Sex Differences in Cancer Incidence Rates by Race and Ethnicity: Results from the Surveillance, Epidemiology, and End Results (SEER) Registry (2000–2019)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
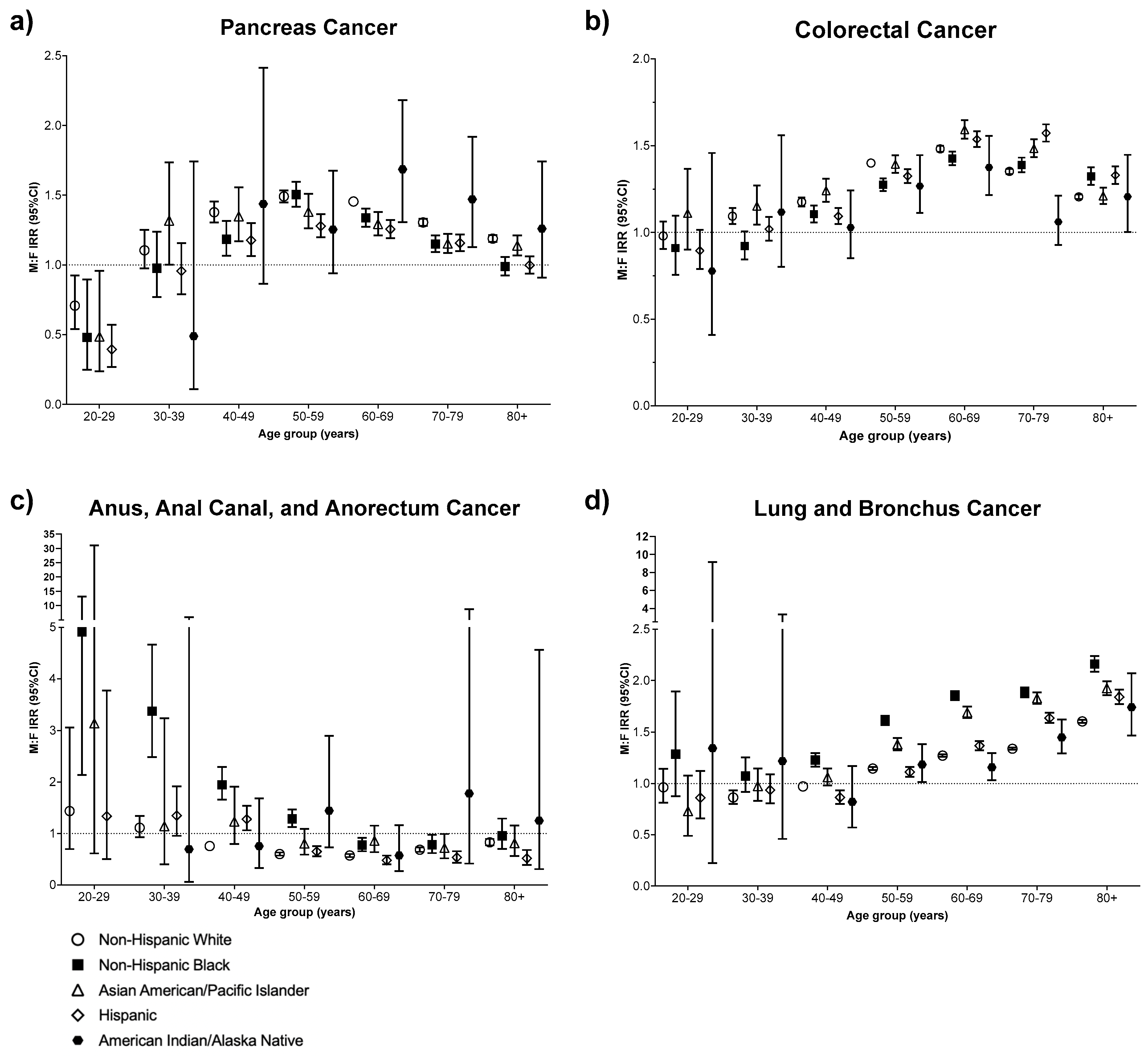
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, M.B.; Dawsey, S.M.; Freedman, N.D.; Inskip, P.D.; Wichner, S.M.; Quraishi, S.M.; Devesa, S.S.; McGlynn, K.A. Sex disparities in cancer incidence by period and age. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1174–1182. [Google Scholar] [CrossRef]
- Cook, M.B.; McGlynn, K.A.; Devesa, S.S.; Freedman, N.D.; Anderson, W.F. Sex disparities in cancer mortality and survival. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1629–1637. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Radkiewicz, C.; Johansson, A.L.V.; Dickman, P.W.; Lambe, M.; Edgren, G. Sex differences in cancer risk and survival: A Swedish cohort study. Eur. J. Cancer 2017, 84, 130–140. [Google Scholar] [CrossRef]
- Edgren, G.; Liang, L.; Adami, H.O.; Chang, E.T. Enigmatic sex disparities in cancer incidence. Eur. J. Epidemiol. 2012, 27, 187–196. [Google Scholar] [CrossRef]
- Jackson, S.S.; Marks, M.A.; Katki, H.A.; Cook, M.B.; Hyun, N.; Freedman, N.D.; Kahle, L.L.; Castle, P.E.; Graubard, B.I.; Chaturvedi, A.K. Sex disparities in the incidence of 21 cancer types: Quantification of the contribution of risk factors. Cancer 2022, 128, 3531–3540. [Google Scholar] [CrossRef]
- Thomson, B.; Emberson, J.; Lacey, B.; Lewington, S.; Peto, R.; Jemal, A.; Islami, F. Association Between Smoking, Smoking Cessation, and Mortality by Race, Ethnicity, and Sex Among US Adults. JAMA Netw. Open 2022, 5, e2231480. [Google Scholar] [CrossRef] [PubMed]
- Stierman, B.; Afful, J.; Carroll, M.D.; Chen, T.C.; Davy, O.; Fink, S.; Fryar, C.D.; Gu, Q.; Hales, C.M.; Hughes, J.P.; et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes; CDC: Atlanta, GA, USA, 2021.
- Surveillance Epidemiology and End Results (SEER) Program. Number of Persons by Race and Hispanic Ethnicity for SEER Participants (2020 Census Data). Available online: https://seer.cancer.gov/registries/data.html (accessed on 19 February 2024).
- Surveillance Epidemiology and End Results (SEER) Program. SEERStat Software; Version 8.4.0.; Surveillance Epidemiology and End Results (SEER) Program: Bethesda, MD, USA, 2022. Available online: https://seer.cancer.gov/seerstat/ (accessed on 21 August 2023).
