Systematic Review of Nutrition Interventions to Improve Short Term Outcomes in Head and Neck Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection Critera
2.2. Study Selection Criteria
2.3. Study Quality Assessment
3. Results
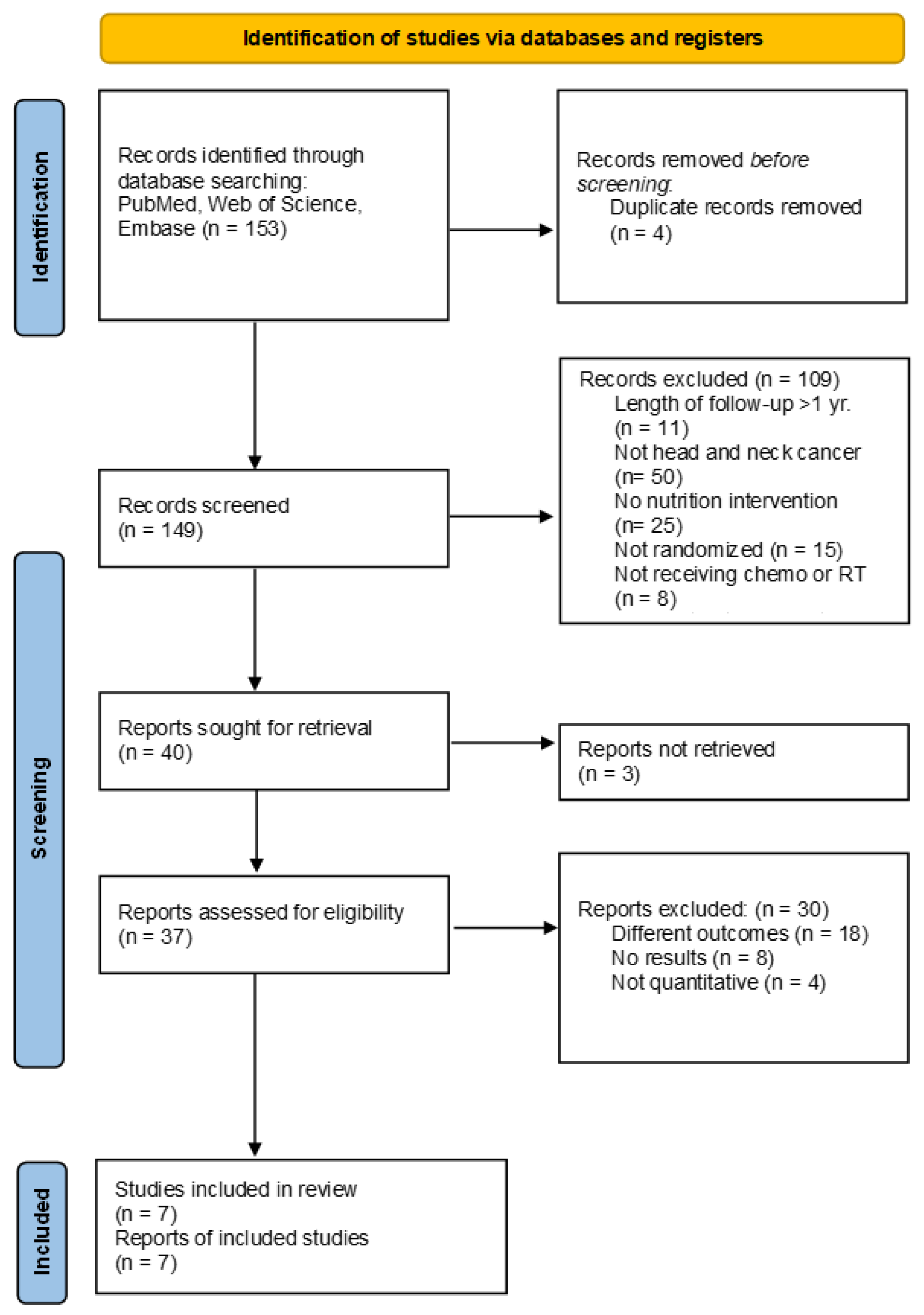
3.1. Study Selection and Characteristics
3.2. Study Quality Assessment
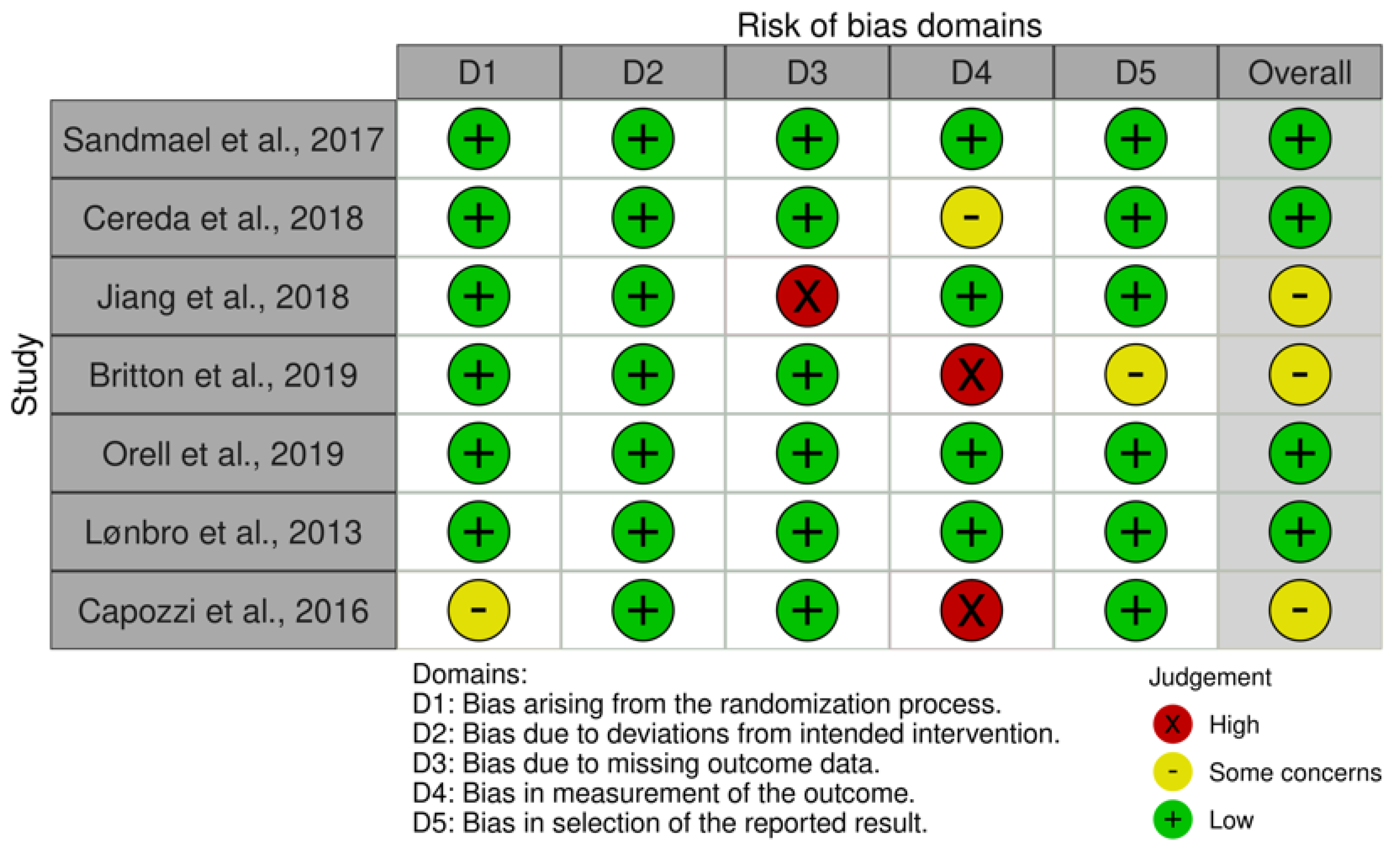
3.3. Risk of Bias Assessment
3.4. Study Characteristics
3.4.1. RD Intervention with ONS
3.4.2. RD Intervention with Motivational Interviewing
3.4.3. Non-RD Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Capuano, G.; Gentile, P.C.; Bianciardi, F.; Tosti, M.; Palladino, A.; Di Palma, M. Prevalence and influence of malnutrition on quality of life and performance status in patients with locally advanced head and neck cancer before treatment. Support. Care Cancer 2010, 18, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Langius, J.A.; Bakker, S.; Rietveld, D.H.; Kruizenga, H.M.; Langendijk, J.A.; Weijs, P.J.; Leemans, C.R. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br. J. Cancer 2013, 109, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.L.; Elliott, L.; Fuchs-Tarlovsky, V.; Levin, R.M.; Voss, A.C.; Piemonte, T. Oncology Evidence-Based Nutrition Practice Guideline for Adults. J. Acad. Nutr. Diet. 2017, 117, 297–310.e247. [Google Scholar] [CrossRef]
- Pfister, D.G.; Spencer, S.; Adelstein, D.; Adkins, D.; Anzai, Y.; Brizel, D.M.; Bruce, J.Y.; Busse, P.M.; Caudell, J.J.; Cmelak, A.J.; et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 873–898. [Google Scholar] [CrossRef]
- Huhmann, M.B.; August, D.A. Review of American Society for Parenteral and Enteral Nutrition (ASPEN) Clinical Guidelines for Nutrition Support in Cancer Patients: Nutrition screening and assessment. Nutr. Clin. Pract. 2008, 23, 182–188. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef]
- Lorton, C.M.; Griffin, O.; Higgins, K.; Roulston, F.; Stewart, G.; Gough, N.; Barnes, E.; Aktas, A.; Walsh, T.D. Late referral of cancer patients with malnutrition to dietitians: A prospective study of clinical practice. Support. Care Cancer 2020, 28, 2351–2360. [Google Scholar] [CrossRef]
- Crowder, S.L.; Douglas, K.G.; Frugé, A.D.; Carroll, W.R.; Spencer, S.A.; Locher, J.L.; Demark-Wahnefried, W.; Rogers, L.Q.; Arthur, A.E. Head and neck cancer survivors’ preferences for and evaluations of a post-treatment dietary intervention. Nutr. J. 2019, 18, 57. [Google Scholar] [CrossRef]
