Simple Summary
Adrenocortical carcinoma (ACC) is a rare and aggressive cancer. There are few treatment options for ACC. Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of different cancers. In this systematic review and meta-analysis, we reviewed studies that used ICIs in the treatment of ACC. We found that the use of ICIs has a modest efficacy but a good safety profile in ACC. In addition, the use of either single-agent ICIs or ICI combinations did not differ in terms of the tumor response. The use of ICIs also showed promising overall survival benefits. We recommend conducting more studies to determine the best candidates for ICI treatment in ACC.
Abstract
Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of different malignancies. However, their efficacy in advanced adrenocortical carcinoma (ACC) remains uncertain. Thus, we conducted a systematic review and meta-analysis to summarize the efficacy and tolerability of ICIs in patients with advanced ACC. We searched PubMed, Scopus, and CENTRAL for studies that used ICIs in ACC. Studies with more than five patients were included in the meta-analysis of the objective response rate (ORR), disease control rate (DCR), overall survival (OS), progression-free survival (PFS), and grade 3/4 adverse events. Twenty studies with 23 treatment arms and 250 patients were included. Single-agent anti-PD1 or anti-PD-L1 treatment was utilized in 13 treatment arms, whereas an anti-PD1 or anti-PD-L1 and anti-CTLA4 combination was used in 4 treatment arms. Other anti-PD1- or anti-PD-L1-based combinations were used in five treatment arms. The ORR was 14% (95% CI = 10–19%, I2 = 0%), and the DCR was 43% (95% CI = 37–50%, I2 = 13%). The combination anti-PD1- or anti-PD-L1-based treatment strategies did not correlate with higher responses compared with monotherapy. The median OS was 13.9 months (95% CI = 7.85–23.05), and the median PFS was 2.8 months (95% CI = 1.8–5.4). ICIs have a modest efficacy in advanced ACC but a good OS. Further studies are needed to investigate predictive biomarkers for ICI response and to compare ICI-based strategies with the current standard of care.
1. Introduction
Adrenocortical carcinoma (ACC) is a rare and highly aggressive tumor arising from the cortex of the adrenal gland [1]. The 5-year survival rates for stage I and stage II are 80% and 60%, respectively. Unfortunately, the detection of ACC in the early stages is not common, and it is usually detected in rather advanced stages, where the 5-year survival rate is less than 15% among patients with metastatic disease [2]. Surgery is the only curative approach to treating localized ACC, but the recurrence rates remain high despite optimal resection [3,4]. Currently, the treatment of metastatic ACC is based on mitotane, which inhibits adrenal steroid production and has a cytotoxic effect on the cancerous adrenal cells. It is the only drug approved by both the US Food and Drug Administration (FDA) and the European Medicine Agencies (EMA) for the treatment of ACC [5]. A combination of mitotane with Etoposide, Doxorubicin, and Cisplatin (EDP) showed a better progression-free survival (PFS) and response rate compared to mitotane and streptozocin in the First International Randomized Trial in Locally Advanced and Metastatic Adrenocortical Carcinoma Treatment (FIRM-ACT) study, the first ever randomized controlled trial on ACC with a large sample size of 304 subjects [3,6]. The median PFS was significantly longer in patients receiving mitotane with EDP compared to patients treated with mitotane and streptozocin (5 vs. 2.1 months, p < 0.001). Interestingly, there was no significant difference in the overall survival (14.8 months vs. 12.0 months, respectively) between the two treatment arms. Both regimens had similar serious adverse event rates and a similar quality of life. Thus, mitotane with EDP became the standard of care in patients with extensive disease ineligible for surgery [7].
ACC is sometimes associated with Lynch syndrome, the presence of one or more mutations in DNA mismatch repair genes [8]. Mutations in these genes have been linked to a superior response to immune checkpoint inhibitors (ICIs) in multiple carcinomas [9,10]. ICIs enhance the immune system to kill tumor cells by blocking the interaction between immune checkpoints, namely PD-1 and CTLA-4, and their ligands. ICIs are being used or investigated in various tumors either as single agents or in combination with other treatments, such as chemotherapy, radiotherapy, or targeted therapy. Figure 1 illustrates the immune microenvironment of ACC and its interaction with some of the other targeted therapies, including lenvatinib and mitotane [11,12,13]. Despite the efficacy of ICI and ICI-based combination therapies, their role in rare tumors like ACC is heterogeneous and less well established [14]. For example, ICIs have been effective in Merkel cell carcinoma and cutaneous squamous cell carcinoma, while they have not been as effective in low-grade neuroendocrine tumors [14,15]. In ACC, Raj. et al. conducted a phase II clinical trial of pembrolizumab in 39 ACC patients, with an impressive median overall survival of 24.9 months [16]. Out of the nine patients that had a meaningful cancer response to pembrolizumab, seven patients had a microsatellite-stabile disease. On the other hand, Le Tourneau et al. investigated the use of avelumab, an anti-PD-L1 treatment, in 50 patients with ACC [17]. The median overall survival was 10.6 months with only three patients achieving a partial response as their best response. In previous trials, FDA-approved biomarkers of immunotherapy such as PD-L1 levels, tumor mutation burden (TMB), and microsatellite stability status were not predictive of the ICI response.
