Six-Month Prostate Cancer Empowerment Program (PC-PEP) Improves Urinary Function: A Randomized Trial
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure
2.2. Primary Outcomes
2.3. Pelvic Floor Compliance
2.4. Prognostic Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leslie, S.W.; Soon-Sutton, T.L.; Anu, R.I.; Sajjad, H.; Skelton, W.P. Prostate Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Critz, F.A.; Benton, J.B.; Shrake, P.; Merlin, M.L. 25-Year disease-free survival rate after irradiation for prostate cancer calculated with the prostate specific antigen definition of recurrence used for radical prostatectomy. J. Urol. 2013, 189, 878–883. [Google Scholar] [CrossRef]
- Michaelson, M.D.; Cotter, S.E.; Gargollo, P.C.; Zietman, A.L.; Dahl, D.M.; Smith, M.R. Management of complications of prostate cancer treatment. CA A Cancer J. Clin. 2008, 58, 196–213. [Google Scholar] [CrossRef]
- Ilie, G.; Rendon, R.; Mason, R.; MacDonald, C.; Kucharczyk, M.J.; Patil, N.; Bowes, D.; Bailly, G.; Bell, D.; Lawen, J.; et al. A Comprehensive 6-mo Prostate Cancer Patient Empowerment Program Decreases Psychological Distress Among Men Undergoing Curative Prostate Cancer Treatment: A Randomized Clinical Trial. Eur. Urol. 2023, 83, 561–570. [Google Scholar] [CrossRef]
- Peyromaure, M.; Ravery, V.; Boccon-Gibod, L. The management of stress urinary incontinence after radical prostatectomy. BJU Int. 2002, 90, 155–161. [Google Scholar] [CrossRef]
- Liu, M.; Pickles, T.; Berthelet, E.; Agranovich, A.; Kwan, W.; Tyldesley, S.; McKenzie, M.; Keyes, M.; Morris, J.; Pai, H.; et al. Urinary incontinence in prostate cancer patients treated with external beam radiotherapy. Radiother. Oncol. 2005, 74, 197–201. [Google Scholar] [CrossRef]
- Horrocks, S.; Somerset, M.; Stoddart, H.; Peters, T.J. What prevents older people from seeking treatment for urinary incontinence? A qualitative exploration of barriers to the use of community continence services. Fam. Pract. 2004, 21, 689–696. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.R. Prevention and management of erectile dysfunction following radical prostatectomy. Urol. Clin. N. Am. 2001, 28, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Zorn, B.H.; Montgomery, H.; Pieper, K.; Gray, M.; Steers, W.D. Urinary incontinence, and depression. J. Urol. 1999, 162, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Urvaylıoğlu, A.; Kutlutürkan, S.; Kılıç, D. Effect of Kegel exercises on the prevention of urinary and fecal incontinence in patients with prostate cancer undergoing radiotherapy. Eur. J. Oncol. Nurs. 2021, 51, 101913. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.E.; Coughlin, G.; Hodges, P.W. Comparison of dynamic features of pelvic floor muscle contraction between men with and without incontinence after prostatectomy and men with no history of prostate cancer. Neurourol. Urodyn. 2020, 39, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.E.; Doorbar-Baptist, S.; Hodges, P.W. The relationship between pre- and postprostatectomy measures of pelvic floor muscle function and development of early incontinence after surgery. Neurourol. Urodyn. 2022, 41, 1722–1730. [Google Scholar] [CrossRef]
- Stafford, R.E.; Hoorn, W.v.D.; Coughlin, G.; Hodges, P.W. Postprostatectomy incontinence is related to pelvic floor displacements observed with trans-perineal ultrasound imaging. Neurourol. Urodyn. 2018, 37, 658–665. [Google Scholar] [CrossRef]
- Wyman, J.F.; Burgio, K.L.; Newman, D.K. Practical aspects of lifestyle modifications and behavioral interventions in the treatment of overactive bladder and urgency urinary incontinence. Int. J. Clin. Pract. 2009, 63, 1177–1191. [Google Scholar] [CrossRef]
- Baumann, F.T.; Reimer, N.; Gockeln, T.; Reike, A.; Hallek, M.; Ricci, C.; Zopf, E.M.; Schmid, D.; Taaffe, D.; Newton, R.U.; et al. Supervised pelvic floor muscle exercise is more effective than unsupervised pelvic floor muscle exercise at improving urinary incontinence in prostate cancer patients following radical prostatectomy—A sys-tematic review and meta-analysis. Disabil. Rehabil. 2022, 44, 5374–5385. [Google Scholar] [CrossRef] [PubMed]