- Langius, J.A.; Zandbergen, M.C.; Eerenstein, S.E.; van Tulder, M.W.; Leemans, C.R.; Kramer, M.H.; Weijs, P.J. Effect of nutritional interventions on nutritional status, quality of life and mortality in patients with head and neck cancer receiving (chemo)radiotherapy: A systematic review. Clin. Nutr. 2013, 32, 671–678. [Google Scholar] [CrossRef]
- Capozzi, L.C.; McNeely, M.L.; Lau, H.Y.; Reimer, R.A.; Giese-Davis, J.; Fung, T.S.; Culos-Reed, S.N. Patient-reported outcomes, body composition, and nutrition status in patients with head and neck cancer: Results from an exploratory randomized controlled exercise trial. Cancer 2016, 122, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Solís-Martínez, O.; Plasa-Carvalho, V.; Phillips-Sixtos, G.; Trujillo-Cabrera, Y.; Hernández-Cuellar, A.; Queipo-García, G.E.; Meaney-Mendiolea, E.; Ceballos-Reyes, G.M.; Fuchs-Tarlovsky, V. Effect of Eicosapentaenoic Acid on Body Composition and Inflammation Markers in Patients with Head and Neck Squamous Cell Cancer from a Public Hospital in Mexico. Nutr. Cancer 2018, 70, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ding, H.; Li, W.; Ling, Y.; Hu, C.; Shen, C. Benefits of Oral Nutritional Supplements in Patients with Locally Advanced Nasopharyngeal Cancer during Concurrent Chemoradiotherapy: An Exploratory Prospective Randomized Trial. Nutr. Cancer 2018, 70, 1299–1307. [Google Scholar] [CrossRef]
- Cereda, E.; Cappello, S.; Colombo, S.; Klersy, C.; Imarisio, I.; Turri, A.; Caraccia, M.; Borioli, V.; Monaco, T.; Benazzo, M.; et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother. Oncol. 2018, 126, 81–88. [Google Scholar] [CrossRef]
- Brown, T.E.; Banks, M.D.; Hughes, B.G.M.; Lin, C.Y.; Kenny, L.M.; Bauer, J.D. Randomised controlled trial of early prophylactic feeding vs standard care in patients with head and neck cancer. Br. J. Cancer 2017, 117, 15–24. [Google Scholar] [CrossRef]
- Britton, B.; Baker, A.L.; Wolfenden, L.; Wratten, C.; Bauer, J.; Beck, A.K.; McCarter, K.; Harrowfield, J.; Isenring, E.; Tang, C.; et al. Eating As Treatment (EAT): A Stepped-Wedge, Randomized Controlled Trial of a Health Behavior Change Intervention Provided by Dietitians to Improve Nutrition in Patients with Head and Neck Cancer Undergoing Radiation Therapy (TROG 12.03). Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 353–362. [Google Scholar] [CrossRef]