- Cushing, L.; Faust, J.; August, L.M.; Cendak, R.; Wieland, W.; Alexeeff, G. Racial/Ethnic Disparities in Cumulative Environmental Health Impacts in California: Evidence From a Statewide Environmental Justice Screening Tool (CalEnviroScreen 1.1). Am. J. Public Health 2015, 105, 2341–2348. [Google Scholar] [CrossRef] [PubMed]
- Jbaily, A.; Zhou, X.; Liu, J.; Lee, T.-H.; Kamareddine, L.; Verguet, S.; Dominici, F. Air pollution exposure disparities across US population and income groups. Nature 2022, 601, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Goding Sauer, A.; Miller, K.D.; Siegel, R.L.; Fedewa, S.A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Ma, J.; Soerjomataram, I.; et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 2018, 68, 31–54. [Google Scholar] [CrossRef]
- Goding Sauer, A.; Siegel, R.L.; Jemal, A.; Fedewa, S.A. Current Prevalence of Major Cancer Risk Factors and Screening Test Use in the United States: Disparities by Education and Race/Ethnicity. Cancer Epidemiol. Biomark. Prev. 2019, 28, 629–642. [Google Scholar] [CrossRef]
- Fu, B.C.; Song, M.; Li, X.; Han, J.; Adami, H.O.; Giovannucci, E.L.; Mucci, L.A. Height as a mediator of sex differences in cancer risk. Ann. Oncol. 2020, 31, 634–640. [Google Scholar] [CrossRef]
- Walter, R.B.; Brasky, T.M.; Buckley, S.A.; Potter, J.D.; White, E. Height as an explanatory factor for sex differences in human cancer. J. Natl. Cancer Inst. 2013, 105, 860–868. [Google Scholar] [CrossRef]
- Zhang, E.R.; Pfeiffer, R.M.; Austin, A.; Clarke, M.A.; Hayes, J.; Horner, M.J.; Monterosso, A.; Pawlish, K.S.; Engels, E.A.; Shiels, M.S. Impact of HIV on Anal Squamous Cell Carcinoma Rates in the United States, 2001-2015. J. Natl. Cancer Inst. 2022, 114, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Colón-López, V.; Shiels, M.S.; Machin, M.; Ortiz, A.P.; Strickler, H.; Castle, P.E.; Pfeiffer, R.M.; Engels, E.A. Anal Cancer Risk Among People With HIV Infection in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 68–75. [Google Scholar] [CrossRef]
- Sasco, A.J.; Secretan, M.B.; Straif, K. Tobacco smoking and cancer: A brief review of recent epidemiological evidence. Lung Cancer 2004, 45 (Suppl. 2), S3–S9. [Google Scholar] [CrossRef]
