Association of Lymphovascular Invasion with Lymph Node Metastases in Prostate Cancer—Lateralization Concept
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Surgical Technique
2.2. Histopathological Examination
2.3. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Unilateral and Bilateral Lymphovascular Invasion
3.3. Unilateral Left and Unilateral Right Lymphovascular Invasion
3.4. Odds Ratios and Patient Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antunes, A.A.; Srougi, M.; Dall’Oglio, M.F.; Crippa, A.; Paranhos, M.; Cury, J.; Nesrallah, L.J.; Leite, K.R. Microvascular Invasion Is an Independent Prognostic Factor in Patients with Prostate Cancer Treated with Radical Prostatectomy. Int. Braz. J. Urol. 2006, 32, 668–675. [Google Scholar] [CrossRef]
- De La Taille, A.; Rubin, M.A.; Buttyan, R.; Olsson, C.A.; Bagiella, E.; Burchardt, M.; Wellisch, O.M.; Katz, A.E. Is Microvascular Invasion on Radical Prostatectomy Specimens a Useful Predictor of PSA Recurrence for Prostate Cancer Patients? Eur. Urol. 2000, 38, 79–84. [Google Scholar] [CrossRef]
- Herman, C.M.; Wilcox, G.E.; Kattan, M.W.; Scardino, P.T.; Wheeler, T.M. Lymphovascular Invasion as a Predictor of Disease Progression in Prostate Cancer. Am. J. Surg. Pathol. 2000, 24, 859–863. [Google Scholar] [CrossRef]
- Shariat, S.F.; Khoddami, S.M.; Saboorian, H.; Koeneman, K.S.; Sagalowsky, A.I.; Cadeddu, J.A.; McConnell, J.D.; Holmes, M.N.; Roehrborn, C.G. Lymphovascular Invasion Is a Pathological Feature of Biologically Aggressive Disease in Patients Treated with Radical Prostatectomy. J. Urol. 2004, 171, 1122–1127. [Google Scholar] [CrossRef]
- Loeb, S.; Roehl, K.A.; Yu, X.; Antenor, J.A.V.; Han, M.; Gashti, S.N.; Yang, X.J.; Catalona, W.J. Lymphovascular Invasion in Radical Prostatectomy Specimens: Prediction of Adverse Pathologic Features and Biochemical Progression. Urology 2006, 68, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, W.; Li, X.; Xu, D.; Liu, L. Prognostic Value of Lymphovascular Space Invasion in Stage IA to IIB Cervical Cancer: A Meta-Analysis. Medicine 2023, 102, E33547. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, L.; Guan, F.; Wang, F.; Zhang, G. Prognostic Value of Lymphovascular Invasion in Upper Urinary Tract Urothelial Carcinoma after Radical Nephroureterectomy: A Systematic Review and Meta-Analysis. Dis. Markers 2019, 2019, 7386140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, D.; Gong, M.; Wen, L.; Liao, C.; Zou, L. High Lymphatic Vessel Density and Presence of Lymphovascular Invasion Both Predict Poor Prognosis in Breast Cancer. BMC Cancer 2017, 17, 335. