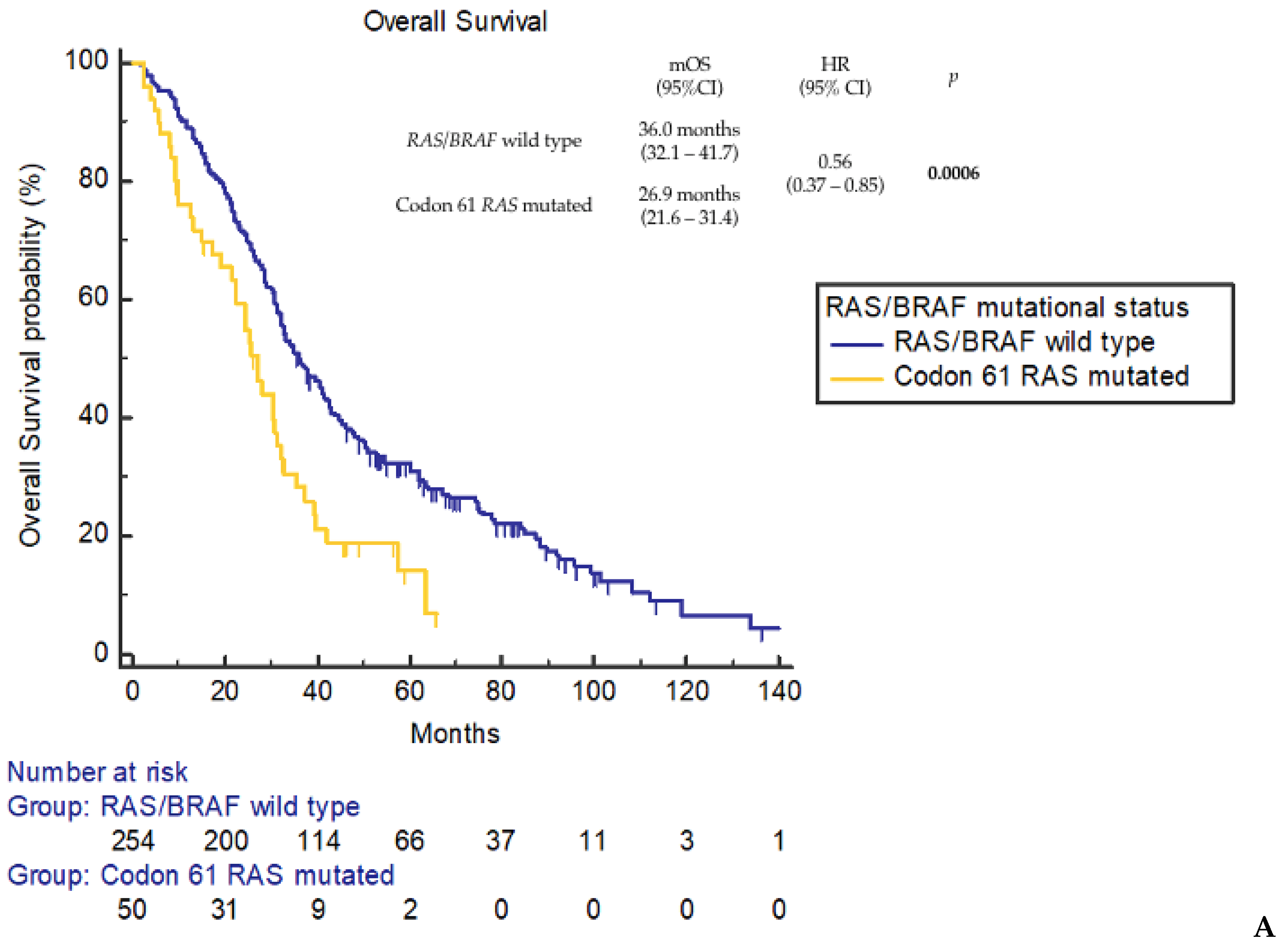
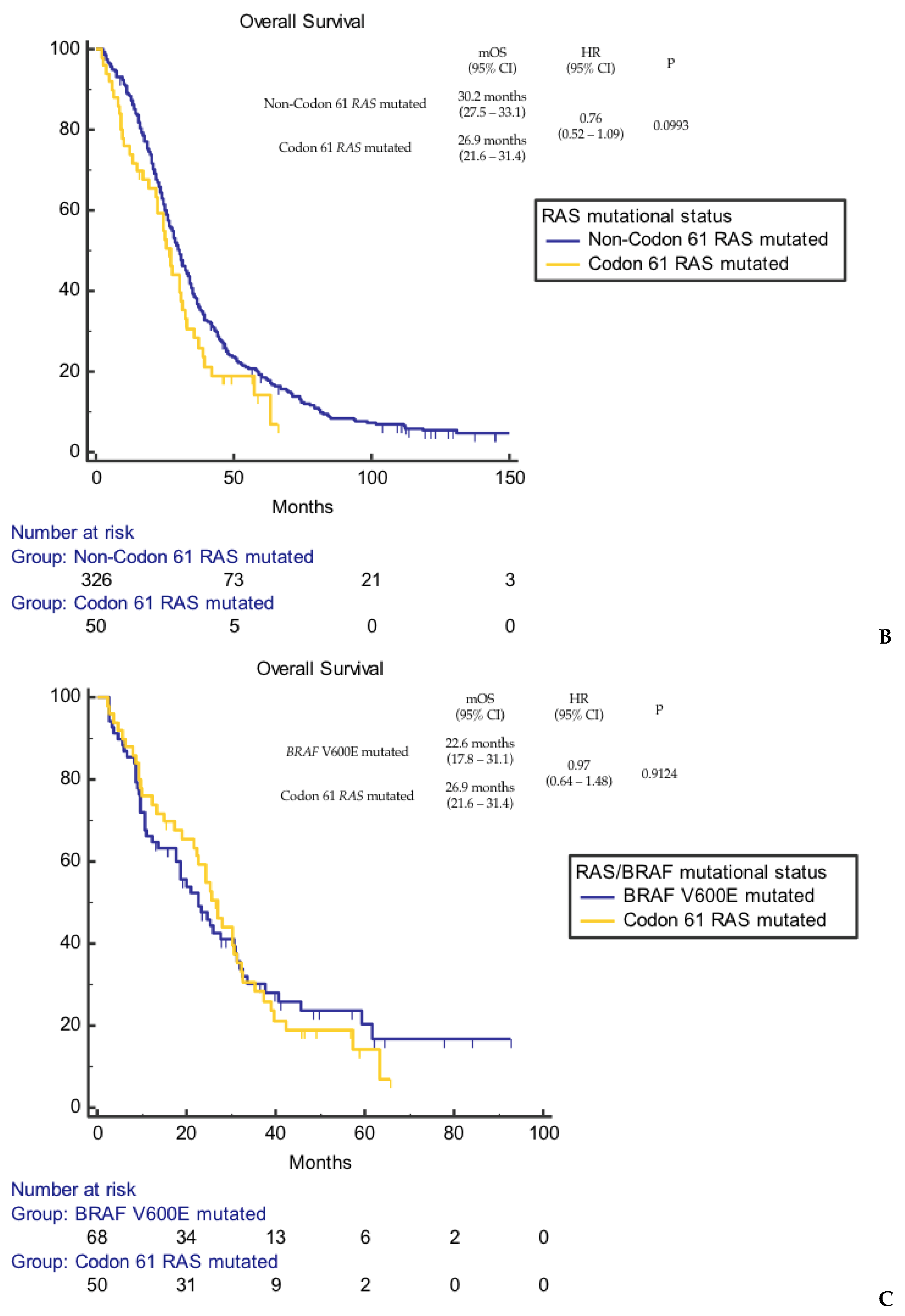
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- De Roock, W.; De Vriendt, V.; Normanno, N.; Ciardiello, F.; Tejpar, S. KRAS, BRAF, PIK3CA, and PTEN mutations: Implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011, 12, 594–603. [Google Scholar] [CrossRef]
- Rosty, C.; Young, J.P.; Walsh, M.D.; Clendenning, M.; Walters, R.J.; Pearson, S.; Pavluk, E.; Nagler, B.; Pakenas, D.; Jass, J.R.; et al. Colorectal carcinomas with KRAS mutation are associated with distinctive morphological and molecular features. Mod. Pathol. 2013, 26, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Karapetis, C.S.; Khambata-Ford, S.; Jonker, D.J.; O’Callaghan, C.J.; Tu, D.; Tebbutt, N.C.; Simes, R.J.; Chalchal, H.; Shapiro, J.D.; Robitaille, S.; et al. K-rasMutations and Benefit from Cetuximab in Advanced Colorectal Cancer. N. Engl. J. Med. 2008, 359, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Amado, R.G.; Wolf, M.; Peeters, M.; Van Cutsem, E.; Siena, S.; Freeman, D.J.; Juan, T.; Sikorski, R.; Suggs, S.; Radinsky, R.; et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- De Roock, W.; Claes, B.; Bernasconi, D.; De Schutter, J.; Biesmans, B.; Fountzilas, G.; Kalogeras, K.T.; Kotoula, V.; Papamichael, D.; Laurent-Puig, P.; et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010, 11, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, A.; Siena, S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J. Clin. Oncol. 2010, 28, 1254–1261. [Google Scholar] [CrossRef]
- Tran, N.H.; Cavalcante, L.L.; Lubner, S.J.; Mulkerin, D.L.; LoConte, N.K.; Clipson, L.; Matkowskyj, K.A.; Deming, D.A. Precision medicine in colorectal cancer: The molecular profile alters treatment strategies. Ther. Adv. Med. Oncol. 2015, 7, 252–262. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Lenz, H.-J.; Köhne, C.-H.; Heinemann, V.; Tejpar, S.; Melezínek, I.; Beier, F.; Stroh, C.; Rougier, P.; van Krieken, J.H.; et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J. Clin. Oncol. 2015, 33, 692–700. [Google Scholar] [CrossRef]
- Allegra, C.J.; Jessup, J.M.; Somerfield, M.R.; Hamilton, S.R.; Hammond, E.H.; Hayes, D.F.; McAllister, P.K.; Morton, R.F.; Schilsky, R.L. American society of clinical oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti–epidermal growth factor receptor monoclonal antibody therapy. J. Clin. Oncol. 2009, 27, 2091–2096. [Google Scholar] [CrossRef]
