1. Introduction
Tumor cell heterogeneity is believed to play a significant role in the progression, metastasis, recurrence, and resistance to anti-tumor therapies [
1,
2,
3]. It is thought that tumors develop from normal cells, which are made up of homogeneous subpopulations with specific functions and limited lifespans [
4]. As the tumor progresses, the cells strive to acquire genotypic and phenotypic heterogeneities. Once the heterogeneity reaches a certain degree, the tumor becomes untreatable because it can now produce a wide range of progenies, some of which will always have advantages in surviving therapeutic insults. Elucidating the driving mechanism for heterogeneity progression may help us understand the causes of tumor progression and metastasis.
How do tumor cells become heterogeneous, specifically prostate tumor cells, which are somatic cells with an endodermal epithelium origin? How do they acquire phenotypic traits typically seen in lineages of mesoderm or the neural crest of the ectoderm? Two theories attempt to address the progression of heterogeneity, but neither fully explains the histopathologic changes observed in clinical tumors.
According to the mutator phenotype theory [
5,
6], tumor cells diversify from each other through successive mutations. However, it is now known that different cells in a tumor have highly heterogeneous mutations with no consensus or cumulative pattern [
7,
8]. On the other hand, the cancer stem cell theory [
9] suggests that heterogeneity arises from the differentiation of pluripotent cancer stem cells upon epigenetic regulation. This theory accounts for the self-renewal perpetuation and lineage plasticity [
10] of cancer cells. However, differentiation may lead to pleomorphism but not the complex aneuploidy and genomic heterogeneity seen in tumors [
11,
12]. The acquisition and progression of tumor cell heterogeneity are complex topics, and the underlying mechanisms are yet to be fully understood.
Alternatively, tumor cell heterogeneity can be induced by interacting with bystander cells, which are non-malignant cells in the tumor microenvironment. These bystander cells include mesenchymal stem/stromal cells, immune cells, endothelial cells, and neural cells, all of which cohabitate with invading tumor cells [
13]. The human prostate cancer LNCaP cells, which are androgen-dependent and non-tumorigenic, can become heterogeneous when co-inoculated [
14] or co-cultured [
15] with non-tumorigenic bone marrow-derived mesenchymal stem/stromal cells. This interaction results in the formation of LNCaP-lineage subclones with distinct genomic changes, androgen-independence, and acquired tumorigenicity. Concurrently, the interaction can convert adjacent bystander cells to malignant cells. Although isogeneity with cancer cells limits identification in clinical tumor specimens, the transformation of bystander cells in the tumor microenvironment can be readily detected in xenograft tumors, as stromal [
16,
17] or endothelial [
18] cells of a mouse host being recruited and reprogrammed into malignant cells.
In our study, we conducted co-cultures and found that LNCaP cells were fusogenic, capable of spontaneously fusing with bystander cell types such as bone marrow- or prostate-derived mesenchymal stem/stromal cells [
19] or neural stem cells [
20]. Most of these fusion hybrids died during the heterokaryon or synkaryon stage, but a few survived to proliferate into subclones with genotypic and phenotypic diversification. Cancer–bystander cell fusion, therefore, has the potential to introduce heterogeneity into the tumor cell population. Since cancer and bystander cells are known to co-evolve [
21], the spontaneity of fusogenicity signifies the reality of an incessant and dynamic heterogeneity progression, which is perhaps the most concerning outcome of cancer cell interaction with the tumor microenvironment.
Through previous studies, we have realized that bystander cells in fusion may have opposite functions. They can either phase out cancer cells by trapping them in the heterokaryon or synkaryon stage or promote tumor cell heterogeneity if the hybrids re-enter the cell cycle. Cancer–bystander cell fusion, therefore, plays intermediating roles in cancer progression and metastasis.
In this current study, we conducted additional co-cultures to investigate how bystander cells could promote the progression of tumor cell heterogeneity. We used the non-tumorigenic LNCaP [
19,
20] and HPE-15 [
22] human prostate cancer cell lines as study subjects and focused on tracking the heterogeneity progression of epithelial cell-like and non-tumorigenic HPE-15 prostate tumor cells through co-culture with prostate mesenchymal stromal cells. Compared to the normal parental control, spontaneously transformed stromal cells showed a significantly enhanced tropism toward HPE-15 heterogeneity progression. The pathophysiological status of bystander cells, therefore, is a determining factor in cancer progression and metastasis.
3. Results
LNCaP and HPE-15 cells share behaviors that are relevant to the early stages of human prostate cancer. As previously mentioned [
19,
20,
22], these cells do not form tumors in immunocompromised mice. However, when co-cultured with bystander cells or other cancer cells, LNCaP and HPE-15 cells become heterogeneous. Some of these cells acquire tumorigenic properties and display signs of metastasis in internal organs. We have identified fusion between cancer cells and bystander cells as a mechanism for heterogeneity progression [
19,
20]. Therefore, we conducted a study to investigate the effects of fusion on LNCaP
RFP and HPE-15
RFP cells when interacting with bystander stromal cells.
3.1. Spontaneous Transformation of Prostate Mesenchymal Stromal Cells
We used a cancer–stromal cell co-culture to model the interaction between LNCaP
RFP or HPE-15
RFP cells and six pair-matched prostate mesenchymal stromal cell lines derived from three prostate cancer patients [
23]. As a control, we used PrSC cells from a healthy donor. All seven stromal cell lines used in this study were between passages 31 and 35 since their original cloning. Our findings showed that approximately 20% of the LNCaP
RFP cells formed fusion hybrids with stromal cells within a week of co-culture, which was consistent with our previous reports [
19].
In contrast, HPE-15
RFP cells exhibited much higher fusogenicity. In fact, after just 24 h of co-culture, all individual HPE-15
RFP cells had fused with the stromal cells. These fusion hybrids appeared as large stromal cells emitting RFP fluorescence, mostly with two nuclei, which aligns with our previous descriptions. Unlike LNCaP
RFP cells, HPE-15
RFP cells exhibited a uniform epithelial cell morphology in cobblestone-like growth [
22]. Using this feature to track cellular heterogeneity, we focused on tracking morphologic changes to monitor heterogeneity progression in HPE-15
RFP cells.
Interestingly, after keeping the co-cultures for eight weeks to track the fate of the fusion hybrids, only the co-culture with HPS-11 cells resulted in hybrid-derived cells. These cells assumed a small stromal cell shape but emitted red fluorescence. At the end of the eight-week observation period, the co-culture with HPS-11 cells showed colonies expressing RFP in various morphologies, indicating that they were derivative clones from the fusion of cancer and stromal cells. In contrast, we found very few derivative clones in the HPS-10, HPS-12, HPS-13, HPS-14, HPS-15, or PrSC co-cultures. In these cases, the large hybrids with mesenchymal stromal morphology and RFP fluorescence remained senescent throughout the entire eight weeks, showing no signs of cell division.
After repeated studies, we concluded that, while all prostate stromal cells were highly susceptible to fusion with HPE-15RFP prostate cancer cells, the HPS-11 cells were unique in their ability to facilitate the proliferation of the hybrids into derivative clones.