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhang, L.; Wu, B.; Zha, Z.; Zhao, H.; Jun, Y.; Jiang, Y. The Impact of Lymphovascular Invasion in Patients with Prostate Cancer Following Radical Prostatectomy and Its Association with Their Clinicopathological Features: An Updated PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2018, 97, e13537. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.; Mahmud, A.; Bass, B.; Brundage, M. Prognostic Significance of Lymphovascular Invasion in Radical Prostatectomy Specimens. BJU Int. 2012, 110, 1507–1514. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, H.; Pan, X.W.; Xu, D.F.; Cui, X.G.; Chen, J.; Hong, Y.; Gao, Y.; Yin, L.; Ye, J.Q.; et al. The Prognostic Value of Lymphovascular Invasion in Radical Prostatectomy: A Systematic Review and Meta-Analysis. Asian J. Androl. 2016, 18, 780–785. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, H.; Yan, L.; Ye, T.; Lu, H.; Sun, X.; Ye, Z.; Xu, H. Prognostic Significance of Six Clinicopathological Features for Biochemical Recurrence after Radical Prostatectomy: A Systematic Review and Meta-Analysis. Oncotarget 2018, 9, 32238–32249. [Google Scholar] [CrossRef]
- Schiavina, R.; Gacci, M.; Briganti, A.; Imbimbo, C.; Simonato, A.; Borghesi, M.; Capitanio, U.; Brunocilla, E.; Martorana, G.; Carini, M.; et al. Can Side-Specific Biopsy Findings Predict the Side of Nodal Metastasis in Clinically Localized Prostate Cancer? Results from a Multicenter Prospective Survey. Eur. J. Surg. Oncol. 2013, 39, 1019–1024. [Google Scholar] [CrossRef]
- Martini, A.; Wever, L.; Soeterik, T.F.W.; Rakauskas, A.; Fankhauser, C.D.; Grogg, J.B.; Checcucci, E.; Amparore, D.; Haiquel, L.; Rodriguez-Sanchez, L.; et al. Unilateral Pelvic Lymph Node Dissection in Prostate Cancer Patients Diagnosed in the Era of Magnetic Resonance Imaging-Targeted Biopsy: A Study That Challenges the Dogma. J. Urol. 2023, 210, 117–126. [Google Scholar] [CrossRef]
- Małkiewicz, B.; Bugla, B.; Czarnecki, M.; Karwacki, J.; Długosz, P.; Gurwin, A.; Kiełb, P.; Lemiński, A.; Krajewski, W.; Jędrzejuk, D.; et al. Diagnostic Value of Radio-Guided Sentinel Node Detection in Patients with Prostate Cancer Undergoing Radical Prostatectomy with Modified-Extended Lymphadenectomy. Cancers 2022, 14, 5012. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA; American Joint Commission on Cancer: Chicago, IL, USA, 2017. [Google Scholar]