- Jemal, A.; Miller, K.D.; Ma, J.; Siegel, R.L.; Fedewa, S.A.; Islami, F.; Devesa, S.S.; Thun, M.J. Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. N. Engl. J. Med. 2018, 378, 1999–2009. [Google Scholar] [CrossRef]
- Woo, J.; Lawrence, E.; Mollborn, S. Racial/ethnic and gender differences in smoking in early middle adulthood. SSM Popul. Health 2022, 18, 101119. [Google Scholar] [CrossRef]
- Jamal, A.; Phillips, E.; Gentzke, A.S.; Homa, D.M.; Babb, S.D.; King, B.A.; Neff, L.J. Current Cigarette Smoking Among Adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 53–59. [Google Scholar] [CrossRef]
- Haupt, S.; Caramia, F.; Klein, S.L.; Rubin, J.B.; Haupt, Y. Sex disparities matter in cancer development and therapy. Nat. Rev. Cancer 2021, 21, 393–407. [Google Scholar] [CrossRef]
- Dunford, A.; Weinstock, D.M.; Savova, V.; Schumacher, S.E.; Cleary, J.P.; Yoda, A.; Sullivan, T.J.; Hess, J.M.; Gimelbrant, A.A.; Beroukhim, R.; et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat. Genet. 2017, 49, 10–16. [Google Scholar] [CrossRef]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Rubin, J.B.; Lagas, J.S.; Broestl, L.; Sponagel, J.; Rockwell, N.; Rhee, G.; Rosen, S.F.; Chen, S.; Klein, R.S.; Imoukhuede, P.; et al. Sex differences in cancer mechanisms. Biol. Sex Differ. 2020, 11, 17. [Google Scholar] [CrossRef]
- Jackson, S.S.; Adami, H.O.; Andreotti, G.; Beane-Freeman, L.E.; de Gonzalez, A.B.; Buring, J.E.; Fraser, G.E.; Freedman, N.D.; Gapstur, S.M.; Gierach, G.; et al. Associations between reproductive factors and biliary tract cancers in women from the Biliary Tract Cancers Pooling Project. J. Hepatol. 2020, 73, 863–872. [Google Scholar] [CrossRef]
- Gabbi, C.; Kim, H.J.; Barros, R.; Korach-Andre, M.; Warner, M.; Gustafsson, J.A. Estrogen-dependent gallbladder carcinogenesis in LXRbeta-/- female mice. Proc. Natl. Acad. Sci. USA 2010, 107, 14763–14768. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, G.G.; Vlantis, A.C.; van Hasselt, C.A. Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway. Cell. Prolif. 2007, 40, 921–935. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of Thyroid Cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results Program. Registry Groupings in SEER Data and Statistics. Available online: https://seer.cancer.gov/registries/terms.html (accessed on 21 October 2022).