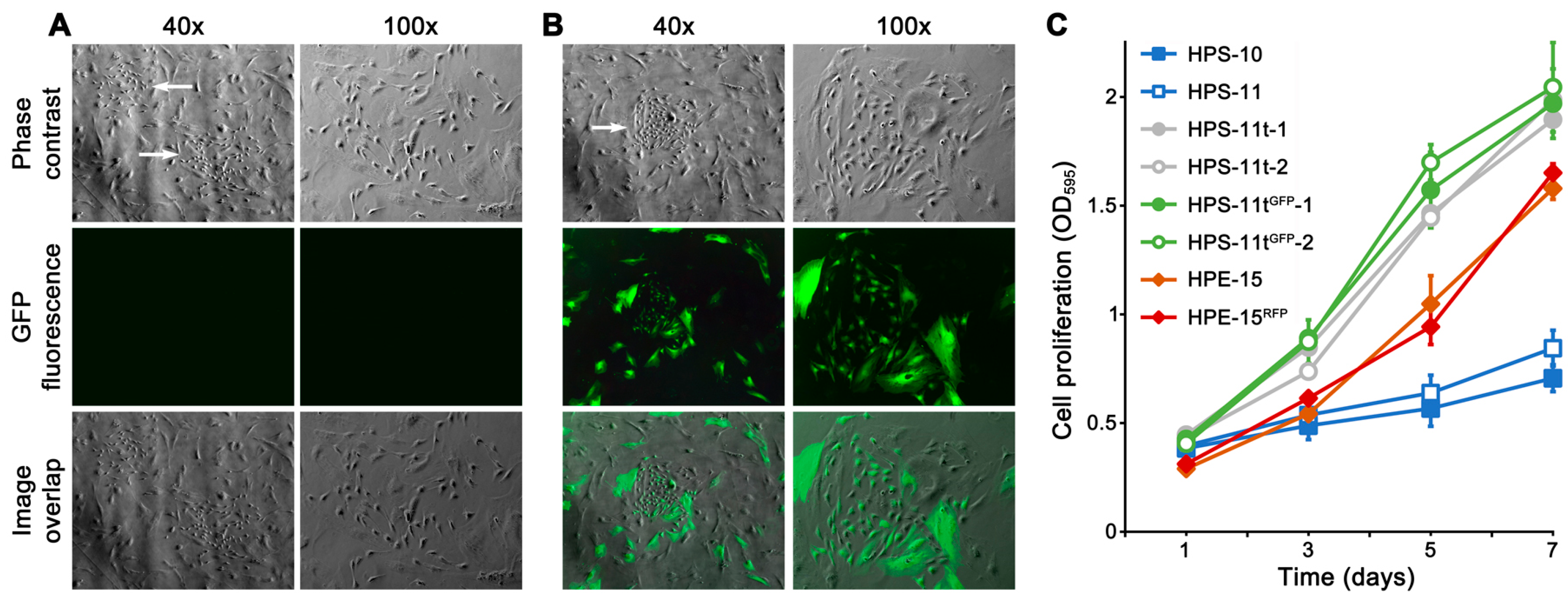
The HPS-11 cell line was isolated as a cancer-associated mesenchymal stromal cell line, paired with HPS-10, which was from the normal zone of the same tumor resection [
23]. Microscopic inspection revealed that the HPS-11 population between passages 31 and 35 consisted of both large and small stromal cells. This distinction became apparent when HPS-11 was replated at low density. While most of the population consisted of large, elongated cells with slow growth, a few small, spindle-shaped cells with accelerated proliferation were also present, forming colonies within 7 days (
Figure 1A,B). In co-culture experiments, it was observed that hybrid colonies were mostly derived from the fusion of HPE-15
RFP with these small stromal cells.
Historically, HPS-11 cells were isolated through cloning, and the small, spindle-shaped cells with a fast growth rate were not identifiable during the first 30 passages [
23]. These cells most likely emerged through spontaneous transformation during extended culture [
24,
25], although no transformation was observed in the other six stromal cell lines after similar passages. We named these transformed cells HPS-11t.
To verify the origin of the transformed cells, we isolated six HPS-11t clones from the low density HPS-11 culture. Another six clones were isolated from the limiting dilution cloning of the GFP-tagged HPS-11 cells and named HPS-11t
GFP clones. The HPS-11 origin of these clones was confirmed with STR profiles identical to the original HPS-11. The HPS-11t clones exhibited significantly faster growth rates compared to the original HPS-11 cells and even faster than the HPE-15 cells (
Figure 1C). Since these cells seemed to be responsible for the increased derivative hybrid colony formation, our focus in the subsequent study was to assess the role of HPS-11t cells.
3.2. Survival and Proliferation of Fusion Hybrids from the Co-Culture with HPS-11t Cells
We conducted co-cultures using HPE-15
RFP cells with either HPS-11t or HPS-11t
GFP clones to observe what happened to cancer–stromal fusion hybrids. Consistent findings were observed in all co-cultures, so we will present representative outcomes from the co-culture of HPE-15
RFP with HPS-11t
GFP-1. Both cell lines were obtained through limiting dilution cloning. The HPE-15
RFP cells had a typical epithelial appearance (
Figure 2A), while the HPS-11t
GFP-1 clone displayed a small spindle shape (
Figure 2B). With distinct fluorescence proteins, we were able to track the cancer–stromal interaction in real-time [
19].