- Szczygielska, D.; Knapik, A.; Pop, T.; Rottermund, J.; Saulicz, E. The Effectiveness of Pelvic Floor Muscle Training in Men after Radical Prostatectomy Measured with the Insert Test. Int. J. Environ. Res. Public Health 2022, 19, 2890. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Winser, S.J.; Fung, B.; Cheing, G. Effectiveness of Pelvic Floor Muscle Training Alone and in Combination with Biofeedback, Electrical Stimulation, or Both Compared to Control for Urinary Incontinence in Men Following Prostatectomy: Systematic Review and Meta-Analysis. Phys. Ther. 2018, 98, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Milios, J.E.; Ackland, T.R.; Green, D.J. Pelvic floor muscle training in radical prostatectomy: A randomized controlled trial of the impacts on pelvic floor muscle function and urinary incontinence. BMC Urol. 2019, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Glazener, C.; Boachie, C.; Buckley, B.; Cochran, C.; Dorey, G.; Grant, A.; Hagen, S.; Kilonzo, M.; McDonald, A.; McPherson, G.; et al. Conservative treatment for urinary incontinence in Men After Prostate Surgery (MAPS): Two parallel randomised controlled trials. Health Technol. Assess. 2011, 15, 1–296. [Google Scholar] [CrossRef] [PubMed]
- Continence Foundation of Australia. Continence and Prostate: A Guide for Men Undergoing Prostate Surgery; Commence Press: Melbourne, VIC, Australia, 2019. [Google Scholar]
- Barry, M.J.; Fowler, F.J., Jr.; O’leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T.; The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Laviana, A.A.; Hernandez, A.; Huang, L.-C.; Zhao, Z.; Koyama, T.; Conwill, R.; Hoffman, K.; Feurer, I.D.; Goodman, M.; Hamilton, A.S.; et al. Interpretation of Domain Scores on the EPIC—How Does the Domain Score Translate into Functional Outcomes? J. Urol. 2019, 202, 1150–1158. [Google Scholar] [CrossRef]
- Chang, P.; Szymanski, K.M.; Dunn, R.L.; Chipman, J.J.; Litwin, M.S.; Nguyen, P.L.; Sweeney, C.J.; Cook, R.; Wagner, A.A.; DeWolf, W.C.; et al. Expanded prostate cancer index composite for clinical practice: Development and validation of a practical health related quality of life instrument for use in the routine clinical care of patients with prostate cancer. J. Urol. 2011, 186, 865–872. [Google Scholar] [CrossRef]
- Agrawal, S.; Stricker, P.; Papa, N. How Does Age Affect Urinary Continence following Robot-Assisted Radical Prostatectomy? A Prospective Multi-Institutional Study Using Independently Collected, Validated Questionnaires. J. Urol. 2022, 207, 1048–1056. [Google Scholar] [CrossRef]
- Wille, S.; Heidenreich, A.; von Knobloch, R.; Hofmann, R.; Engelmann, U. Impact of comorbidities on post-prostatectomy incontinence. Urol. Int. 2006, 76, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Abdollah, F.; Hansen, J.; Trinh, Q.; Bianchi, M.; Tian, Z.; Briganti, A.; Shariat, S.F.; Montorsi, F.; Perrotte, P.; et al. Is a treatment delay in radical prostatectomy safe in individuals with low-risk prostate cancer? J. Sex. Med. 2012, 9, 2961–2969. [Google Scholar] [CrossRef]
- Li, K.; Magnani, C.J.; Bozkurt, S.; Seto, T.; Blayney, D.W.; Brooks, J.D.; Hernandez-Boussard, T. Practice-based evidence for factors associated with urinary incontinence following prostate cancer care. J. Clin. Oncol. 2018, 36 (Suppl. S6), 106. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Adjustment for Baseline Covariates in Clinical Trials. Committee for Medicinal Products for Human Use (CHMP); 2015. EMA/CHMP/295050/2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-adjustment-baseline-covariates-clinical-trials_en.pdf (accessed on 22 February 2024).
- U.S. Food and Drug Administration. Adjusting for Covariates in Randomized Clinical Trials for Drugs and Biological Products. Draft Guidance for Industry; Center for Drug Evaluation and Research: Rockville, MD, USA, 2021. Available online: https://www.fda.gov/media/123801/download (accessed on 22 February 2024).