- Tejpar, S.; Celik, I.; Schlichting, M.; Sartorius, U.; Bokemeyer, C.; Van Cutsem, E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J. Clin. Oncol. 2012, 30, 3570–3577. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Köhne, C.-H.; Ciardiello, F.; Lenz, H.-J.; Heinemann, V.; Klinkhardt, U.; Beier, F.; Duecker, K.; van Krieken, J.; Tejpar, S. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur. J. Cancer 2015, 51, 1243–1252. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014, 25, 1346–1355. [Google Scholar] [CrossRef]
- Buhrman, G.; Wink, G.; Mattos, C. Transformation Efficiency of RasQ61 Mutants Linked to Structural Features of the Switch Regions in the Presence of Raf. Structure 2007, 15, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Loupakis, F.; Ruzzo, A.; Cremolini, C.; Vincenzi, B.; Salvatore, L.; Santini, D.; Masi, G.; Stasi, I.; Canestrari, E.; Rulli, E.; et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br. J. Cancer 2009, 101, 715–721. [Google Scholar] [CrossRef]
- Douillard, J.-Y.; Oliner, K.S.; Siena, S.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N. Engl. J. Med. 2013, 369, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Pietrantonio, F.; Lonardi, S.; Mussolin, B.; Rua, F.; Crisafulli, G.; Bartolini, A.; Fenocchio, E.; Amatu, A.; Manca, P.; et al. Circulating tumor DNA to guide rechallenge with panitumumab in metastatic colorectal cancer: The phase 2 CHRONOS trial. Nat. Med. 2022, 28, 1612–1618. [Google Scholar] [CrossRef] [PubMed]
- Misale, S.; Yaeger, R.; Hobor, S.; Scala, E.; Janakiraman, M.; Liska, D.; Valtorta, E.; Schiavo, R.; Buscarino, M.; Siravegna, G.; et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 2012, 486, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Sartore-Bianchi, A.; Garcia-Carbonero, R.; Karthaus, M.; Smith, D.; Tabernero, J.; Van Cutsem, E.; Guan, X.; Boedigheimer, M.; Ang, A.; et al. Dynamic molecular analysis and clinical correlates of tumor evolution within a phase II trial of panitumumab-based therapy in metastatic colorectal cancer. Ann. Oncol. 2018, 29, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kaltenbrun, E.; Anderson, G.R.; Stephens, S.J.; Arena, S.; Bardelli, A.; Counter, C.M.; Wood, K.C. Codon bias imposes a targetable limitation on KRAS-driven therapeutic resistance. Nat. Commun. 2017, 8, 15617. [Google Scholar] [CrossRef]