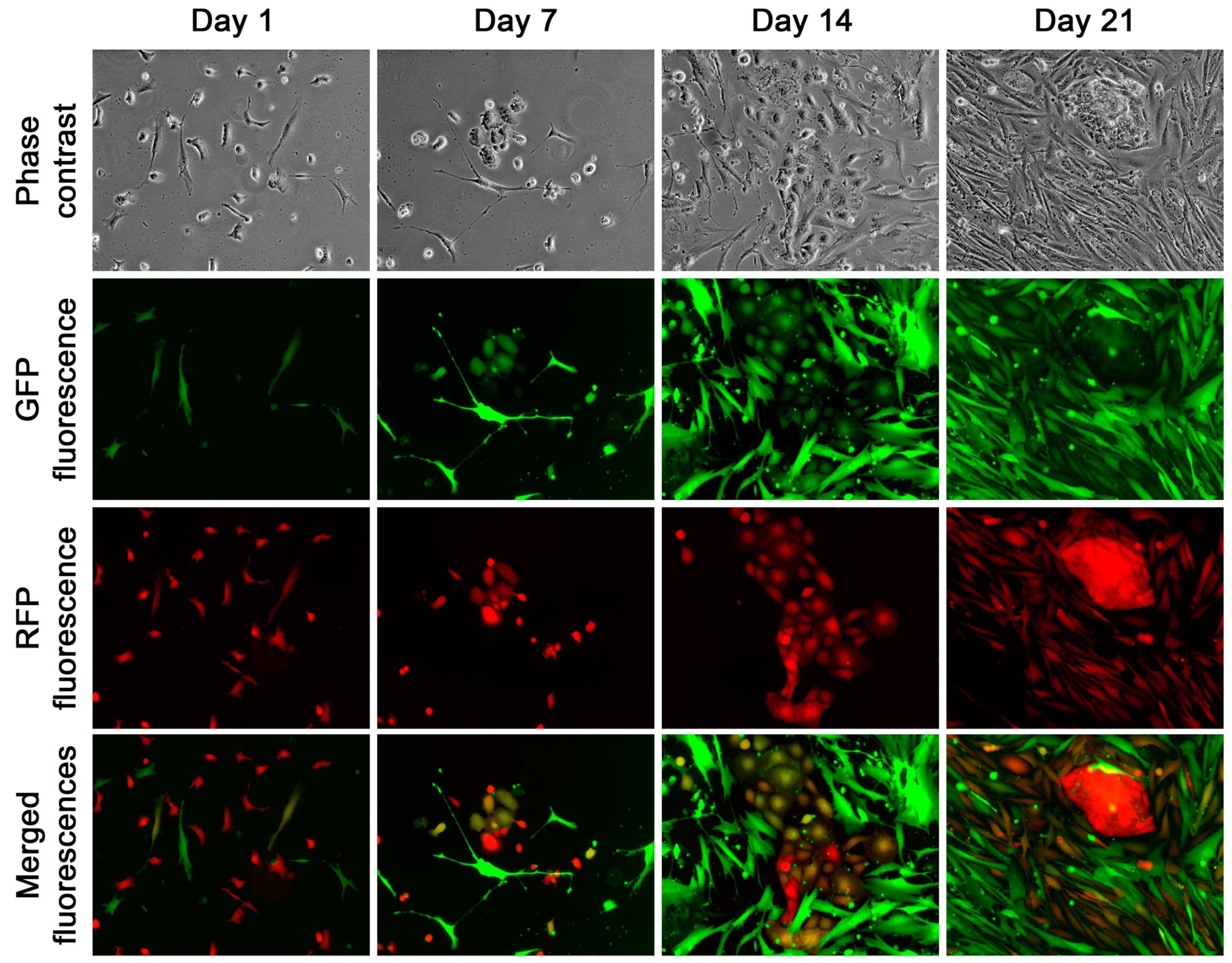
Real-time fluorescence microscopic tracking revealed rapid fusion between cancer and stromal cells, as cells with both red and green fluorescence could be seen as early as 12 h after the co-culture began (
Figure 3A). At this time, most of the hybrids appeared as small and rounded cells. Subsequently, hybrids with typical stromal morphology started to appear and became more frequent around 7 days of the co-culture (
Figure 3B). After that, the co-culture became more complex, with clusters of dual fluorescent cells in various shapes emerging in the epithelial HPE-15
RFP and stromal HPE-11t
GFP-1 co-culture. We continuously tracked this and found that it was caused by fusion hybrids that began to divide. Although the co-culture often contained dead cells with dual fluorescence, indicating hybrid death [
19,
20], the division of fusion hybrids in the HPE-15
RFP and HPS-11t
GFP-1 co-culture was equally effective. Within 21 days, the entire co-culture was scattered with clusters of fusion hybrid derivative cells (
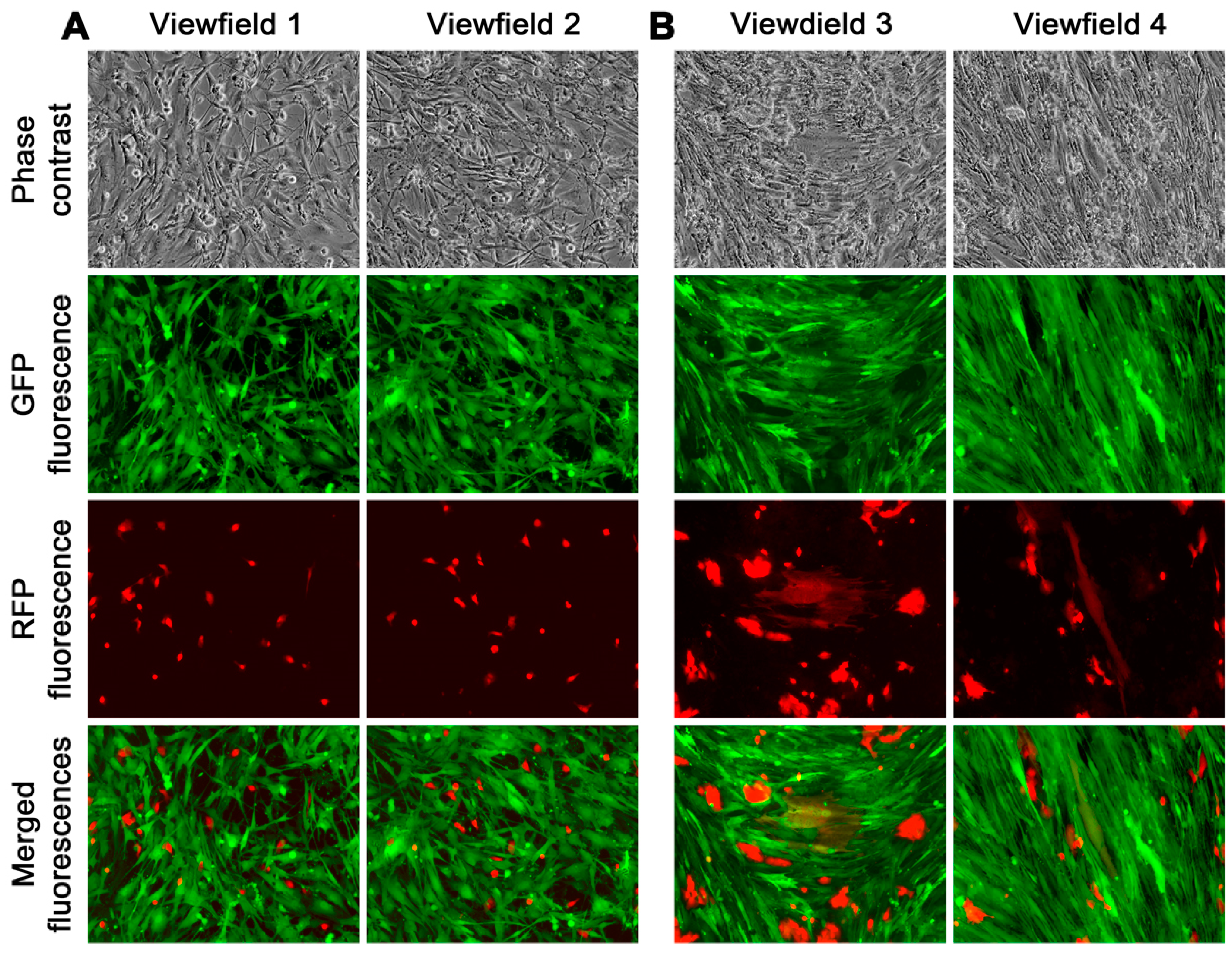
Figure 4A,B).
The derivative cells obtained from the fusion hybrids exhibited diverse morphologies and distinct growth behaviors. Some cells had an epithelial morphology, arranged in a cobble stone pattern, and had a slow growth rate (
Figure 4A). Other cells took on a stromal cell shape, forming colonies that grew rapidly and were scattered (
Figure 4B). Additionally, there were derivative cells that had transitional shapes, transitioning from epithelial to stromal cells, and displayed varying growth rates. The fusion of cancer and stromal cells, therefore, could give rise to hybrid progeny with diverse morphologies and growth behaviors.