| Non-Hispanic White | Non-Hispanic Black | Non-Hispanic Asian Pacific Islander | Latino | Non-Hispanic American Indian/Alaskan Native | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MF IRR | 95% CI | MF IRR | 95% CI | MF IRR | 95% CI | MF IRR | 95% CI | MF IRR | 95% CI | |
| Lip | 3.45 | (3.30, 3.61) | 2.62 | (1.82, 3.80) | 1.43 | (1.05, 1.96) | 2.90 | (2.46, 3.44) | 3.03 | (1.36, 7.28) |
| Tongue | 2.80 | (2.74, 2.86) | 3.19 | (2.96, 3.44) | 1.74 | (1.62, 1.87) | 2.15 | (2.01, 2.31) | 2.78 | (2.00, 3.89) |
| Salivary Gland | 1.93 | (1.86, 1.99) | 1.17 | (1.06, 1.29) | 1.19 | (1.08, 1.31) | 1.31 | (1.19, 1.44) | 1.17 | (0.75, 1.80) |
| Floor of Mouth | 2.41 | (2.29, 2.54) | 3.71 | (3.19, 4.34) | 2.06 | (1.64, 2.60) | 2.69 | (2.24, 3.24) | 2.30 | (1.29, 4.27) |
| Gum and Other Mouth | 1.41 | (1.37, 1.45) | 1.59 | (1.46, 1.73) | 1.78 | (1.62, 1.95) | 1.49 | (1.36, 1.63) | 1.59 | (1.07, 2.39) |
| Nasopharynx | 2.59 | (2.42, 2.77) | 2.96 | (2.59, 3.39) | 2.78 | (2.59, 2.97) | 2.66 | (2.28, 3.10) | 2.15 | (1.47, 3.18) |
| Tonsil | 5.03 | (4.87, 5.20) | 5.12 | (4.65, 5.64) | 4.30 | (3.69, 5.03) | 4.77 | (4.30, 5.30) | 5.50 | (3.50, 9.00) |
| Oropharynx | 3.84 | (3.61, 4.10) | 4.00 | (3.45, 4.66) | 3.14 | (2.32, 4.28) | 4.64 | (3.76, 5.76) | 4.22 | (1.85, 10.97) |
| Hypopharynx | 4.03 | (3.81, 4.26) | 5.88 | (5.14, 6.74) | 6.17 | (4.97, 7.71) | 8.18 | (6.68, 10.1) | 5.56 | (2.98, 11.03) |
| Other Oral Cavity and Pharynx | 3.25 | (2.99, 3.53) | 3.55 | (2.85, 4.44) | 3.53 | (2.30, 5.53) | 3.58 | (2.65, 4.90) | 2.26 | (0.81, 7.60) |
| Esophagus | 4.43 | (4.34, 4.53) | 3.11 | (2.96, 3.28) | 3.60 | (3.34, 3.89) | 4.57 | (4.27, 4.89) | 3.16 | (2.50, 4.03) |
| Stomach | 2.19 | (2.16, 2.23) | 1.84 | (1.78, 1.90) | 1.71 | (1.66, 1.76) | 1.60 | (1.55, 1.64) | 1.87 | (1.62, 2.15) |
| Small Intestine | 1.38 | (1.35, 1.42) | 1.32 | (1.25, 1.39) | 1.58 | (1.44, 1.74) | 1.28 | (1.19, 1.37) | 1.44 | (0.99, 2.11) |
| Colon and Rectum | 1.32 | (1.31, 1.33) | 1.32 | (1.30, 1.34) | 1.39 | (1.37, 1.41) | 1.40 | (1.38, 1.42) | 1.18 | (1.10, 1.26) |
| Anus, Anal Canal, and Anorectum | 0.67 | (0.66, 0.69) | 1.24 | (1.15, 1.33) | 0.85 | (0.74, 0.98) | 0.63 | (0.58, 0.69) | 0.98 | (0.67, 1.42) |
| Liver | 3.47 | (3.41, 3.54) | 3.75 | (3.61, 3.90) | 2.94 | (2.85, 3.04) | 2.84 | (2.76, 2.93) | 2.53 | (2.23, 2.88) |
| Gallbladder | 0.62 | (0.60, 0.65) | 0.66 | (0.60, 0.73) | 0.71 | (0.64, 0.78) | 0.46 | (0.43, 0.50) | 0.64 | (0.47, 0.86) |