- Van Leenders, G.J.L.H.; Van Der Kwast, T.H.; Grignon, D.J.; Evans, A.J.; Kristiansen, G.; Kweldam, C.F.; Litjens, G.; McKenney, J.K.; Melamed, J.; Mottet, N.; et al. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2020, 44, E87–E99. [Google Scholar] [CrossRef] [PubMed]
- Yee, D.S.; Shariat, S.F.; Lowrance, W.T.; Maschino, A.C.; Savage, C.J.; Cronin, A.M.; Scardino, P.T.; Eastham, J.A. Prognostic Significance of Lymphovascular Invasion in Radical Prostatectomy Specimens. BJU Int. 2011, 108, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Kryvenko, O.N.; Epstein, J.I. Histologic Criteria and Pitfalls in the Diagnosis of Lymphovascular Invasion in Radical Prostatectomy Specimens. Am. J. Surg. Pathol. 2012, 36, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B.; Chen, H.J. A Comparative Study of Various Tests for Normality. J. Am. Stat. Assoc. 1968, 63, 1343. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, T.Y.; Kim, W.B.; Gong, G.; Kim, S.C.; Hong, S.J.; Shong, Y.K. Lymphovascular Invasion Is Associated with Lateral Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma. Laryngoscope 2006, 116, 2081–2085. [Google Scholar] [CrossRef]
- Ryu, Y.J.; Kang, S.J.; Cho, J.S.; Yoon, J.H.; Park, M.H. Identifying Risk Factors of Lateral Lymph Node Recurrence in Clinically Node-Negative Papillary Thyroid Cancer. Medicine 2018, 97, e13435. [Google Scholar] [CrossRef]
- Masui, T.; Adachi, S.; Uemura, H.; Kimura, T.; Kitahara, T. Risk Factors for the Lateral Cervical Lymph Node Metastasis of Papillary Thyroid Carcinoma: A Clinical Study. Mol. Clin. Oncol. 2023, 18, 25. [Google Scholar] [CrossRef]
- Caliskan, O. Predictive Factors Affecting the Development of Lateral Lymph Node Metastasis in Papillary Thyroid Cancer. SiSli Etfal Hastan. Tip Bul./Med. Bull. Sisli Hosp. 2023, 57, 312–319. [Google Scholar] [CrossRef]
- Miccio, J.A.; Verma, V.; Kelly, J.; Kann, B.H.; An, Y.; Park, H.S.; Eskander, A.; Burtness, B.; Husain, Z. Impact of Contralateral Lymph Nodal Involvement and Extranodal Extension on Survival of Surgically Managed HPV-Positive Oropharyngeal Cancer Staged with the AJCC Eighth Edition. Oral. Oncol. 2019, 99, 104447. [Google Scholar] [CrossRef]