The widespread proliferation of hybrids was confirmed through continued passaging with low density replating. Daily inspection revealed two distinct behaviors of the fusion hybrids. About 40% of the hybrids displayed limited cell division, undergoing a single or no division following replating. The hybrids with no division were generally large in size, with large singular or plural nuclei indicating growth arrest in the heterokaryon or synkaryon state. Similarly, the hybrids with a singular division were growth arrested as well. These hybrids remained in the two-cell state until the end of an entire 28-day culture period without signs of additional division (
Figure 5).
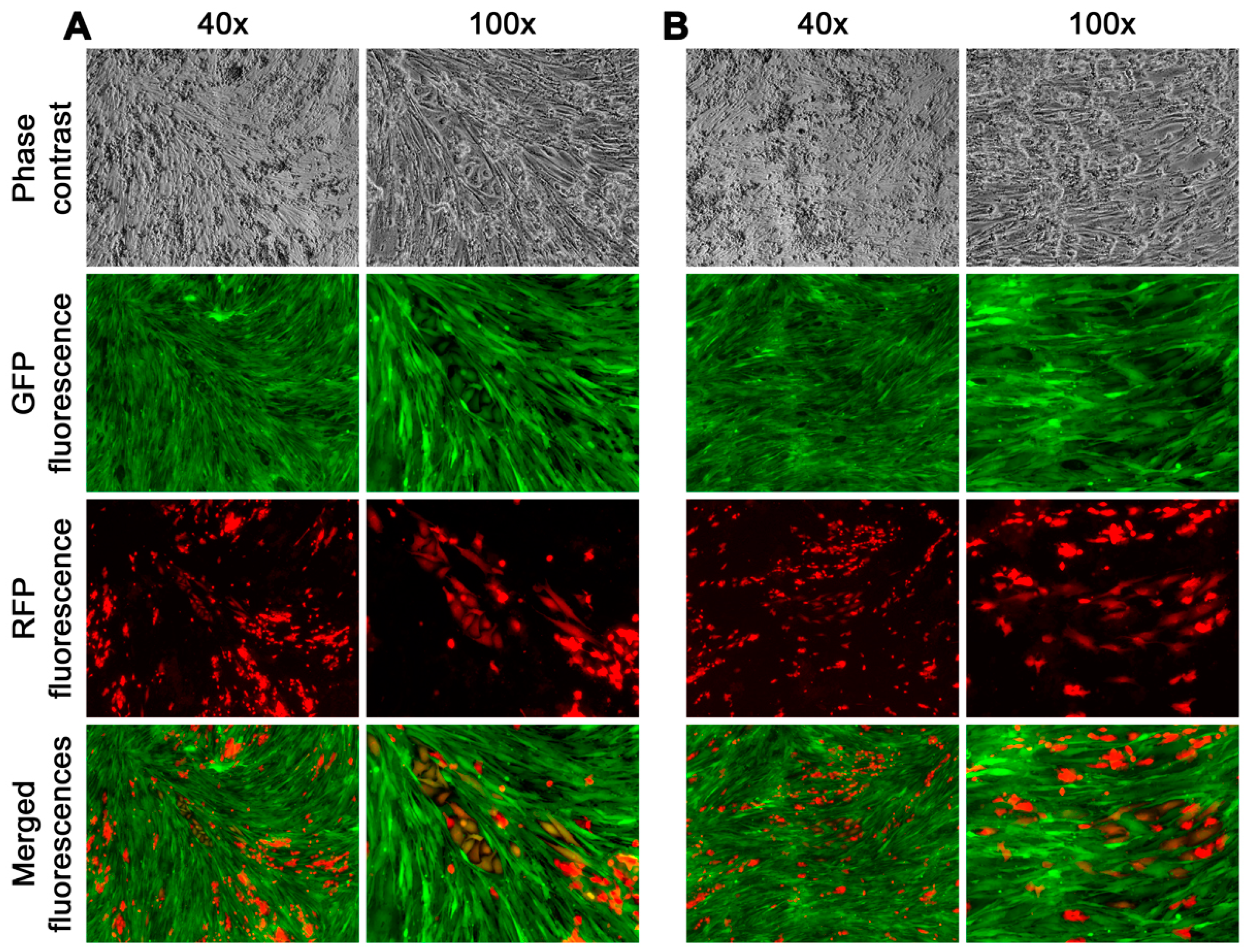
In contrast, about 60% of the fusion hybrids displayed the capability of repeated division, forming colonies individually with morphologies and growth behaviors that diversified from each other (
Figure 6).
These results demonstrated that, while some lacked replication capability, a substantial number of fusion hybrids from the HPE-15RFP and HPS-11tGFP-1 co-cultures could proliferate to form a derivative population with diversified morphology and growth behavior. Recognizing the implications for clinical cancer heterogeneity progression, we focused on this phenomenon by tracking the fate of fusion hybrids with unlimited proliferation.
3.3. Heterogeneity of the Fusion Hybrid Derivative Clones
Our co-culture of fluorescently tagged cells provides a unique opportunity to study the effects of cancer–bystander cell interaction. Our objective was to isolate derivative clones in order to measure heterogeneity among the individual fusion hybrids.