| Other Biliary | 1.57 | (1.52, 1.62) | 1.36 | (1.25, 1.48) | 1.54 | (1.44, 1.64) | 1.34 | (1.26, 1.42) | 1.14 | (0.84, 1.54) |
| Pancreas | 1.33 | (1.31, 1.34) | 1.19 | (1.16, 1.23) | 1.21 | (1.17, 1.25) | 1.14 | (1.11, 1.17) | 1.40 | (1.22, 1.61) |
| Retroperitoneum | 1.27 | (1.19, 1.35) | 0.78 | (0.65, 0.93) | 0.98 | (0.82, 1.17) | 1.14 | (0.99, 1.32) | 0.54 | (0.22, 1.27) |
| Peritoneum, Omentum, and Mesentery | 0.08 | (0.07, 0.09) | 0.21 | (0.16, 0.27) | 0.06 | (0.04, 0.09) | 0.12 | (0.09, 0.15) | 0.11 | (0.02, 0.41) |
| Other Digestive Organs | 1.35 | (1.28, 1.41) | 1.29 | (1.14, 1.46) | 1.36 | (1.18, 1.55) | 1.17 | (1.05, 1.31) | 1.25 | (0.81, 1.93) |
| Nose, Nasal Cavity, and Middle Ear | 1.77 | (1.69, 1.85) | 1.93 | (1.69, 2.21) | 1.81 | (1.58, 2.07) | 1.63 | (1.45, 1.83) | 1.37 | (0.77, 2.48) |
| Larynx | 4.38 | (4.27, 4.49) | 5.64 | (5.32, 5.99) | 8.68 | (7.54, 10.03) | 7.69 | (7.05, 8.39) | 4.38 | (3.13, 6.23) |
| Lung and Bronchus | 1.32 | (1.32, 1.33) | 1.83 | (1.81, 1.86) | 1.71 | (1.68, 1.74) | 1.53 | (1.51, 1.56) | 1.37 | (1.28, 1.47) |
| Pleura | 2.23 | (1.79, 2.79) | 0.91 | (0.37, 2.07) | 0.76 | (0.27, 2.01) | 5.23 | (2.63, 11.55) | 4.38 | (0.28, 230.79) |
| Trachea, Mediastinum, and Other Respiratory Organs | 2.62 | (2.35, 2.93) | 2.29 | (1.71, 3.08) | 2.57 | (1.92, 3.46) | 2.63 | (2.06, 3.39) | ~ | ~ |
| Bones and Joints | 1.36 | (1.30, 1.43) | 1.28 | (1.12, 1.46) | 1.30 | (1.13, 1.51) | 1.29 | (1.16, 1.42) | 1.34 | (0.77, 2.29) |
| Soft Tissue Including Heart | 1.56 | (1.53, 1.60) | 1.21 | (1.15, 1.28) | 1.38 | (1.30, 1.48) | 1.28 | (1.21, 1.34) | 1.70 | (1.29, 2.25) |
| Skin Excluding Basal and Squamous | 1.60 | (1.59, 1.62) | 1.19 | (1.11, 1.28) | 1.32 | (1.24, 1.41) | 1.06 | (1.02, 1.10) | 1.23 | (1.05, 1.45) |
| Melanoma of the Skin | 1.57 | (1.56, 1.58) | 1.25 | (1.12, 1.39) | 1.26 | (1.16, 1.37) | 1.03 | (0.99, 1.07) | 1.25 | (1.05, 1.49) |
| Other Non-Epithelial Skin | 2.14 | (2.08, 2.19) | 1.14 | (1.04, 1.26) | 1.42 | (1.28, 1.59) | 1.20 | (1.10, 1.31) | 1.17 | (0.76, 1.79) |
| Urinary Bladder | 4.15 | (4.11, 4.18) | 3.00 | (2.91, 3.11) | 4.17 | (4.00, 4.34) | 3.86 | (3.74, 3.99) | 4.14 | (3.49, 4.92) |
| Kidney and Renal Pelvis | 2.06 | (2.04, 2.08) | 1.99 | (1.94, 2.04) | 2.11 | (2.04, 2.19) | 1.81 | (1.77, 1.85) | 1.87 | (1.7, 2.06) |
| Ureter | 2.17 | (2.06, 2.27) | 1.72 | (1.39, 2.15) | 1.68 | (1.46, 1.95) | 1.90 | (1.60, 2.26) | 1.69 | (0.62, 5.17) |