- Contrera, K.J.; Huang, A.T.; Shenson, J.A.; Tang, C.; Roberts, D.; Myers, J.N.; Weber, R.S.; Lai, S.Y.; Williams, M.; El-Hallal, M.; et al. Primary and Recurrent Regional Metastases for Lateralized Oral Cavity Squamous Cell Carcinoma. Surg. Oncol. 2022, 44, 101804. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.F.; Hizuta, A.; Iwagaki, H.; Tanaka, N.; Orita, K. Lateral Lymph Node Dissection for Rectal Carcinoma below the Peritoneal Reflection. Br. J. Surg. 1994, 81, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Chang, J.S.; Yoon, H.I.; Jang, D.S.; Kim, N.K.; Lim, J.S.; Min, B.S.; Huh, H.; Shin, S.J.; Ahn, J.B.; et al. Mapping of Lateral Pelvic Lymph Node Recurrences in Rectal Cancer: A Radiation Oncologist’s Perspective. J. Cancer Res. Clin. Oncol. 2018, 144, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Furrer, M.A.; Mulholland, C.J.; Katsios, A.; Soliman, C.; Lawrentschuk, N.; Peters, J.S.; Zargar, H.; Costello, A.J.; Hovens, C.M.; et al. Lymphovascular Invasion at the Time of Radical Prostatectomy Adversely Impacts Oncological Outcomes. Cancers 2024, 16, 123. [Google Scholar] [CrossRef]
- Rakic, N.; Jamil, M.; Keeley, J.; Sood, A.; Vetterlein, M.; Dalela, D.; Arora, S.; Modonutti, D.; Bronkema, C.; Novara, G.; et al. Evaluation of Lymphovascular Invasion as a Prognostic Predictor of Overall Survival after Radical Prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 495.e1–495.e6. [Google Scholar] [CrossRef]
- Jamil, M.; Rakic, N.; Sood, A.; Keeley, J.; Modonutti, D.; Novara, G.; Jeong, W.; Menon, M.; Rogers, C.G.; Abdollah, F. Impact of Lymphovascular Invasion on Overall Survival in Patients With Prostate Cancer Following Radical Prostatectomy: Stage-per-Stage Analysis. Clin. Genitourin. Cancer 2021, 19, e319–e325. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kawakami, S.; Yonese, J.; Fujii, Y.; Ohkubo, Y.; Suyama, T.; Komai, Y.; Kijima, T.; Ishikawa, Y.; Fukui, I. Lymphovascular Invasion Is an Independent Predictor of Prostate-Specific Antigen Failure after Radical Prostatectomy in Patients with PT3aN0 Prostate Cancer. Int. J. Urol. 2008, 15, 895–899. [Google Scholar] [CrossRef]
- Kang, M.; Oh, J.J.; Lee, S.; Hong, S.K.; Lee, S.E.; Byun, S.S. Perineural Invasion and Lymphovascular Invasion Are Associated with Increased Risk of Biochemical Recurrence in Patients Undergoing Radical Prostatectomy. Ann. Surg. Oncol. 2016, 23, 2699–2706. [Google Scholar] [CrossRef]