Derivative clones were isolated from low-density plating for further examination. A total of 192 dual red- and green-fluorescent clones (referred to as RG-clones) were picked and cultured for 30 continuous passages from a single HPE-15
RFP and HPS-11t
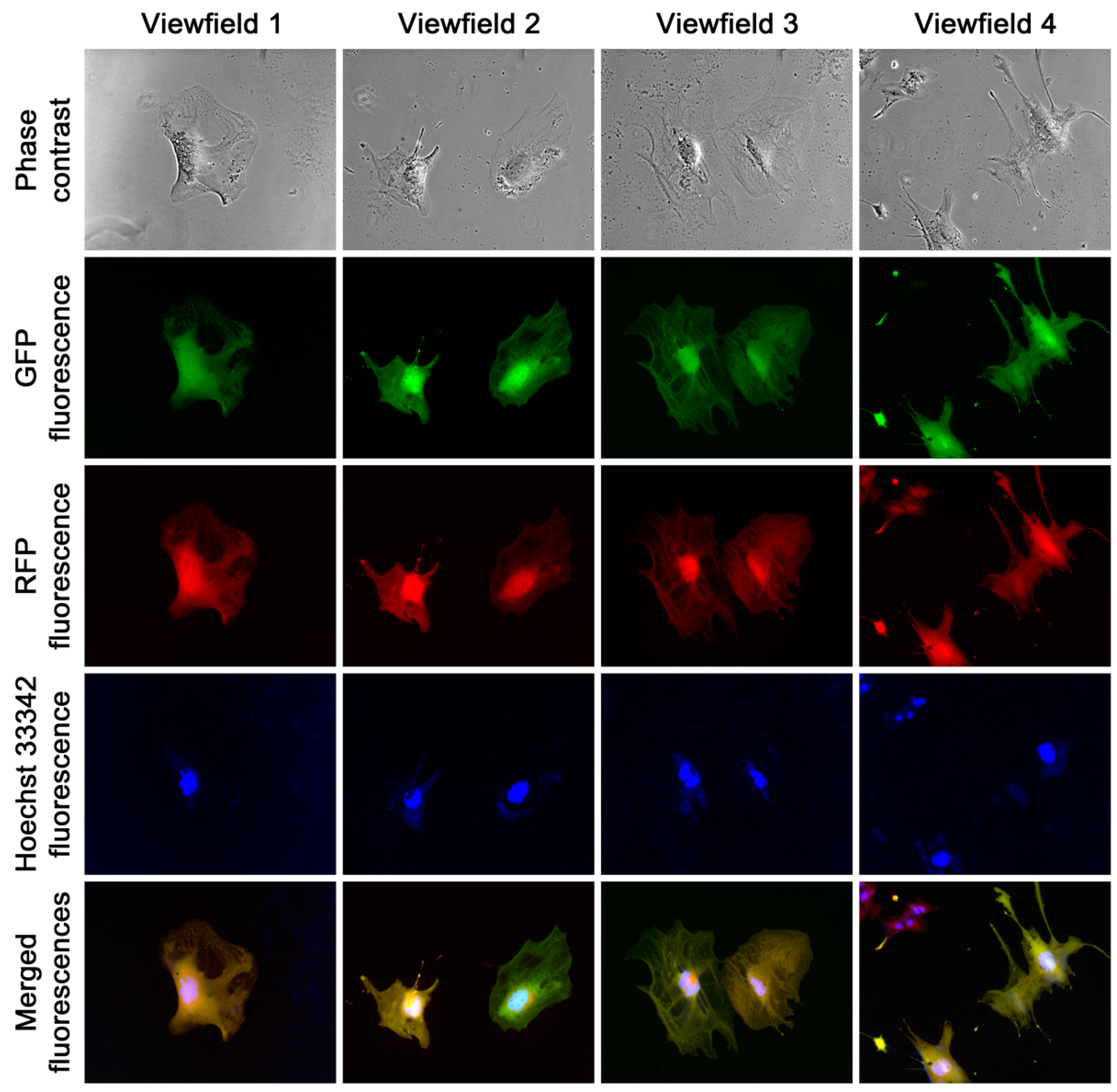
GFP-1 co-culture. These clones exhibited epithelial, stromal, or transitional morphologies to varying degrees. The analysis of this series of studies revealed a marked difference in growth rates. Clones with stromal or transitional shape displayed a stable morphology and exhibited fast proliferation. Epithelial clones in early passages showed noticeable instability, with individual cells in each clone exhibiting diversification in terms of cell size, nucleus sizes or numbers, and green or red fluorescence intensity (
Figure 7). Furthermore, the growth of epithelial clones was much slower compared to stromal clones. However, this instability was attenuated over time, as cells in epithelial clones became smaller and polykaryons became less frequent during successive passaging. After 10 passages, cells in the epithelial clones became predominantly uniform and all these clones survived the continuous passaging. Both phase contrast microscopic examination and Hoechst 33342 staining indicated the presence of a singular nucleus in most cells. These results demonstrated that a substantial number of the HPE-15
RFP and HPS-11t
GFP-1 fusion hybrids were able to perpetuate and propagate successfully.
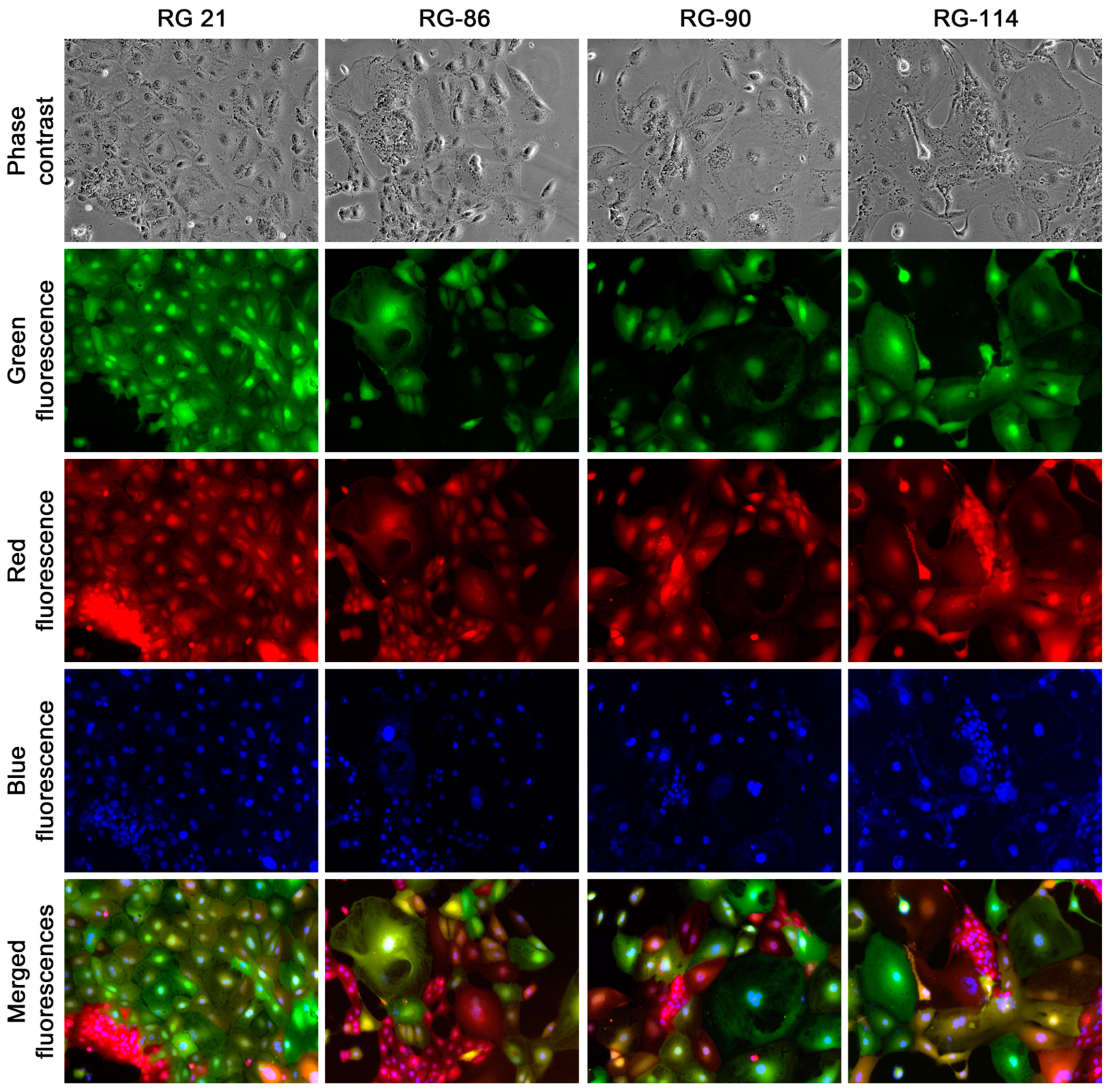
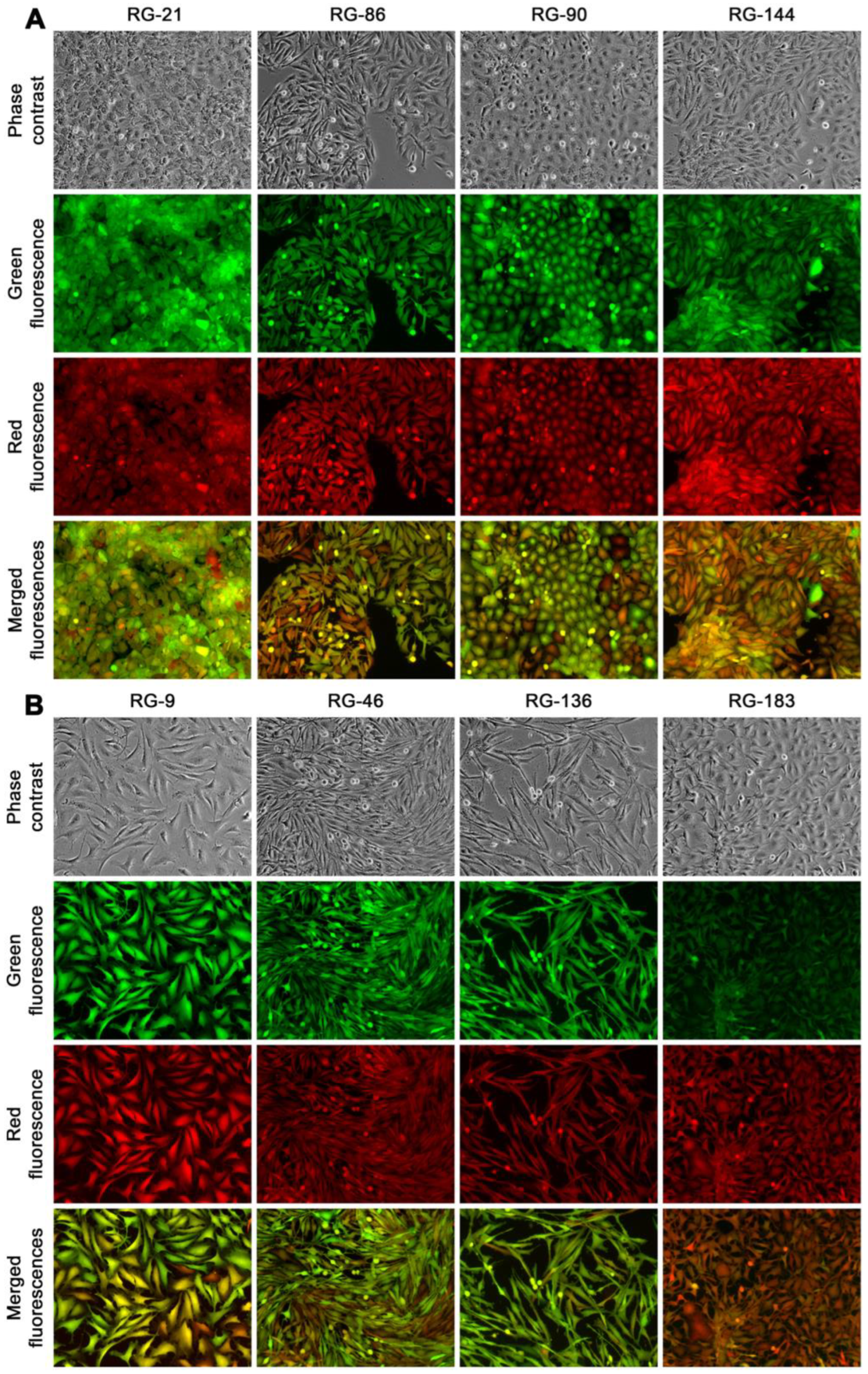
At passage 30, the clones were categorized into three groups based on their morphologic attributes: epithelial-like, transitional, and stromal. For further characterization, four clones were randomly selected from the epithelial group (RG-21, 86, 90, and 144,