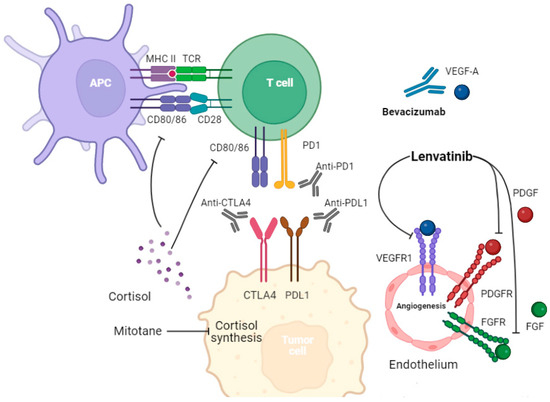
Figure 1.
The interaction between immune cells, endothelium, and tumor cells highlights possible treatment strategies. Tumor cells express PD-L1 and CTLA-4, which inhibit the immune system. Endogenous cortisol excess can decrease antigen presentation, inhibit T cell activation, decrease CD8+ T cell infiltration, weaken immune attack, and cause potential immunotherapy failure. Mitotane works by inhibiting the CYP11A1, CYP11B1, CYP11B2, and 3β-HSD enzymes, leading to decreased cortisol secretion. Targeting angiogenesis through multikinase inhibitors can also be helpful in controlling disease. In addition to inhibiting angiogenesis, lenvatinib can alter the immune tumor microenvironment.
Unfortunately, there are few treatment options, variable responses, and a scarcity of randomized clinical trials in this disease space; hence, there is an urgent need to find novel therapeutic options for these patients. Considering all of these points, we conducted a systematic review and meta-analysis to assess the efficacy and safety of using immune checkpoint inhibitors in patients with ACC.
2. Methodology
2.1. Search Strategy
This study protocol was registered via PROSPERO (ID: CRD42022303733). This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The PubMed, Scopus, and CENTRAL electronic databases were searched for articles published from their inception up to 28 September 2022 and later updated to up to 6 October 2023. In addition, we searched the ASCO annual meeting library (2014–2022), ESMO annual congress and special meetings (2014–2022), and the European Network for the Study of Adrenal Tumors (ENSAT) 2022 meeting. The references and new citations of the included studies were also screened to ensure no missed studies. The primary endpoints were the objective response rate (ORR), disease control rate (DCR), overall survival (OS), and progression-free survival (PFS). The secondary endpoints were adverse events. The main search terms used in the databases search were the following: “adrenocortical”, “immune checkpoint inhibitors”, “ipilimumab”, “nivolumab”, “pembrolizumab”, “avelumab”, “cemiplimab”, “durvalumab”, “tremelimumab”, “atezolizumab”, “spartalizumab”, “immunotherapy”. The full search algorithms are reported in Table S1. Two reviewers independently appraised the eligibility criteria and extracted the data.
2.2. Eligibility Criteria
We included case reports, case series, retrospective studies, cohort studies, and clinical trials. Studies had to report at least one primary outcome to be included. Non-English studies and studies that used radiotherapy as part of an immunotherapy combination were excluded. Studies with less than 5 patients were excluded from the meta-analysis.
2.3. Quality Assessment
Two reviewers independently assessed the risk of bias in each study. Discrepancies between the reviewers’ judgments throughout the process were resolved according to discussions with a third reviewer. The methodological index for non-randomized studies (MINORS) tool was used for case series, non-randomized controlled trials, and single-arm clinical trials [18]. The MINORS evaluation criteria items that we used to assess the quality of the studies included (1) a clear aim, (2) the inclusion of patients in a consecutive manner, (3) prospectively collected clinical data, (4) appropriate endpoints, (5) unbiased assessment of endpoints, (6) an appropriate follow-up time, (7) less than 5% loss during follow-up, and (8) calculation of the study size before study initiation. In the case of single-arm studies, the following items were not applicable: (1) a suitable control arm, (2) contemporary groups, (3) baseline similarity between arms, and (4) appropriate statistical analyses. The items were scored 0 if not reported, 1 if reported but inadequate, or 2 if reported and adequate. Thus, a total score would be 16 for single-arm studies and 24 points for comparative studies. We considered scores of 12 points or more high-quality with a low risk of bias; scores between 8 and 12 points at medium risk of bias; and scores of 8 points or less at high risk of bias.