- Małkiewicz, B.; Kiełb, P.; Kobylański, M.; Karwacki, J.; Poterek, A.; Krajewski, W.; Zdrojowy, R.; Szydełko, T. Sentinel Lymph Node Techniques in Urologic Oncology: Current Knowledge and Application. Cancers 2023, 15, 2495. [Google Scholar] [CrossRef]
- Wit, E.M.K.; Acar, C.; Grivas, N.; Yuan, C.; Horenblas, S.; Liedberg, F.; Valdes Olmos, R.A.; van Leeuwen, F.W.B.; van den Berg, N.S.; Winter, A.; et al. Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy. Eur. Urol. 2017, 71, 596–605. [Google Scholar] [CrossRef]
- Harrison, S.H.; Seale-Hawkins, C.; Schum, C.W.; Kay Dunn, J.; Scardino, P.T. Correlation between Side of Palpable Tumor and Side of Pelvic Lymph Node Metastasis in Clinically Localized Prostate Cancer. Cancer 1992, 69, 750–754. [Google Scholar] [CrossRef][Green Version]
- Małkiewicz, B.; Kiełb, P.; Karwacki, J.; Czerwińska, R.; Długosz, P.; Lemiński, A.; Nowak, Ł.; Krajewski, W.; Szydełko, T. Utility of Lymphadenectomy in Prostate Cancer: Where Do We Stand? J. Clin. Med. 2022, 11, 2343. [Google Scholar] [CrossRef] [PubMed]
- Fossati, N.; Willemse, P.P.M.; Van den Broeck, T.; van den Bergh, R.C.N.; Yuan, C.Y.; Briers, E.; Bellmunt, J.; Bolla, M.; Cornford, P.; De Santis, M.; et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef] [PubMed]
- Preisser, F.; Marchioni, M.; Nazzani, S.; Bandini, M.; Tian, Z.; Pompe, R.S.; Montorsi, F.; Saad, F.; Abdollah, F.; Steuber, T.; et al. The Impact of Lymph Node Metastases Burden at Radical Prostatectomy. Eur. Urol. Focus. 2019, 5, 399–406. [Google Scholar] [CrossRef]
- EAU Guidelines. Edn. In Proceedings of the EAU Annual Congress Milan 2023, Milan, Italy, 10–13 March 2023; EAU Guidelines Office: Arnhem, The Netherlands. [Google Scholar]
- National Comprehensive Cancer Network. Guidelines: Prostate Cancer; 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459 (accessed on 28 December 2023).
- Lestingi, J.F.P.; Guglielmetti, G.B.; Trinh, Q.D.; Coelho, R.F.; Pontes, J.; Bastos, D.A.; Cordeiro, M.D.; Sarkis, A.S.; Faraj, S.F.; Mitre, A.I.; et al. Extended Versus Limited Pelvic Lymph Node Dissection During Radical Prostatectomy for Intermediate- and High-Risk Prostate Cancer: Early Oncological Outcomes from a Randomized Phase 3 Trial. Eur. Urol. 2021, 79, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Karwacki, J.; Stodolak, M.; Nowak, Ł.; Kiełb, P.; Krajewski, W.; Szydełko, T.; Lemiński, A.; Małkiewicz, B. Preoperative Factors for Lymphovascular Invasion in Prostate Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 856. [Google Scholar] [CrossRef]