- Yeh, K.Y.; Wang, H.M.; Chang, J.W.; Huang, J.S.; Lai, C.H.; Lan, Y.J.; Wu, T.H.; Chang, P.H.; Wang, H.; Wu, C.J.; et al. Omega-3 fatty acid-, micronutrient-, and probiotic-enriched nutrition helps body weight stabilization in head and neck cancer cachexia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 41–48. [Google Scholar] [CrossRef]
- Sandmael, J.A.; Bye, A.; Solheim, T.S.; Stene, G.B.; Thorsen, L.; Kaasa, S.; Lund, J.; Oldervoll, L.M. Feasibility and preliminary effects of resistance training and nutritional supplements during versus after radiotherapy in patients with head and neck cancer: A pilot randomized trial. Cancer 2017, 123, 4440–4448. [Google Scholar] [CrossRef] [PubMed]
- Orell, H.; Schwab, U.; Saarilahti, K.; Österlund, P.; Ravasco, P.; Mäkitie, A. Nutritional Counseling for Head and Neck Cancer Patients Undergoing (Chemo) Radiotherapy-A Prospective Randomized Trial. Front. Nutr. 2019, 6, 22. [Google Scholar] [CrossRef]
- Lønbro, S.; Dalgas, U.; Primdahl, H.; Overgaard, J.; Overgaard, K. Feasibility and efficacy of progressive resistance training and dietary supplements in radiotherapy treated head and neck cancer patients--the DAHANCA 25A study. Acta Oncol. 2013, 52, 310–318. [Google Scholar] [CrossRef]
- Ho, Y.W.; Yeh, K.Y.; Hsueh, S.W.; Hung, C.Y.; Lu, C.H.; Tsang, N.M.; Wang, H.M.; Hung, Y.S.; Chou, W.C. Impact of early nutrition counseling in head and neck cancer patients with normal nutritional status. Support. Care Cancer 2021, 29, 2777–2785. [Google Scholar] [CrossRef]
- van den Berg, M.G.; Rasmussen-Conrad, E.L.; Wei, K.H.; Lintz-Luidens, H.; Kaanders, J.H.; Merkx, M.A. Comparison of the effect of individual dietary counselling and of standard nutritional care on weight loss in patients with head and neck cancer undergoing radiotherapy. Br. J. Nutr. 2010, 104, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Ravasco, P.; Monteiro-Grillo, I.; Marques Vidal, P.; Camilo, M.E. Impact of nutrition on outcome: A prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005, 27, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Lee, M.S.; Cheng, H.L.; Chou, H.Y.; Chan, L.C. More Frequent Nutrition Counseling Limits Weight Loss and Improves Energy Intake During Oncology Management: A Longitudinal Inpatient Study in Taiwan. Nutr. Cancer 2019, 71, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Guller, M.; Herberg, M.; Amin, N.; Alkhatib, H.; Maroun, C.; Wu, E.; Allen, H.; Zheng, Y.; Gourin, C.; Vosler, P.; et al. Nutritional Status as a Predictive Biomarker for Immunotherapy Outcomes in Advanced Head and Neck Cancer. Cancers 2021, 13, 5772. [Google Scholar] [CrossRef]