Figure 8A), and another four were chosen from the stromal group (RG-9, 46, 136, and 183,
Figure 8B). All these randomly selected clones appeared to be unique, displaying distinctive phenotypic attributes and growth behaviors. This suggested a significant inter-clonal heterogeneity. These eight clones were used in the following studies.
To determine clonality and clonal stability, the eight clones were cultured continuously until passage 60, following the previously described method [
19]. The stromal clones took approximately eight months to culture from passage 30 to passage 60, using a replating ratio of 1:5. The epithelial clones, on the other hand, took about twelve months to reach the same passage. Throughout this culturing process, no changes in morphology or growth behavior were observed.
STR genotyping analyses were conducted to examine the hybridization between the HPE-15
RFP and HPS-11t
GFP-1 cells (
Table 1). Results showed multiple allelism in all the loci examined and in all eight randomly selected clones, indicating the presence of genomic materials from both parental cells. Meanwhile, there was extensive genomic heterogeneity, with individual clones displaying different combinations of allelic polymorphism. No clones were found to have an identical genotyping profile. This heterogeneity was so thorough that no correlation could be established between the genotypic profile and the phenotypic traits of the clones.
To evaluate the inheritance of fusogenicity, the eight clones were subjected to another round of co-culture. Once again, rapid and effective fusion was observed with the parental HPS-11tGFP-1 stromal cells. However, the fate of the fusion hybrids was not tracked, and the fusogenicity of the eight clones was not compared to their parental HPE-15RFP cancer cells.