- IBM Corp. IBM SPSS Statistics for Windows; IBM Corp.: Armonk, NY, USA, 2020. [Google Scholar]
- Wang, L.; Li, Y.; Qi, Z.; Wang, W. Barriers and facilitators of the implementation of the application of pelvic floor muscle training in patients with prostate cancer: A scoping review. Front. Public Health 2023, 11, 1191508. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Funnell, M.M. Patient empowerment: Reflections on the challenge of fostering the adoption of a new paradigm. Patient Educ. Couns. 2005, 57, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.; Hibbard, J.; Tusler, M. How do People with Different Levels of Activation Self-Manage their Chronic Conditions? Patient—Patient-Centered Outcomes Res. 2009, 2, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Smither, A.R.; Guralnick, M.L.; Davis, N.B.; See, W.A. Quantifying the natural history of post-radical prostatectomy incontinence using objective pad test data. BMC Urol. 2007, 7, 2. [Google Scholar] [CrossRef]



















| PC-PEP Median (Quartile) n, % (n = 66) | Control Median (Quartile) n, % (n = 62) | p | |
|---|---|---|---|
| Urologic self-reported outcomes | |||
| IPSS a sum scores | 66, 7 (3, 12) | 62, 8 (4, 12) | 0.3 |
| IPSS a bother score | 66, 2 (1, 4) | 62, 2 (1, 3) | 1.0 |
| EPIC b Urinary Incontinence | 66, 100 (62, 139) | 62, 100 (74, 126) | 0.7 |
| EPIC b Urinary Irritative/Obstructive | 66, 94 (85, 104) | 62, 94 (85, 104) | 0.4 |
| EPIC b Bowel function | 66, 100 (96,104) | 62, 100 (96,104) | 0.8 |
| EPIC b Sexual function | 66, 62 (37, 87) | 62, 61 (37, 84) | 0.9 |
| EPIC b Hormonal function | 66, 95 (89, 101) | 62, 95 (88, 103) | 0.5 |
| Demographic characteristics | |||
| Age (yr) | 66, 66 (60, 70) | 62, 68 (61, 72) | 0.2 |
| Body Mass Index | 66, 29 (24, 34) | 62, 27 (23, 31) | 0.5 |
| Household Income at baseline, >30,000 CAD/past year | 54, 82% | 52, 84% | 0.5 |
| Race, White | 60, 91% | 61, 98% | 0.068 |
| Education, university or above | 31, 47% | 37, 60% | 0.16 |
| Employed (part of full time) | 22, 33% | 23, 37% | 0.7 |
| Charlson Comorbidity Index | 66, 2 (2, 3) | 62, 3 (2, 3) | 0.5 |
| Self-identified as a cigarette smoker | 5, 8% | 3, 5% | 0.7 |
| Diagnosis and treatment-relevant characteristics | |||
| Stage of cancer | |||
| Risk Category (RP c + primary RT d ± TH e) f | 0.6 | ||
| Low | 1, 1.5% | 2, 3.2% | |
| Intermediate | 42, 75% | 40, 67% | |
| High | 13, 23% | 18, 30% | |