We used the Joanna Briggs Institute (JBI) critical appraisal tools for the case reports [19]. Items in the tool were scored either “yes”, “no”, or “not clear”. All items are shown in the table. The total risk of bias was considered “high-risk” when “yes” was the answer for less than 50% of the questions for a study, “moderate” if “yes” was the answer for 50–69% of questions, and “low-risk” if “yes” answers were more than 70%.
2.4. Statistical Analyses
The ORR, DCR, PR, SD, and PD incidence rates were calculated using proportional meta-analysis. The degree of statistical heterogeneity between studies was evaluated using the I2 statistic. A fixed-effects model was employed if there was no statistical heterogeneity (I2 < 50%) across the studies. A random-effects model was utilized if there was statistical heterogeneity (I2 ≥ 50%) between studies. The publication bias was represented using funnel plots and statistically evaluated using both Egger’s and Begg’s tests. The META package from R software version 4.2.1 (https://www.R-project.org (accessed on 13 November 2023)) was used to analyze the data and build the figures. For pooling the OS and PFS, we used the median of medians methods performed using ‘metamedian’ as described by McGrath et al. [20]. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Search Results and Characteristics of the Included Trials
Our systematic search retrieved a total of 2451 reports. After removing duplicates and checking the titles and abstracts, 25 articles were identified. Among these, 20 unique studies with 22 treatment arms were included (Figure 2). There were eight phase II trials [16,21,22,23,24,25,26,27], two phase I trials [17,28], three retrospective cohorts [29,30,31], one case series [32], and six case reports [33,34,35,36,37,38], with a total of 248 ACC patients. Monotherapy of an anti-PD-L1 or anti-PD1 treatment was used in 12 treatment arms. An anti-PD1/anti-PD-L1 and anti-CTLA4 combination was used in four treatment arms, and one study had most of its patients receiving an anti-PD-L1 or anti-PD1 treatment, while 4% received an anti-PD-L1 or anti-PD1 with anti-CTLA4 combination. An anti-PD-L1 or anti-PD1 treatment was used with other agents in five treatment arms. Since most of the patients in the Remde et al. study [31] received single-agent ICIs, the study was considered a monotherapy ICI study in subsequent meta-analyses. The characteristics of the included studies are summarized in Table 1.
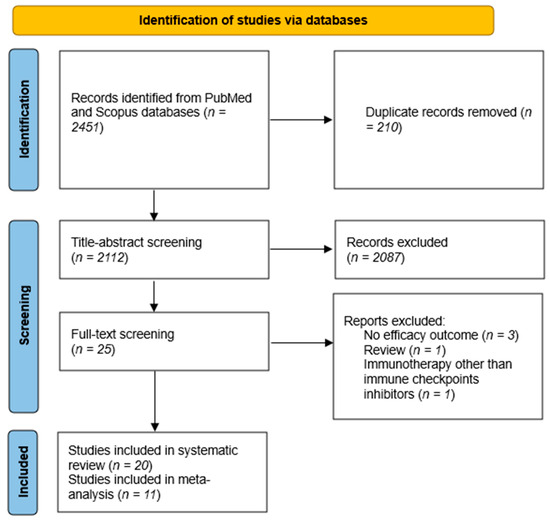
Figure 2.
PRISMA flow diagram. After applying our search strategy, 2112 unique reports were found. A total of 20 reports satisfied our eligibility criteria, of which 11 studies had sufficient data to be included in our meta-analysis.

Table 1.
Included studies characteristics. *: These studies included multiple tumors, and data regarding ACC were not separated. †: High PDL1 was defined as PD-L1 protein levels of 1% or more. ACC: adrenocortical carcinoma, MSI-H/MMR-D: microsatellite-instability-high/mismatch repair-deficient, NR: not reported.
3.2. Risk of Bias Assessment
All studies evaluated using the MINORS tool scored > 12 and thus were regarded to be at a low risk of bias except for three studies with a score less than 12, which were regarded as at medium risk of bias. Regarding the case reports, all of them had a low risk of bias according to the JBI tool; Please refer to Supplementary Materials Tables S2 and S3 here.
3.3. Meta-Analysis
Meta-analysis is a statistical tool that combines the results of multiple studies to come up with an aggregate, more precise value of an effect. In the following sections, we report the results of our meta-analyses for the ORR, DCR, PR, SD, and PD.