- Jeet, V.; Parkinson, B.; Song, R.; Sharma, R.; Hoyle, M. Histopathologically Validated Diagnostic Accuracy of PSMA-PET/CT in the Primary and Secondary Staging of Prostate Cancer and the Impact of PSMA-PET/CT on Clinical Management: A Systematic Review and Meta-Analysis. Semin. Nucl. Med. 2023, 53, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, P.; Ghezzo, S.; Spataro, A.; Bezzi, C.; Samanes Gajate, A.M.; Chiti, A.; Picchio, M. Systematic Review and Metanalysis on the Role of Prostate-Specific Membrane Antigen Positron Emission Tomography/Magnetic Resonance Imaging for Intraprostatic Tumour Assessment. Magn. Reson. Imaging Clin. N. Am. 2023, 31, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Buckle, T.; Brouwer, O.R.; Valdés Olmos, R.A.; Van Der Poel, H.G.; Van Leeuwen, F.W.B. Relationship between Intraprostatic Tracer Deposits and Sentinel Lymph Node Mapping in Prostate Cancer Patients. J. Nucl. Med. 2012, 53, 1026–1033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Briganti, A.; Suardi, N.; Capogrosso, P.; Passoni, N.; Freschi, M.; Di Trapani, E.; Gallina, A.; Capitanio, U.; Abdollah, F.; Tutolo, M.; et al. Lymphatic Spread of Nodal Metastases in High-Risk Prostate Cancer: The Ascending Pathway from the Pelvis to the Retroperitoneum. Prostate 2012, 72, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Swanson, G.P.; Hubbard, J.K. A Better Understanding of Lymphatic Drainage of the Prostate with Modern Imaging and Surgical Techniques. Clin. Genitourin. Cancer 2013, 11, 431–440. [Google Scholar] [CrossRef]
- Park, J.M.; Charnsangavej, C.; Yoshimitsu, K.; Herron, D.H.; Robinson, T.J.; Wallace, S. Pathways of Nodal Metastasis from Pelvic Tumors: CT Demonstration. Radiographics 1994, 14, 1309–1321. [Google Scholar] [CrossRef]


| Clinicopathological Data | All Patients (n = 96) |
|---|---|
| Age | 64.3 ± 6.8; 64.5 (41–78) |
| Preoperative PSA | 27.9 ± 25.8; 22.0 (2.3–174) |
| cT stage | |
| cT1 | 3 (3.1%) |
| cT2 | 52 (54.2%) |
| cT3 | 37 (38.5%) |
| cT4 | 4 (4.2%) |
| Biopsy GGG | |
| 1 | 17 (17.7%) |
| 2 | 22 (22.9%) |
| 3 | 22 (22.9%) |
| 4 | 17 (17.7%) |
| 5 | 18 (18.8%) |
| pT stage | |
| pT2a | 1 (1.0%) |
| pT2c | 5 (5.2%) |
| pT3a | 14 (14.6%) |
| pT3b | 76 (79.2%) |
| Pathological GGG | |
| 1 | 0 (0.0%) |
| 2 | 13 (13.5%) |
| 3 | 30 (31.3%) |
| 4 | 12 (12.5%) |
| 5 | 41 (42.7%) |
| Number of removed LNs | 21.5 ± 10.5; 20.0 (5–74) |