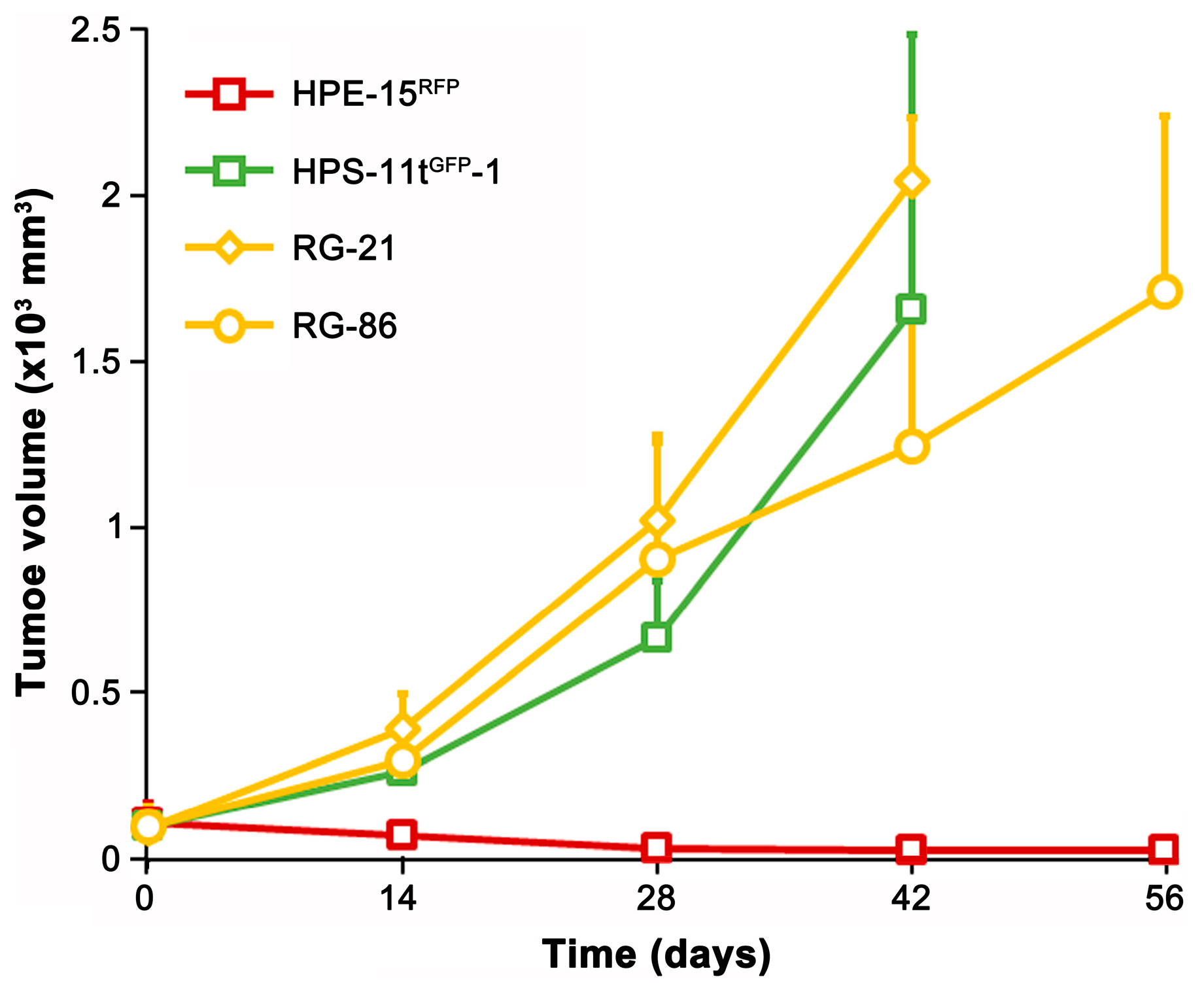
As a proof-of-concept study to assess tumor formation potential, we tested two epithelial clones (RLG-21 and RLG-86) for xenograft tumor formation. The parental HPE-15
RFP cells were non-tumorigenic [
22]. However, both derivative clones acquired tumorigenicity (
Figure 9). Interestingly, while the parental HPS-11 cells were non-tumorigenic [
23], the HPS-11t
GFP-1 clone formed subcutaneous tumors. Whether the tumorigenicity in the derivative clones is inherited from the transformed stromal cells or acquired through the process of cell fusion remains to be determined.
4. Discussion
This study investigated the acquisition of heterogeneity in HPE-15
RFP prostate tumor cells through fusion with mesenchymal stem/stromal cells. While fusion hybrids were common in the co-culture with all stromal cells evaluated, only the hybrids with transformed stromal cells proliferated into derivative clones. These clones displayed significant inter-clonal heterogeneities and retained the ability to produce more hybrid progenies in further co-culture. The observed genomic heterogeneity and cellular complexity were more extensive than our previous estimates, supporting the “dark matter” hypothesis [
26]. This hypothesis suggests that cell fusion in clinical tumors is frequent and dynamic but difficult to detect or quantify due to isogenicity between the involved cells. As cancer interacts with bystander cells in the tumor microenvironment and co-evolves with stromal cells [
21], the progression of heterogeneity induced by cell fusion must be a continuous process throughout the entire history of disease progression. The implications of our study will be carefully considered.
4.1. Transformed Bystander Cells Are More Permissive to Heterogeneity Progression
By tracking the formation and fate of fusion hybrids, we have established an experimental system to investigate the natural history of cancer–bystander cell fusion in real time. We have found that fusion with mesenchymal stem/stromal cells can have opposing effects [
19,
20]. It can either prevent the progression of heterogeneity by trapping cancer cells in the heterokaryon or synkaryon state or facilitate progression if the fusion hybrids manage to escape the trap and re-enter the cell cycle. Mesenchymal stem/stromal cells are known to be prone to spontaneous transformation, especially during long-term expansion [
24,
25]. In our comparative analysis, we have discovered that transformed stromal cells have a significantly increased capacity to facilitate the re-entry of fusion hybrids into the cell cycle. In clinical tumors, chronic interaction with tumor cells likely leads to the transformation of bystander mesenchymal stromal cells, which can be recruited through fusion with tumor cells and reprogrammed into heterogeneous constituents of the tumor cell compartment. This study identifies abnormalities in bystander cells as contributing factors to the progression of heterogeneity.
How did the transformed stromal cells facilitate the proliferation of fusion hybrids? Although the underlying mechanism remains to be elucidated, it is conceivable that the accelerated growth rate in transformed stromal cells is compatible with hybrid cell division. Transformed HPS-11t
GFP-1 cells exhibited enhanced proliferative activity, with a much faster growth rate than the parental HPS-11 stromal cells. Coincidentally, the transformed cells showed a remarkable capacity to facilitate the proliferation of fusion hybrids into derivative clones. Since the parental stromal cells grow much more slowly than the HPE-15
RFP cells, their fusion hybrids are controlled by two different cell cycle timings. The division of the hybrids becomes challenging due to dysregulated cytokinesis or karyokinesis, leading to growth arrest and mitotic catastrophe [
27]. However, in the co-culture with transformed stromal cells, both the cancer and stromal cells were actively proliferating, despite their cycles not being synchronized. As a result, the division of their fusion hybrids is now controlled by similar or compatible cytokinesis and karyokinesis rhythms, providing an opportunity for the hybrids to re-enter the cell cycle. The growth rate of bystanders is a critical determinant of the fate of fusion hybrids.
This proposition is supported by our unpublished observations from previous co-culture studies. While LNCaP prostate cancer cells could fuse with slow-growing bone marrow- or prostate-derived mesenchymal stromal cells, the fusion hybrids had limited chances to proliferate into derivative clones [
19]. In contrast, many derivative clones were observed from the LNCaP co-culture with fast-growing virally transformed HS-5 and HS-27a bone marrow-derived mesenchymal stromal cells [
28]. Further investigation is necessary to uncover the mechanism by which transformed stromal cells facilitate the proliferation of fusion hybrids.
4.2. Individual Fusion Events Are the Modular Units of Tumor Cell Heterogeneity
Our studies have revealed several clues suggesting that individual cell fusion events have the potential to introduce heterogeneity directly into the tumor population. The HPE-15 is a non-tumorigenic prostate tumor cell line with a karyotype that is more similar to virally transformed prostate epithelial cells than commonly used prostate cancer cell lines. It is intriguing that such a cell line can acquire tumorigenicity simply through cell–cell interaction [
22]. In addition to this study, our unpublished works have detected HPE-15
RFP fusion with other prostate cancer cells, in agreement with findings that malignancy can spread among tumor cells [
29,
30]. HPE-15 cells can also fuse with each other. These findings may explain the fact that, even in a newly established cancer cell line like HPE-15, individual cells have heterogeneous genomic makeups [
22], while the karyotype description for a given cancer cell line always includes structural and numerical variations [
31]. Cell fusion is a straightforward way for tumor cells to acquire heterogeneity.