| Other Urinary Organs | 3.31 | (3.09, 3.55) | 1.76 | (1.49, 2.07) | 2.11 | (1.68, 2.67) | 2.63 | (2.14, 3.24) | 4.11 | (1.40, 15.14) |
| Eye and Orbit | 1.41 | (1.35, 1.46) | 1.62 | (1.21, 2.17) | 1.54 | (1.21, 1.97) | 1.44 | (1.26, 1.65) | 1.20 | (0.55, 2.54) |
| Brain | 1.49 | (1.47, 1.51) | 1.48 | (1.39, 1.57) | 1.49 | (1.41, 1.58) | 1.36 | (1.31, 1.42) | 1.50 | (1.19, 1.91) |
| Cranial Nerves Other Nervous System | 1.02 | (0.95, 1.09) | 1.05 | (0.87, 1.27) | 1.05 | (0.86, 1.28) | 1.02 | (0.87, 1.19) | 0.84 | (0.31, 2.07) |
| Thyroid | 0.37 | (0.37, 0.37) | 0.30 | (0.28, 0.31) | 0.32 | (0.31, 0.33) | 0.28 | (0.27, 0.29) | 0.29 | (0.25, 0.35) |
| Other Endocrine Including Thymus | 1.25 | (1.18, 1.31) | 1.02 | (0.91, 1.15) | 1.32 | (1.17, 1.47) | 1.18 | (1.05, 1.34) | 1.49 | (0.71, 3.20) |
| Hodgkin Lymphoma | 1.27 | (1.24, 1.30) | 1.34 | (1.25, 1.42) | 1.33 | (1.22, 1.47) | 1.40 | (1.33, 1.48) | 1.13 | (0.76, 1.68) |
| Non-Hodgkin Lymphoma | 1.47 | (1.45, 1.48) | 1.47 | (1.43, 1.51) | 1.46 | (1.42, 1.50) | 1.32 | (1.30, 1.35) | 1.26 | (1.11, 1.42) |
| Myeloma | 1.67 | (1.65, 1.70) | 1.39 | (1.35, 1.43) | 1.47 | (1.39, 1.55) | 1.44 | (1.39, 1.49) | 1.19 | (0.98, 1.43) |
| Lymphocytic Leukemia | 1.92 | (1.89, 1.95) | 2.00 | (1.90, 2.11) | 1.76 | (1.64, 1.90) | 1.56 | (1.49, 1.63) | 1.87 | (1.44, 2.44) |
| Myeloid and Monocytic Leukemia | 1.58 | (1.55, 1.60) | 1.40 | (1.34, 1.47) | 1.55 | (1.48, 1.62) | 1.44 | (1.38, 1.49) | 1.38 | (1.12, 1.71) |
| Other Leukemia | 1.43 | (1.37, 1.50) | 1.28 | (1.13, 1.44) | 1.69 | (1.45, 1.98) | 1.43 | (1.26, 1.62) | 1.62 | (0.98, 2.69) |
| Mesothelioma | 4.18 | (4.01, 4.36) | 4.25 | (3.61, 5.03) | 3.35 | (2.76, 4.08) | 3.44 | (3.09, 3.85) | 3.20 | (1.70, 6.29) |
| Kaposi Sarcoma | 12.86 | (11.58, 14.32) | 13.5 | (11.43, 16.06) | 13.81 | (9.08, 22.00) | 7.96 | (6.78, 9.40) | 6.33 | (2.57, 20.83) |
| Non-Hispanic White | Non-Hispanic Black | Latino | Non-Hispanic Asian Pacific Islander | Non-Hispanic American Indian/Alaskan Native | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MF IRR | 95% CI | MF IRR | 95% CI | MF IRR | 95% CI | MF IRR | 95% CI | MF IRR | 95% CI | |
| Esophagus Adenocarcinoma | 7.71 | (7.48, 7.95) | 3.80 | (3.30, 4.38) | 6.10 | (5.08, 7.37) | 6.10 | (5.51, 6.76) | 4.07 | (2.85, 5.95) |
| Esophagus SCC | 1.75 | (1.69, 1.81) | 2.98 | (2.81, 3.16) | 3.13 | (2.86, 3.43) | 3.46 | (3.12, 3.84) | 2.87 | (1.98, 4.20) |