| PSA (ng/mL) at time of RT (salvage group only) | 10, 0.11 (0.065, 0.16) | 2, 0.28 (0.18, 0.37) | 0.5 |
| Prescribed ADT g | 27, 41% | 21, 34% | 0.4 |
| Treatment modality | 0.067 | ||
| RP c | 29, 44% | 33, 53% | |
| RT d | 27, 41% | 27, 44% | |
| Salvage RT h | 10, 15% | 2, 3.2% | |
| Nr. days between RP c and standard of care PFMT i appointment (referral) | 5, 27 (12, 65) | 15, 34 (18, 87) | 0.4 |
| No. of visits to the PFMT i nurse specialist at the hospital | 5, 1 (1, 7) | 15, 2 (1, 5) | 0.9 |
| Time between the first and last PFMT visit to the hospital (days) | 5, 0 (0, 726) | 15, 49 (0, 202) | 0.7 |
| Time between randomization and RP c or RT d treatment (days) | 66, 61 (34, 99) | 62, 73 (29, 101) | 0.3 |
| Intake of prescribed medication for depression, anxiety, or both at the time of entering the trial | 12, 18% | 7, 11% | 0.3 |
| Absence of cancer recurrence at 6 months post-randomization | 63, 96% | 58, 94% | 0.6 |
| The specific reasons for patients’ visits to the Hospital’s PFMT i Nurse as part of their standard of care during the duration of the trial | |||
| PFMT i biofeedback | 1 | 11 | |
| PFMT i verbal education provided, trial of void, and PFMT i handout provided—single visit | 3 | 3 | |
| Removal of catheter and PFMT i provided—single visit | 1 | 1 | |
| I-PSS Sum Score | ||||
|---|---|---|---|---|
| Level | Parameter Estimate | 95% Confidence Interval | p | |
| Lower | Upper | |||
| Group (Control vs. PC-PEP) | 2.8 | 0.58 | 4.9 | 0.013 |
| Time (baseline vs. 6 months) | −0.076 | −1.8 | 1.7 | 0.9 |
| Time × Group (Control) | −2.4 | −4.9 | 0.097 | 0.059 |
| Surgery | ||||
| Group (Control vs. PC-PEP) | 5.4 | 2.1 | 8.7 | 0.02 |
| Time (baseline vs. 6 months) | 0.69 | −2.1 | 3.5 | 0.6 |
| Time × Group (Control) | −3.4 | −7.2 | 3.5 | 0.077 |
| Radiation | ||||
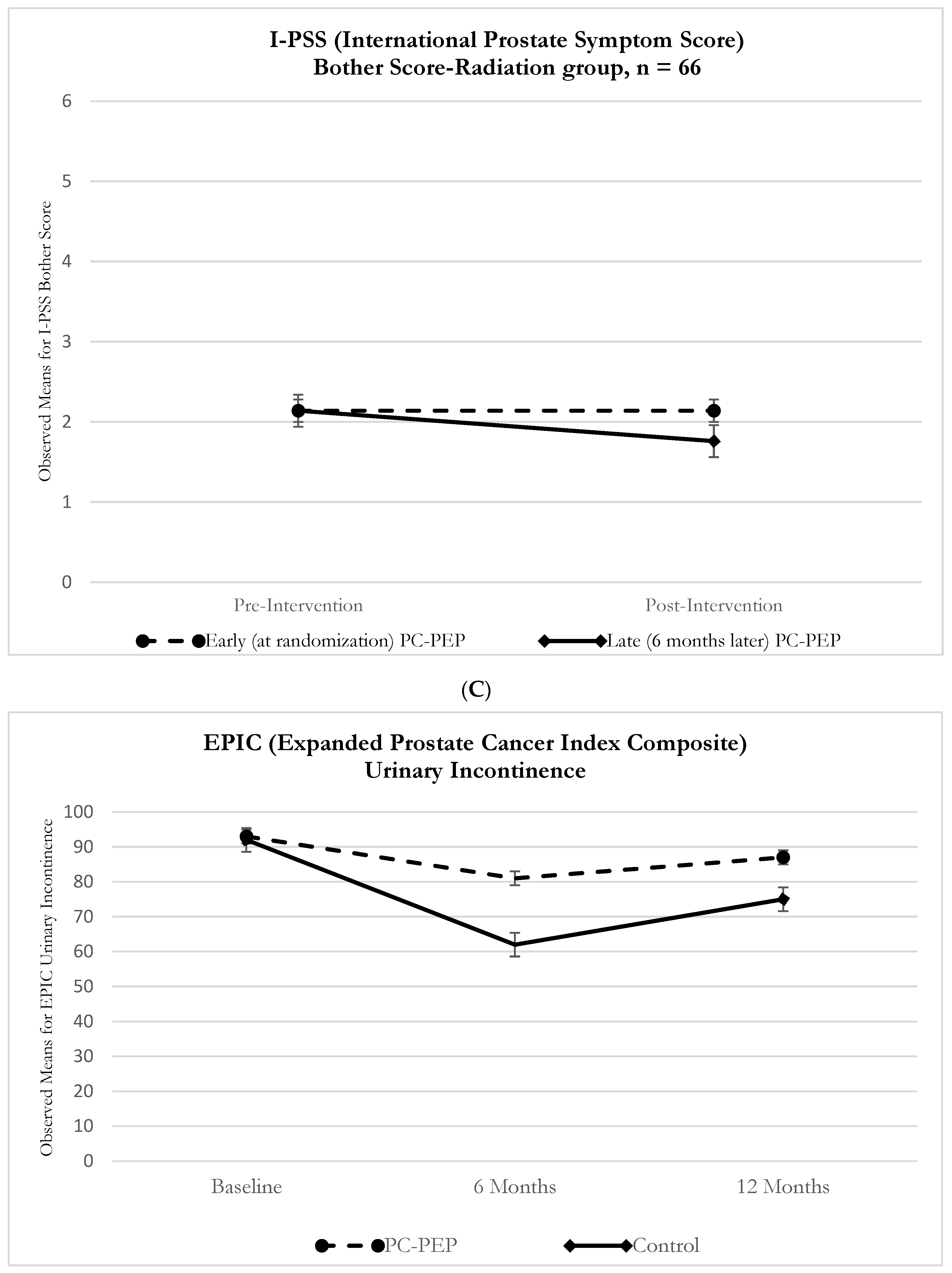
| Group (Control vs. PC-PEP) | −0.28 | −3.1 | 2.6 | 0.9 |
| Time (baseline vs. 6 months) | −0.68 | −3.0 | 1.6 | 0.6 |
| Time × Group (Control) | −1.5 | −5.0 | 1.9 | 0.4 |
| IPSS Bother score | ||||
| Group (Control vs. PC-PEP) | 0.88 | 0.35 | 1.4 | 0.001 |
| Time (baseline vs. 6 months) | −0.11 | −0.51 | 0.30 | 0.6 |
| Time × Group (PCPEP) | −0.88 | −1.5 | −0.29 | 0.004 |
| Surgery | ||||
| Group (Control vs. PC-PEP) | 1.8 | 1.02 | 2.5 | <0.001 |
| Time (baseline vs. 6 months) | −0.24 | −0.91 | 0.43 | 0.5 |
| Time × Group (Control) | −1.03 | −1.9 | 0.12 | 0.028 |
| Radiation | ||||