- Lavacchi, D.; Fancelli, S.; Roviello, G.; Castiglione, F.; Caliman, E.; Rossi, G.; Venturini, J.; Pellegrini, E.; Brugia, M.; Vannini, A.; et al. Mutations matter: An observational study of the prognostic and predictive value of KRAS mutations in metastatic colorectal cancer. Front. Oncol. 2022, 12, 1055019. [Google Scholar] [CrossRef]
- Zeng, J.; Fan, W.; Li, J.; Wu, G.; Wu, H. KRAS/NRAS Mutations Associated with Distant Metastasis and BRAF/PIK3CA Mutations Associated with Poor Tumor Differentiation in Colorectal Cancer. Int. J. Gen. Med. 2023, 16, 4109–4120. [Google Scholar] [CrossRef]
- Serebriiskii, I.G.; Connelly, C.; Frampton, G.; Newberg, J.; Cooke, M.; Miller, V.; Ali, S.; Ross, J.S.; Handorf, E.; Arora, S.; et al. Comprehensive characterization of RAS mutations in colon and rectal cancers in old and young patients. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Lochhead, P.; Yamauchi, M.; Kuchiba, A.; Qian, Z.R.; Liao, X.; Nishihara, R.; Jung, S.; Wu, K.; Nosho, K.; et al. Analyses of clinicopathological, molecular, and prognostic associations of KRAS codon 61 and codon 146 mutations in colorectal cancer: Cohort study and literature review. Mol. Cancer 2014, 13, 135. [Google Scholar] [CrossRef]
- Morris, V.K.; Lucas, F.A.S.; Overman, M.J.; Eng, C.; Morelli, M.P.; Jiang, Z.-Q.; Luthra, R.; Meric-Bernstam, F.; Maru, D.; Scheet, P.; et al. Clinicopathologic characteristics and gene expression analyses of non-KRAS 12/13, RAS-mutated metastatic colorectal cancer. Ann. Oncol. 2014, 25, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.; Seymour, M.; Magill, L.; Handley, K.; Glasbey, J.; Glimelius, B.; Palmer, A.; Seligmann, J.; Laurberg, S.; Murakami, K.; et al. Preoperative Chemotherapy for Operable Colon Cancer: Mature Results of an International Randomized Controlled Trial. J. Clin. Oncol. 2023, 41, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef]
- Goéré, D.; Glehen, O.; Quenet, F.; Guilloit, J.-M.; Bereder, J.-M.; Lorimier, G.; Thibaudeau, E.; Ghouti, L.; Pinto, A.; Tuech, J.-J.; et al. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP–PRODIGE 15): A randomised, phase 3 study. Lancet Oncol. 2020, 21, 1147–1154. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Lenos, K.J.; Bach, S.; Moreno, L.F.; Hoorn, S.T.; Sluiter, N.R.; Bootsma, S.; Braga, F.A.V.; Nijman, L.E.; Bosch, T.v.D.; Miedema, D.M.; et al. Molecular characterization of colorectal cancer related peritoneal metastatic disease. Nat. Commun. 2022, 13, 4443. [Google Scholar] [CrossRef]