Each fusion event has the potential to introduce unique heterogeneity to the tumor cell population. In the current study, one round of HPE-15
RFP co-culture with transformed stromal cells yielded abundant fusion hybrid progenies with extensive genomic hybridization and inter-clonal heterogeneity. All eight randomly selected clones were found to have multiple allelism, which is consistent with hyperdiploidy in clinical tumor cells. Although STR analysis cannot determine copy numbers, the presence of multiple allelism suggests a minimum doubling of the genome. About 60% of the fusion hybrids were able to undergo repeated cell division, which is a critical requirement for somatic mutation [
32]. Through cell division, heterokaryons and synkaryons undergo extensive genomic reorganization to reduce the chromosome number [
33], likely using a meiosis-like process of diploidization [
34], during which genetic recombination occurs at random locations [
35]. The inherent aneuploidy of the parental cancer cell, combined with chromosomal mismatches in the hybrid, makes the meiosis-like process incomplete and unstable, resulting in hybrid progenies with dynamic aneuploidy, perceived as genomic instability [
20]. At the same time, the spontaneity of fusogenicity inherited by hybrid progenies may drive cell fusion in the tumor microenvironment to yield more heterokaryons, perpetuating the dynamic aneuploidy throughout the clinical history of disease progression.
In clinical tumors, cancer cells may fuse with neighboring bystander cells. In the simplest scenario, somatic fusion involves a single cancer cell and a specific bystander cell. Although the cancer and bystander cells involved are isogenic, their resulting fusion hybrid becomes heterogeneous. This is because each fusion represents an isolated event of genomic hybridization. The subsequent genomic reorganization, diploidization, and genetic recombination take place within the hybrid, separate from any neighboring cells or fusion hybrids. Since recombination events are randomly distributed [
35], each fusion event creates a derivative clone with a mutated genomic makeup that is unique and distinct from other cancer–bystander cell fusion hybrids in the same tumor. The findings of this study suggest that individual fusion events may potentially contribute to the progression of tumor cell heterogeneity.
4.3. The Dynamic Heterogeneity Confounds Tumor Marker Identification
The aberrant reactivation of gene expression is a well-known phenomenon related to tumors and is a clear indication of dysregulated genetic control. Eukaryotic cells, including tumor cells, appear to be highly tolerant of reactivated gene expression. Except for a few lethality-related genes [
36] or those involved in synthetic lethality [
37], the reactivation and even overexpression of most protein-encoding genes do not affect the survival of tumor cells. Interestingly, although many non-housekeeping genes are found to be abnormally expressed in tumors, only a few can be defined as tumor markers and even fewer as companion biomarkers. This is because the candidate genes always fail to reach a consensus pattern of temporal or spatial expression in the majority of tumor cells [
38]. Few candidates can be scored with a prevalent expression or a close correlation to the progression of the disease through histopathologic quantification. The reactivation and overexpression of genes in cancers seem to be rather erratic or random [
39].
The results of this study suggest that heterogeneity is a result of random events at the level of individual tumor cells. The randomness and individuality make it impossible to profile tumor cell heterogeneity with a consensus pattern. We propose that the spontaneity of fusogenicity in cancer–bystander cell fusion, combined with the randomness of subsequent genetic recombination, may explain the confounding issue in tumor marker identification. Gene expression and protein production are controlled by various biochemical mechanisms, which cause expression noises and fluctuations due to the stochastic nature of biochemical reactions with finite substrate molecules [
40,
41,
42]. Even in normal tissues, the expression of a gene among individual cells is often heterogeneous. Gene expression and protein levels can be transiently increased or decreased by epigenetic regulation and other non-mutational mechanisms [
43]. In fusion hybrid derivative cells, the expression heterogeneity could only be strengthened, while the complete genomic heterogeneity and prevalent multiple allelism may cause drastic changes in gene expression. Most importantly, although a given gene is regulated by numerous factors at different levels, the output of its expression is inherently binary, limited to an “on” or “off” mode in an all-or-none fashion [
44]. Instead of widespread expression, a completely dysregulated marker gene would be “on” in 50% of the tumor cell population. A combinatorial marker analysis will only decrease the positivity score because, in cancer cells, each marker expression is abnormally regulated by independent mechanisms, following the multiplication rule of probability.
4.4. Limitations of the Study
This study has suggested that, compared to their parental cells, transformed prostate stromal cells are more effective in promoting the proliferation of cancer–stromal fusion hybrid progenies in the tumor microenvironment. These hybrid progenies exhibit a high level of heterogeneity in terms of genotype and phenotype. Heterogeneity has the potential to evolve if cancer cells continue to fuse. Heterogeneous hybrids may acquire additional cancer hallmarks [
45] and be positively selected. Further research is needed to fully understand the clinical significance of cancer–bystander cell fusion, while the treatment of tumor progression and metastasis through inhibiting spontaneous cancer–bystander cell fusion remains to be tested.
One perplexing observation in this study is the choice between growth arrest and proliferation. While transformed stromal cells are more supportive than parental stromal cells, approximately 40% of the fusion hybrids displayed limited division, while the remaining 60% exhibited unrestricted proliferation. Comparative studies are needed to explore the underlying mechanism and identify the determinants that are essential for tumor cell survival and proliferation.
Fusion with HPS-11tGFP-1 cells resulted in a significant number of derivative clones, whereas fusion with non-transformed prostate stromal cells did not produce any hybrid progenies. This contrasts with previous studies where fusion between RFP-tagged LNCaP cells and non-transformed stromal cells did generate hybrid derivative clones, although with limited success. Both LNCaP and HPE-15 are non-tumorigenic prostate cancer cell lines. The cell fusion mechanism may be constitutively expressed in HPE-15RFP cells, which facilitates rapid (less than 24 h of co-culture) and complete (100% of the HPE-15RFP cells) fusion with stromal cells. On the other hand, the slow (over 48 h of co-culture) and incomplete (around 20% of the cells) fusion observed with RFP-tagged LNCaP cells indicates an induced mechanism. Comparative analyses are required to determine the mechanism responsible for cancer–stromal cell fusion.
While a large number of derivative hybrid clones were obtained from the fusion between HPE-15RFP and HPS-1tGFP-1 cells, the heterogeneity of these clones has only been characterized in terms of clonal morphology, growth behavior, and genotyping. The extent of phenotypic heterogeneity among the clones needs to be evaluated. Representative clones should be tested for their ability to form xenograft tumors and their sensitivity to anti-tumor drug treatments. Comparative analyses should be conducted to demonstrate the impact of prostate cancer cell fusion with transformed stromal cells on the progression and metastasis of the disease.
It is important to note that this work is mostly observational and qualitative, as few quantitative parameters on the cancer–bystander cell fusion were analyzed. Future studies will aim to perform a molecular and pathophysiological characterization of fusion-derived cancer cells.