| Gastric Cardia Adenocarcinoma | 4.88 | (4.72, 5.04) | 3.05 | (2.72, 3.41) | 3.82 | (3.44, 4.26) | 2.98 | (2.74, 3.24) | 3.10 | (2.15, 4.53) |
| Gastric Non-Cardia Adenocarcinoma | 1.62 | (1.58, 1.66) | 1.95 | (1.87, 2.03) | 1.61 | (1.56, 1.67) | 1.59 | (1.54, 1.65) | 1.90 | (1.60, 2.26) |
| Liver HCC | 4.07 | (3.99, 4.16) | 4.17 | (4.00, 4.36) | 3.13 | (3.03, 3.24) | 3.11 | (3.01, 3.21) | 2.70 | (2.35, 3.10) |
| Liver ICC | 1.26 | (1.21, 1.31) | 1.36 | (1.20, 1.53) | 1.35 | (1.23, 1.47) | 1.08 | (0.99, 1.17) | 1.29 | (0.85, 1.95) |
| Lung and Bronchus Adenocarcinoma | 1.09 | (1.08, 1.09) | 1.48 | (1.45, 1.51) | 1.26 | (1.23, 1.29) | 1.22 | (1.19, 1.25) | 1.06 | (0.94, 1.21) |
| Lung and Bronchus SCC | 2.01 | (1.99, 2.04) | 2.59 | (2.51, 2.66) | 3.46 | (3.29, 3.64) | 2.36 | (2.26, 2.47) | 1.84 | (1.60, 2.12) |
| Lung and Bronchus Small Cell Carcinoma | 0.79 | (0.76, 0.81) | 1.09 | (0.99, 1.19) | 1.44 | (1.26, 1.65) | 0.83 | (0.76, 0.91) | 0.96 | (0.59, 1.54) |
| Lung and Bronchus Large Cell Carcinoma | 1.72 | (1.25, 2.37) | 1.39 | (0.54, 3.60) | 17.76 | (2.48, 753.32) | 3.89 | (1.13, 17.56) | ~ | ~ |
| Urinary Bladder TCC | 4.28 | (4.24, 4.32) | 3.20 | (3.09, 3.32) | 4.35 | (4.17, 4.53) | 4.08 | (3.95, 4.23) | 4.86 | (4.01, 5.92) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosakoon, S.; Lawrence, W.R.; Shiels, M.S.; Jackson, S.S. Sex Differences in Cancer Incidence Rates by Race and Ethnicity: Results from the Surveillance, Epidemiology, and End Results (SEER) Registry (2000–2019). Cancers 2024, 16, 989. https://doi.org/10.3390/cancers16050989
Tosakoon S, Lawrence WR, Shiels MS, Jackson SS. Sex Differences in Cancer Incidence Rates by Race and Ethnicity: Results from the Surveillance, Epidemiology, and End Results (SEER) Registry (2000–2019). Cancers. 2024; 16(5):989. https://doi.org/10.3390/cancers16050989
Chicago/Turabian StyleTosakoon, Sararat, Wayne R. Lawrence, Meredith S. Shiels, and Sarah S. Jackson. 2024. "Sex Differences in Cancer Incidence Rates by Race and Ethnicity: Results from the Surveillance, Epidemiology, and End Results (SEER) Registry (2000–2019)" Cancers 16, no. 5: 989. https://doi.org/10.3390/cancers16050989
APA StyleTosakoon, S., Lawrence, W. R., Shiels, M. S., & Jackson, S. S. (2024). Sex Differences in Cancer Incidence Rates by Race and Ethnicity: Results from the Surveillance, Epidemiology, and End Results (SEER) Registry (2000–2019). Cancers, 16(5), 989. https://doi.org/10.3390/cancers16050989