- Garland-Kledzik, M.; Uppal, A.; Naeini, Y.B.; Stern, S.; Erali, R.; Scholer, A.J.; Khader, A.M.; Santamaria-Barria, J.A.; Cummins-Perry, K.; Zhou, Y.; et al. Prognostic Impact and Utility of Immunoprofiling in the Selection of Patients with Colorectal Peritoneal Carcinomatosis for Cytoreductive Surgery (CRS) and Heated Intraperitoneal Chemotherapy (HIPEC). J. Gastrointest. Surg. 2021, 25, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Witkiewicz, A.K.; McMillan, E.A.; Balaji, U.; Baek, G.; Lin, W.-C.; Mansour, J.; Mollaee, M.; Wagner, K.-U.; Koduru, P.; Yopp, A.; et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015, 6, 6744. [Google Scholar] [CrossRef]
- Nusrat, M.; Yaeger, R. KRAS inhibition in metastatic colorectal cancer: An update. Curr. Opin. Pharmacol. 2023, 68, 102343. [Google Scholar] [CrossRef] [PubMed]
- Vangala, D.; Ladigan, S.; Liffers, S.T.; Noseir, S.; Maghnouj, A.; Götze, T.-M.; Verdoodt, B.; Klein-Scory, S.; Godfrey, L.; Zowada, M.K.; et al. Secondary resistance to anti-EGFR therapy by transcriptional reprogramming in patient-derived colorectal cancer models. Genome Med. 2021, 13, 116. [Google Scholar] [CrossRef]
- Parseghian, C.M.; Sun, R.; Woods, M.; Napolitano, S.; Lee, H.M.; Alshenaifi, J.; Willis, J.; Nunez, S.; Raghav, K.P.; Morris, V.K.; et al. Resistance Mechanisms to Anti–Epidermal Growth Factor Receptor Therapy in RAS/RAF Wild-Type Colorectal Cancer Vary by Regimen and Line of Therapy. J. Clin. Oncol. 2023, 41, 460–471. [Google Scholar] [CrossRef]
- Cremolini, C.; Rossini, D.; Dell’aquila, E.; Lonardi, S.; Conca, E.; Del Re, M.; Busico, A.; Pietrantonio, F.; Danesi, R.; Aprile, G.; et al. Rechallenge for Patients with RAS and BRAF Wild-Type Metastatic Colorectal Cancer with Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol. 2019, 5, 343–350. [Google Scholar] [CrossRef]
- Hobbs, G.A.; Der, C.J.; Rossman, K.L. RAS isoforms and mutations in cancer at a glance. J. Cell Sci. 2016, 129, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Sacher, A.; LoRusso, P.; Patel, M.R.; Miller, W.H.; Garralda, E.; Forster, M.D.; Santoro, A.; Falcon, A.; Kim, T.W.; Paz-Ares, L.; et al. Single-Agent Divarasib (GDC-6036) in Solid Tumors with a KRAS G12C Mutation. N. Engl. J. Med. 2023, 389, 710–721. [Google Scholar] [CrossRef]