| Group (Control vs. PC-PEP) | −0.062 | −0.76 | 0.63 | 0.9 |
| Time (baseline vs. 6 months) | 0.00 | −0.50 | 0.50 | 1.0 |
| Time × Group (Control) | −0.66 | −1.4 | 0.10 | 0.089 |
| EPIC Urinary Incontinence score | ||||
| Group (Control vs. PC-PEP) | −18 | −25 | −11 | <0.001 |
| Time (baseline vs. 6 months) | 11 | 3.9 | 17 | 0.002 |
| Time × Group (Control) | 20 | 10 | 29 | <0.001 |
| Surgery | ||||
| Group (Control vs. PC-PEP) | −31 | −41 | −22 | <0.001 |
| Time (baseline vs. 6 months) | 25 | 15 | 34 | <0.001 |
| Time × Group (Control) | 27 | 14 | 40 | <0.001 |
| Radiation | ||||
| Group (Control vs. PC-PEP) | −1.9 | −9.8 | 6.05 | 0.6 |
| Time (baseline vs. 6 months) | −0.34 | −4.9 | 4.2 | 0.9 |
| Time × Group (Control) | 6.2 | −0.65 | 13 | 0.075 |
| EPIC Urinary Irritative/Obstruction score | ||||
| Group (Control vs. PC-PEP) | −7.6 | −12 | −3.4 | <0.001 |
| Time (baseline vs. 6 months) | −1.4 | −4.7 | 1.9 | 0.4 |
| Time × Group (Control) | 6.5 | 1.7 | 11 | 0.008 |
| Surgery | ||||
| Group (Control vs. PC-PEP) | −15 | −21 | −8.3 | <0.001 |
| Time (baseline vs. 6 months) | −2.3 | −7.7 | −8.3 | 0.4 |
| Time × Group (Control) | 9.2 | 1.7 | 17 | 0.017 |
| Radiation | ||||
| Group (Control vs. PC-PEP) | −0.45 | −6.1 | 5.3 | 0.9 |
| Time (baseline vs. 6 months) | −0.73 | −4.91 | 3.45 | 0.7 |
| Time × Group (Control) | 3.7 | −2.6 | 10 | 0.2 |
| EPIC Bowel scores | ||||
| Group (Control vs. PC-PEP) | −2.8 | −6.7 | 1.2 | 0.17 |
| Time (baseline vs. 6 months) | 2.5 | −0.30 | 5.3 | 0.079 |
| Time × Group (Control) | 2.03 | −2.02 | 6.09 | 0.3 |
| Surgery | ||||
| Group (Control vs. PC-PEP) | −2.7 | −7.9 | 2.5 | 0.3 |
| Time (baseline vs. 6 months) | −0.53 | −3.2 | 2.1 | 0.7 |
| Time × Group (Control) | 2.1 | −1.5 | 5.7 | 0.3 |
| Radiation | ||||
| Group (Control vs. PC-PEP) | −3.1 | −9.2 | 2.9 | 0.3 |
| Time (baseline vs. 6 months) | 4.9 | 0.28 | 9.6 | 0.038 |
| Time × Group (Control) | 3.04 | −4.0 | 10 | 0.4 |
| EPIC Sexual score | ||||
| Group (Control vs. PC-PEP) | −2.7 | −12 | 6.2 | 0.6 |
| Time (baseline vs. 6 months) | 30 | 23 | 37 | <0.001 |
| Time × Group (Control) | 4.8 | −5.7 | 15 | 0.4 |
| Surgery | ||||
| Group (Control vs. PC-PEP) | −12 | −23 | −1.7 | 0.024 |
| Time (baseline vs. 6 months) | 43 | 32 | 54 | <0.001 |
| Time × Group (Control) | 4.3 | −10 | 19 | 0.6 |
| Radiation | ||||
| Group (Control vs. PC-PEP) | 10 | −3.4 | 24 | 0.14 |
| Time (baseline vs. 6 months) | 20 | 11 | 29 | <0.001 |
| Time × Group (Control) | 0.89 | −12 | 14 | 0.9 |