| Number of positive LNs | 4.2 ± 4.7; 3 (1–30) |
| % of positive LNs | 19.5 ± 17.2%; 13.4% (2–100%) |
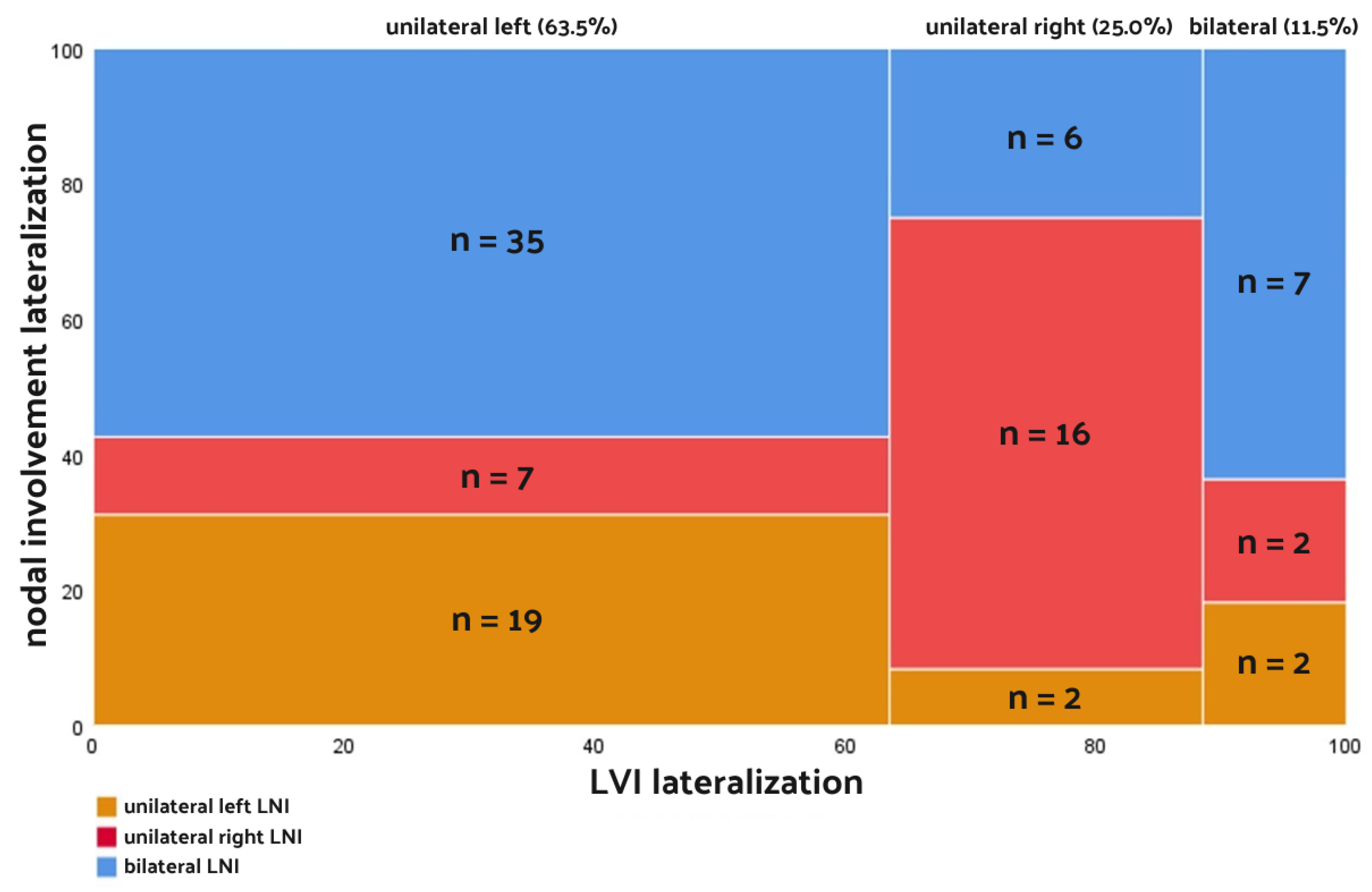
| LVI laterality | |
| Unilateral | 85 (88.5%) |
| Bilateral | 11 (11.5%) |
| LNI laterality | |
| Unilateral | 48 (50.0%) |
| Bilateral | 48 (50.0%) |
| Clinicopathological Data | Patients with Unilateral LVI (n = 85) | Patients with Bilateral LVI (n = 11) |
|---|---|---|
| Age | 64.6 ± 6.5; 64.6 (42–78) | 61.3 ± 8.6; 64.2 (41–71) |
| Preoperative PSA | 28.1 ± 24.8; 22.9 (2.3–174.0) | 26.3 ± 33.7; 14.3 (7.9–124.9) |
| Clinical T stage | ||
| cT1 | 3 (3.5%) | 0 (0.0%) |
| cT2 | 45 (52.9%) | 7 (63.6%) |
| cT3 | 34 (40.0%) | 3 (27.3%) |
| cT4 | 3 (3.5%) | 1 (9.1%) |
| Biopsy GGG | ||
| 1 | 17 (20.0%) | 0 (0.0%) |
| 2 | 17 (20.0%) | 5 (45.5%) |
| 3 | 18 (21.2%) | 4 (36.4%) |
| 4 | 16 (18.8%) | 1 (9.1%) |
| 5 | 17 (20.0%) | 1 (9.1%) |
| Laterality | ||
| Left | 61 (71.8%) | - |
| Right | 24 (28.2%) | - |
| Pathological T stage | ||
| pT2a | 1 (1.2%) | 0 (0.0%) |
| pT2c | 5 (5.9%) | 0 (0.0%) |
| pT3a | 14 (16.5%) | 0 (0.0%) |
| pT3b | 65 (76.5%) | 11 (100.0%) |
| Pathological GGG | ||
| 1 | 0 (0.0%) | 0 (0.0%) |
| 2 | 12 (27.1%) | 1 (9.1%) |
| 3 | 23 (27.1%) | 7 (63.6%) |
| 4 | 11 (12.9%) | 1 (9.1%) |
| 5 | 39 (45.9%) | 2 (18.2%) |
| Number of removed LNs | 21.2 ± 10.9; 20.0 (5–74) | 23.5 ± 7.2; 24.0 (12–35) |
| Number of positive LNs | 4.1 ± 5.0; 3.0 (1–30) | 4.4 ± 2.7; 5.0 (1–9) |
| % of positive LNs | 19.7 ± 18.0%; 13.3% (2.4–100%) | 17.7 ± 9.2%; 20.0% (4.5–31.6%) |
| Clinicopathological Data | Patients with Unilateral Left LVI (n = 61) | Patients with Unilateral Right LVI (n = 24) | p-Value |
|---|---|---|---|
| Age | 64.6 ± 6.4; 64.9 (42.2–76.8) | 64.8 ± 6.8; 63.8 (54.2–78.0) | 0.792 |
| Preoperative PSA | 30.2 ± 27.4; 24.2 (2.3–174.0) | 22.9 ± 15.9; 19.7 (4.4–76.0) | 0.287 |
| Clinical T stage | 0.463 | ||
| cT1 | 3 (4.9%) | 0 (0.0%) | |
| cT2 | 31 (50.8%) | 14 (58.3%) | |
| cT3 | 24 (39.3%) | 10 (41.7%) | |
| cT4 | 3 (4.9%) | 0 (0.0%) | |
| Biopsy GGG | 0.143 | ||
| 1 | 9 (14.8%) | 8 (33.3%) | |
| 2 | 14 (23.0%) | 3 (12.5%) | |
| 3 | 13 (21.3%) | 5 (20.8%) | |
| 4 | 10 (16.4%) | 6 (25.0%) | |
| 5 | 15 (24.6%) | 2 (8.3%) | |
| Pathological T stage | 0.047 | ||
| pT2a | 0 (0.0%) | 1 (4.2%) | |
| pT2c | 2 (3.3%) | 3 (12.5%) | |
| pT3a | 8 (13.1%) | 6 (25.0%) | |
| pT3b | 51 (83.6%) | 14 (58.3%) | |
| Pathological GGG | 0.464 | ||