- Johannet, P.; Sawyers, A.; Qian, Y.; Kozloff, S.; Gulati, N.; Donnelly, D.; Zhong, J.; Osman, I. Baseline prognostic nutritional index and changes in pretreatment body mass index associate with immunotherapy response in patients with advanced cancer. J. Immunother. Cancer 2020, 8, e001674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rui, M.; Lin, C.; Li, Z.; Wei, D.; Han, R.; Ju, H.; Ren, G. The association between body mass index and efficacy of pembrolizumab as second-line therapy in patients with recurrent/metastatic head and neck squamous cell carcinoma. Cancer Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Han, H.R.; Hermann, G.M.; Ma, S.J.; Iovoli, A.J.; Wooten, K.E.; Arshad, H.; Gupta, V.; McSpadden, R.P.; Kuriakose, M.A.; Markiewicz, M.R.; et al. Matched pair analysis to evaluate weight loss during radiation therapy for head and neck cancer as a prognostic factor for survival. Ann. Transl. Med. 2021, 9, 914. [Google Scholar] [CrossRef]
- Lang, S.; Schimansky, S.; Beynon, R.; Penfold, C.; Davies, A.; Waylen, A.; Thomas, S.; Pring, M.; Pawlita, M.; Waterboer, T.; et al. Dietary behaviors and survival in people with head and neck cancer: Results from Head and Neck 5000. Head Neck 2019, 41, 2074–2084. [Google Scholar] [CrossRef]
- Arthur, A.E.; Peterson, K.E.; Rozek, L.S.; Taylor, J.M.; Light, E.; Chepeha, D.B.; Hébert, J.R.; Terrell, J.E.; Wolf, G.T.; Duffy, S.A. Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. Am. J. Clin. Nutr. 2013, 97, 360–368. [Google Scholar] [CrossRef]
| Acronym: | Definition: | Description: |
|---|---|---|
| P | Patient or problem | Human subjects with a head and neck cancer diagnosis |
| I | Intervention | (1) Oral nutrition supplements (ONS) and MNT by a registered dietitian (RD), (2) enteral nutrition and MNT by an RD, (3) motivational interviewing by an RD, and (4) ONS and no RD. |
| C | Comparison | Not applicable |
| O | Outcomes | How nutrition intervention is associated with nutrition status, QOL, and treatment tolerance |
| S | Study Design | Quantitative studies |
| Author, Year | Intervention | Main Inclusion Criteria | Sample Size | Length of Follow-Up | Nutrition Status | Treatment Tolerance | Quality of Life | Country |
|---|---|---|---|---|---|---|---|---|
| RD intervention with ONS | ||||||||