- Yaeger, R.; Weiss, J.; Pelster, M.S.; Spira, A.I.; Barve, M.; Ou, S.-H.I.; Leal, T.A.; Bekaii-Saab, T.S.; Paweletz, C.P.; Heavey, G.A.; et al. Adagrasib with or without Cetuximab in Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 388, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Kopetz, S.; Kuboki, Y.; Kim, T.W.; Munster, P.N.; Krauss, J.C.; Falchook, G.S.; Han, S.-W.; Heinemann, V.; Muro, K.; et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK100): A prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 2022, 23, 115–124. [Google Scholar] [CrossRef]
- Kuboki, Y.; Yaeger, R.; Fakih, M.; Strickler, J.; Masuishi, T.; Kim, E.; Bestvina, C.; Langer, C.; Krauss, J.; Puri, S.; et al. 315O Sotorasib in combination with panitumumab in refractory KRAS G12C-mutated colorectal cancer: Safety and efficacy for phase Ib full expansion cohort. Ann. Oncol. 2022, 33, S680–S681. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.G.; Salvatore, L.; Esaki, T.; Modest, D.P.; Lopez-Bravo, D.P.; Taieb, J.; Karamouzis, M.V.; Ruiz-Garcia, E.; Kim, T.-W.; Kuboki, Y.; et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N. Engl. J. Med. 2023, 389, 2125–2139. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | N = 50 (%) | KRAS (n = 28) | NRAS (n = 22) | |
|---|---|---|---|---|
| Age (at metastatic diagnosis), median (range) | 65 yrs (34–86 yrs) | 65 yrs (41–86 yrs) | 63 yrs (34–84 yrs) | |
| ECOG PS | 0 | 25 (50%) | 15 (53%) | 10 (45%) |
| 1 | 19 (38%) | 8 (28%) | 11 (50%) | |
| 2 | 6 (12%) | 5 (19%) | 1 (5%) | |
| Sex | Male | 19 (38%) | 10 (36%) | 9 (41%) |
| Female | 31 (62%) | 18 (64%) | 13 (59%) | |
| Previous surgery | Y | 40 (80%) | 22 (79%) | 18 (82%) |
| N | 10 (20%) | 6 (21%) | 4 (18%) | |
| Metastatic at diagnosis | Y | 33 (66%) | 19 (68%) | 14 (64%) |
| N | 17 (34%) | 9 (32%) | 8 (36%) | |
| Primary tumor location | Right | 14 (28%) | 8 (29%) | 6 (27%) |
| Left | 36 (72%) | 20 (71%) | 16 (73%) | |
| Sites of metastatic disease at diagnosis | Liver | 24 (48%) | 12 (43%) | 12 (54%) |
| Lung | 11 (22%) | 7 (25%) | 4 (18%) | |
| Nodes | 15 (30%) | 8 (28%) | 7 (32%) | |
| Peritoneum/Ovary | 16 (32%) | 7 (25%) | 9 (41%) | |