| 1 | 0 (0.0%) | 0 (0.0%) | |
| 2 | 8 (13.1%) | 4 (16.7%) | |
| 3 | 14 (23.0%) | 9 (37.5%) | |
| 4 | 9 (14.8%) | 2 (8.3%) | |
| 5 | 30 (49.2%) | 9 (37.5%) | |
| Number of removed LNs | 21.5 ± 10.2; 20.0 (5–67) | 20.5 ± 12.6; 18.5 (9–74) | 0.379 |
| Number of positive LNs | 4.3 ± 4.5; 3.0 (1–23) | 3.8 ± 6.2; 2.0 (1–30) | 0.069 |
| % of positive LNs | 20.8% ± 18.5%; 16.7% (2.4–100%) | 16.9% ± 16.7%; 10% (3.6–61.1%) | 0.135 |
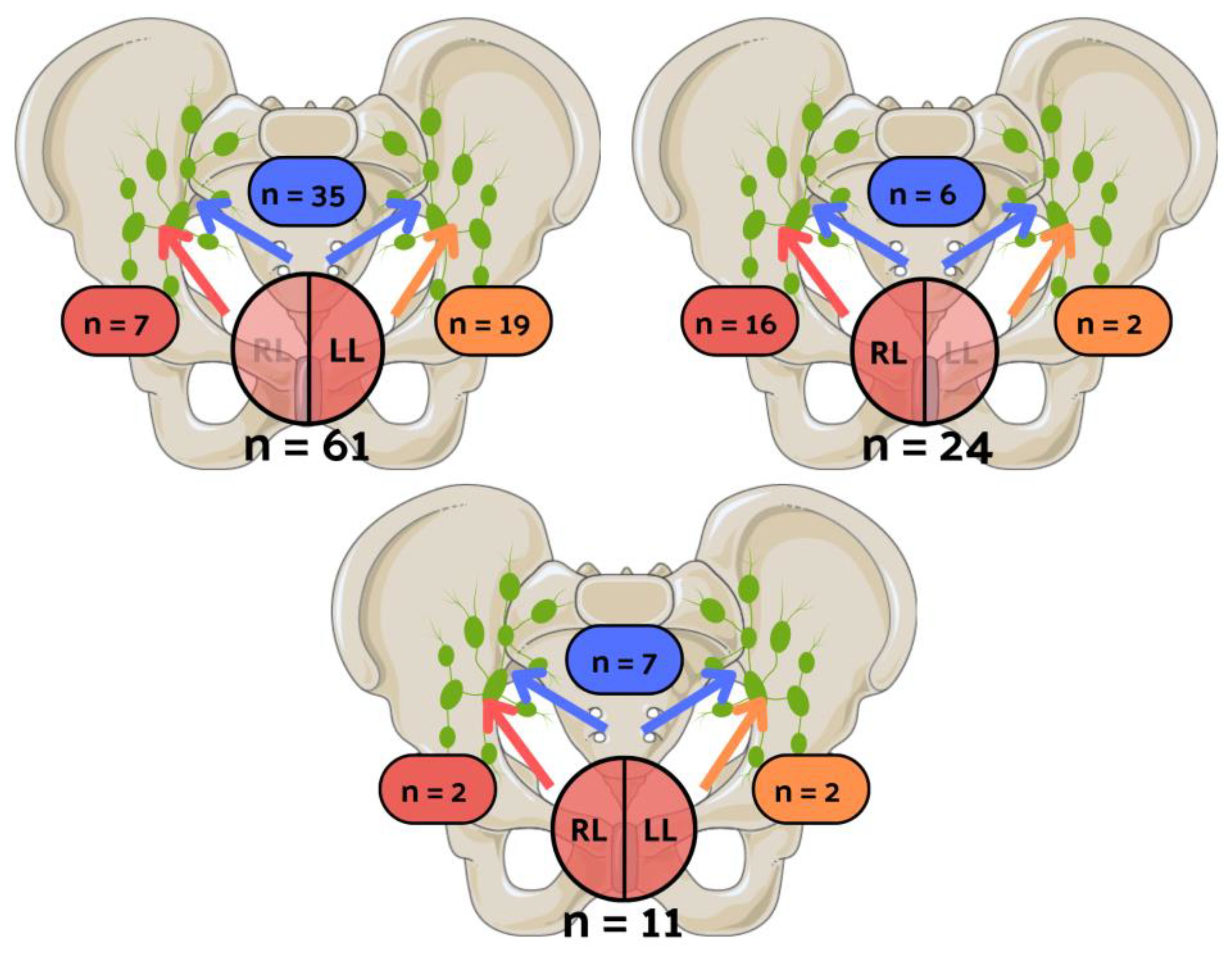
| LNI laterality | <0.001 | ||
| Unilateral left | 19 (31.1%) | 2 (8.3%) | |
| Unilateral right | 7 (11.5%) | 16 (66.7%) | |
| Bilateral | 35 (56.4%) | 6 (25.0%) |
| LNI Laterality | Patients with Unilateral Left LVI (n = 61) | OR (95% CI) | Patients with Unilateral Right LVI (n = 24) | OR (95% CI) |
|---|---|---|---|---|
| Unilateral left | 19 (31.1%) | 3.609 (0.925–14.077) | 2 (8.3%) | 0.725 (0.579–0.908) |
| Unilateral right | 7 (11.5%) | 0.185 (0.092–0.374) | 16 (66.7%) | 2.862 (1.531–5.348) |
| Bilateral | 35 (57.4%) | 2.795 (1.231–6.348) | 6 (25.0%) | 0.692 (0.525–0.913) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwacki, J.; Gurwin, A.; Jaworski, A.; Jarocki, M.; Stodolak, M.; Dłubak, A.; Szuba, P.; Lemiński, A.; Kaczmarek, K.; Hałoń, A.; et al. Association of Lymphovascular Invasion with Lymph Node Metastases in Prostate Cancer—Lateralization Concept. Cancers 2024, 16, 925. https://doi.org/10.3390/cancers16050925
Karwacki J, Gurwin A, Jaworski A, Jarocki M, Stodolak M, Dłubak A, Szuba P, Lemiński A, Kaczmarek K, Hałoń A, et al. Association of Lymphovascular Invasion with Lymph Node Metastases in Prostate Cancer—Lateralization Concept. Cancers. 2024; 16(5):925. https://doi.org/10.3390/cancers16050925
Chicago/Turabian StyleKarwacki, Jakub, Adam Gurwin, Arkadiusz Jaworski, Michał Jarocki, Marcel Stodolak, Andrzej Dłubak, Przemysław Szuba, Artur Lemiński, Krystian Kaczmarek, Agnieszka Hałoń, and et al. 2024. "Association of Lymphovascular Invasion with Lymph Node Metastases in Prostate Cancer—Lateralization Concept" Cancers 16, no. 5: 925. https://doi.org/10.3390/cancers16050925
APA StyleKarwacki, J., Gurwin, A., Jaworski, A., Jarocki, M., Stodolak, M., Dłubak, A., Szuba, P., Lemiński, A., Kaczmarek, K., Hałoń, A., Szydełko, T., & Małkiewicz, B. (2024). Association of Lymphovascular Invasion with Lymph Node Metastases in Prostate Cancer—Lateralization Concept. Cancers, 16(5), 925. https://doi.org/10.3390/cancers16050925