| Sandmael et al., 2017 [18] | Intervention: PRT and nutrition intervention of RD consult and 1 supplement/day during RT; Control: Post-treatment intervention | Diagnosis of HNSCC with referral for curative RT with or without chemo | 41 | Two months | No difference between the two groups from baseline to week 14 for change body weight (p = 0.818) or change in muscle mass (p = 0.821) | Norway | ||
| Cereda et al., 2018 [14] | Intervention: Nutrition counseling and two ONS per day; Control: Nutrition Counseling | Newly diagnosed HNC patients suitable for RT or RT plus systematic treatment | 159 | Three months after the end of RT | Counseling plus ONS resulted in less change in body weight (p = 0.006), increased protein-calorie intake (p < 0.001), and increased protein intake (p < 0.001) than nutritional counseling alone | No difference in tolerance to anti-cancer treatments were in the two groups, however, patients receiving ONS were less likely to require RT and/or ST dose reduction or complete suspension | Counseling plus ONS resulted in higher QOL scores (p < 0.001) | Italy |
| Jiang et al., 2018 [13] | Intervention: ONS once daily; Control: No extra nutritional supplements were provided | HNC (NPC), stage 3 or 4 receiving chemoradiation | 100 | Three months after the end of CRT | ONS group had higher body weight (p = 0.036) and BMI (p = 0.021) at the end of CRT; No difference between groups at three months post-CRT in weight (p = 0.71), BMI (p = 0.608), FFM (p = 0.809), FFMI (p = 0.800) | Patients in the ONS group had higher QOL at the end of CRT (p = 0.045); No between group difference at three months post-CRT in QOL (p = 0.294) | China | |
| RD intervention with Motivational Interviewing (MI) | ||||||||
| Britton et al., 2019 [16] | Intervention: RD provides MI and cognitive behavioral therapy (CBT); Control: RD provides treatment as usual | HNC requiring RT or concurrent chemoradiation with curative intent | 307 | 12 weeks after the end of RT | Patients who received MI and CBT had significantly better (lower) PG-SGA scores (p = 0.03) and less percent weight loss (p = 0.03) than control | Patients who received MI and cognitive behavioral therapy had significantly less interruptions in RT treatment (p = 0.04). | Patients who received MI and cognitive behavioral therapy had significantly better QOL (p < 0.01) | Australia |