| EPIC Hormonal score | ||||
| Group (Control vs. PC-PEP) | −2.08 | −6.6 | 2.4 | 0.4 |
| Time (baseline vs. 6 months) | 4.6 | 1.4 | 7.7 | 0.005 |
| Time × Group (Control) | 1.3 | −3.2 | 5.7 | 0.6 |
| Surgery | ||||
| Group (Control vs. PC-PEP) | −5.9 | −12 | 0.32 | 0.063 |
| Time (baseline vs. 6 months) | −0.34 | −4.5 | 3.9 | 0.9 |
| Time × Group (Control) | 3.7 | −2.08 | 9.4 | 0.2 |
| Radiation | ||||
| Group (Control vs. PC-PEP) | 1.3 | −5.1 | 7.7 | 0.7 |
| Time (baseline vs. 6 months) | 8.4 | 4.01 | 13 | <0.001 |
| Time × Group (Control) | 0.24 | −6.3 | 6.8 | 0.9 |
| Tests of Fixed Effects | |||
|---|---|---|---|
| Average number of days of PFMF compliance per week | |||
| Time | 1 | 4.2 | 0.04 |
| Group | 1 | 2.0 | 0.16 |
| Group × Time | 1 | 1.08 | 0.3 |
| Average number of days of PFMF compliance per week 1 | |||
| Time | 1 | 3.9 | 0.052 |
| Group | 1 | 1.2 | 0.3 |
| Average daily duration in minutes of PFMT compliance per week | |||
| Time | 1 | 0.27 | 0.6 |
| Group | 1 | 3.2 | 0.08 |
| Group × Time | 1 | 2 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawen, T.; Ilie, G.; Mason, R.; Rendon, R.; Spooner, J.; Champion, E.; Davis, J.; MacDonald, C.; Kucharczyk, M.J.; Patil, N.; et al. Six-Month Prostate Cancer Empowerment Program (PC-PEP) Improves Urinary Function: A Randomized Trial. Cancers 2024, 16, 958. https://doi.org/10.3390/cancers16050958
Lawen T, Ilie G, Mason R, Rendon R, Spooner J, Champion E, Davis J, MacDonald C, Kucharczyk MJ, Patil N, et al. Six-Month Prostate Cancer Empowerment Program (PC-PEP) Improves Urinary Function: A Randomized Trial. Cancers. 2024; 16(5):958. https://doi.org/10.3390/cancers16050958
Chicago/Turabian StyleLawen, Tarek, Gabriela Ilie, Ross Mason, Ricardo Rendon, Jesse Spooner, Emmi Champion, Jessica Davis, Cody MacDonald, Michael J. Kucharczyk, Nikhilesh Patil, and et al. 2024. "Six-Month Prostate Cancer Empowerment Program (PC-PEP) Improves Urinary Function: A Randomized Trial" Cancers 16, no. 5: 958. https://doi.org/10.3390/cancers16050958
APA StyleLawen, T., Ilie, G., Mason, R., Rendon, R., Spooner, J., Champion, E., Davis, J., MacDonald, C., Kucharczyk, M. J., Patil, N., Bowes, D., Bailly, G., Bell, D., Lawen, J., Wilke, D., Kephart, G., & Rutledge, R. D. H. (2024). Six-Month Prostate Cancer Empowerment Program (PC-PEP) Improves Urinary Function: A Randomized Trial. Cancers, 16(5), 958. https://doi.org/10.3390/cancers16050958