| Other | 5 (10%) | 3 (10%) | 2 (9%) | |
| Peritoneal and/or ovarian metastasis | Y | 27 (54%) | 13 (46%) | 14 (64%) |
| N | 23 (46%) | 15 (54%) | 8 (36%) | |
| First line chemotherapy regimen | FOLFOXIRI +/− bevacizumab | 3 (6%) | 0 | 3 (14%) |
| FOLFOX +/− bevacizumab | 29 (58%) | 20 (71%) | 9 (41%) | |
| FOLFIRI +/− bevacizumab | 9 (18%) | 3 (11%) | 6 (27%) | |
| Other | 9 (18%) | 5 (18%) | 4 (18%) | |
| Total number of treatment lines | 1 | 20 (40%) | 15 (53%) | 5 (23%) |
| 2 | 10 (20%) | 5 (18%) | 5 (23%) | |
| 3 | 13 (26%) | 6 (21%) | 7 (32%) | |
| 4 | 5 (10%) | 1 (4%) | 4 (18%) | |
| 5 | 2 (4%) | 1 (4%) | 1 (4%) | |
| RAS mutation | KRAS | 28 (56%) | ||
| Q61X | 15 (30%) | |||
| Q61H | 6 (12%) | |||
| Q61L | 3 (6%) | |||
| Q61R | 2 (4%) | |||
| G61X | 2 (4%) | |||
| NRAS | 22 (44%) | |||
| Q61R | 8 (16%) | |||
| Q61K | 8 (16%) | |||
| Q61L | 5 (10%) | |||
| G61H | 1 (2%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schietroma, F.; Anghelone, A.; Valente, G.; Beccia, V.; Caira, G.; Spring, A.; Trovato, G.; Di Bello, A.; Ceccarelli, A.; Chiofalo, L.; et al. Focus on RAS Codon 61 Mutations in Metastatic Colorectal Cancer: A Retrospective Analysis. Cancers 2024, 16, 988. https://doi.org/10.3390/cancers16050988
Schietroma F, Anghelone A, Valente G, Beccia V, Caira G, Spring A, Trovato G, Di Bello A, Ceccarelli A, Chiofalo L, et al. Focus on RAS Codon 61 Mutations in Metastatic Colorectal Cancer: A Retrospective Analysis. Cancers. 2024; 16(5):988. https://doi.org/10.3390/cancers16050988
Chicago/Turabian StyleSchietroma, Francesco, Annunziato Anghelone, Giustina Valente, Viria Beccia, Giulia Caira, Alexia Spring, Giovanni Trovato, Armando Di Bello, Anna Ceccarelli, Laura Chiofalo, and et al. 2024. "Focus on RAS Codon 61 Mutations in Metastatic Colorectal Cancer: A Retrospective Analysis" Cancers 16, no. 5: 988. https://doi.org/10.3390/cancers16050988
APA StyleSchietroma, F., Anghelone, A., Valente, G., Beccia, V., Caira, G., Spring, A., Trovato, G., Di Bello, A., Ceccarelli, A., Chiofalo, L., Perazzo, S., Bensi, M., Minucci, A., Urbani, A., Larocca, L. M., Basso, M., Pozzo, C., Salvatore, L., Calegari, M. A., & Tortora, G. (2024). Focus on RAS Codon 61 Mutations in Metastatic Colorectal Cancer: A Retrospective Analysis. Cancers, 16(5), 988. https://doi.org/10.3390/cancers16050988