| Orell et al., 2019 [19] | Intervention: RD provides intensive nutritional counseling (INC); Control: RD provides on-demand nutrition counseling (ODC) | Locally advanced HNC receiving curative treatment with combined surgery and adjuvant (chemo) radiotherapy, or definitive (chemo) radiotherapy | 65 | Primary outcomes assessed at end of treatment; Survival measured—median of 43 months | No difference in nutrition measures between groups. PG-SGA scores increased for all patients during treatment (p < 0.001), 77% of patients had critical weight loss in the INC vs. 67% in the ODC group. Weight loss was greater in the group with baseline OW vs. normal BMI (p < 0.001). | Finland | ||
| Non-RD intervention | ||||||||
| Lønbro et al., 2013 [20] | Intervention: PRT + ONS consisting of creatine and protein powder; Control: PRT + isocaloric placebo (maltodextrin) | HNC, terminated curative RT treatment +/− chemotherapy | 30 | 12 weeks | Both groups had increased LBM, intervention group had LBM increase (p < 0.0001), but not BW. | Denmark | ||
| Capozzi et al., 2016 [11] | Intervention: 12-week immediate lifestyle intervention (ILI); Control: 12-week delayed intervention (DLI) | HNC scheduled to receive radiation or concurrent chemoradiation treatment | 60 | 12 months | No difference between the two groups across the 24 weeks for lean body mass (p = 0.756), BMI (p = 0.698), percent body fat (p = 0.741), or nutrition status (p = 0.846) | No difference between the two groups across the 24 weeks for physical, functional, or anemia-specific QOL (p = 0.751) or for HNC-specific QOL (p = 0.503) | Canada | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leis, C.; Arthur, A.E.; Chen, X.; Greene, M.W.; Frugé, A.D. Systematic Review of Nutrition Interventions to Improve Short Term Outcomes in Head and Neck Cancer Patients. Cancers 2023, 15, 822. https://doi.org/10.3390/cancers15030822
Leis C, Arthur AE, Chen X, Greene MW, Frugé AD. Systematic Review of Nutrition Interventions to Improve Short Term Outcomes in Head and Neck Cancer Patients. Cancers. 2023; 15(3):822. https://doi.org/10.3390/cancers15030822
Chicago/Turabian StyleLeis, Claire, Anna E. Arthur, Xin Chen, Michael W. Greene, and Andrew D. Frugé. 2023. "Systematic Review of Nutrition Interventions to Improve Short Term Outcomes in Head and Neck Cancer Patients" Cancers 15, no. 3: 822. https://doi.org/10.3390/cancers15030822
APA StyleLeis, C., Arthur, A. E., Chen, X., Greene, M. W., & Frugé, A. D. (2023). Systematic Review of Nutrition Interventions to Improve Short Term Outcomes in Head and Neck Cancer Patients. Cancers, 15(3), 822. https://doi.org/10.3390/cancers15030822