3.3.1. ORR and DCR
Eleven studies with more than five patients, totaling 228 patients, reported data on the ORR. Using the fixed-effects model, the ORR was 14% (95% CI = 10–19%, I2 = 0%); see Figure 3A. The subgroup analysis did not show a difference between monotherapy and combination therapy (14%, 95% CI = 10–19%, I2 = 0% vs. 17%, 95% CI = 7–34%, I2 = 17%, respectively). The DCR was reported in 10 trials. The pooled DCR was 43% (95% CI = 37–50%, I2 = 13%); see Figure 3B. The DCR was not different between monotherapy and combination therapy (42%, 95% CI = 36–49%, I2 = 27% vs. 50%, 95% CI = 33–67%, I2 = 0%, respectively). Both the ORR and DCR were similar based on the type of drugs used; see Figures S1 and S2.

Figure 3.
Forest plots demonstrating (A) ORR and (B) DCR. ORR was 14% with no difference between monotherapy and combination therapy (14% vs. 17%, p-value = 0.66). DCR was 43% with no difference between monotherapy and combination therapy (42% vs. 50%, p-value = 0.44). Each red square represents the effect size of a study, and the black diamond represents the pooled effect size. ORR: objective response rate, DCR: disease control rate [16,17,21,23,24,25,29,30,31,32].
3.3.2. Best Overall Response
We evaluated each response rate individually; see Figure 4A–C. Interestingly, no patient achieved a complete response (CR). Partial response (PR) and stable disease (SD) were seen in 14% (95% CI = 10–19%, I2 = 0%) and 29% (95% CI = 19–41%, I2 = 54%), respectively. A total of 115 patients had progressive disease (PD) with a rate of 50% (95% CI = 44–57%, I2 = 53%). Whether patients received monotherapy or combination therapy did not change the response rates.
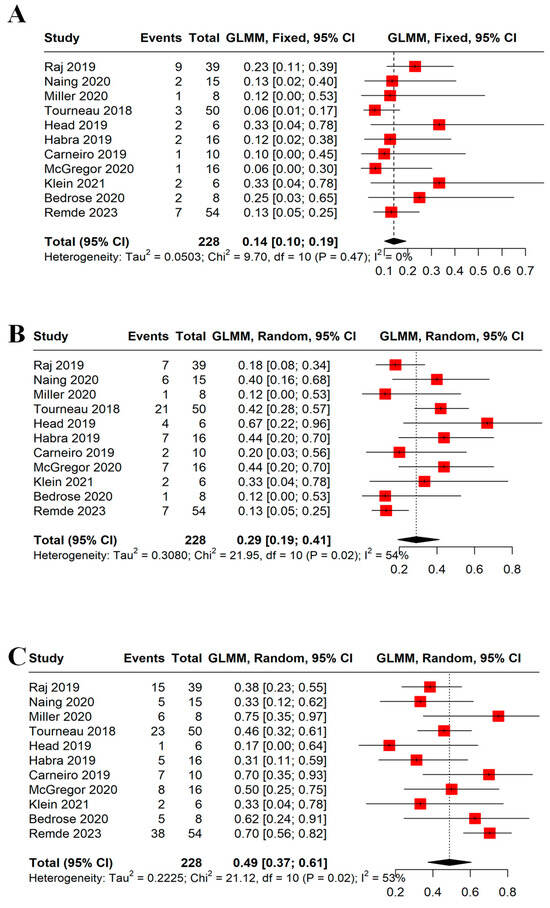
Figure 4.
Forest plots demonstrating (A) PR, (B) SD, and (C) PD. The PR, SD, and PD rates were 14%, 29%, and 49%, respectively. A random model was used for SD and PD due to significant heterogeneity (I2 > 50%). Each red square represents the effect size of a study and the black diamond represents the pooled effect size. PR: partial response, SD: stable disease, PD: progressive disease [16,17,21,23,24,25,29,30,31,32].
3.3.3. PFS and OS Results
The median OS (mOS) was reported in six arms with 173 patients. The pooled mOS was 13.9 months (95% CI = 7.85–23.05); see Figure 5A. On the other hand, eight arms with 194 patients had a median PFS (mPFS) of 2.8 months (95% CI = 1.8–5.4); see Figure 5B. The time-specific survival rates were mostly not reported or were reported at different intervals. Thus, we only analyzed the 6-month OS and PFS. The 6-month OS was reported in two studies and was 71% (95% CI = 59–82%, I2 = 0%); see Figure S3. Meanwhile, the 6-month PFS was reported by three trials and was 21% (95% CI = 14–30%, I2 = 0%); see Figure S4.
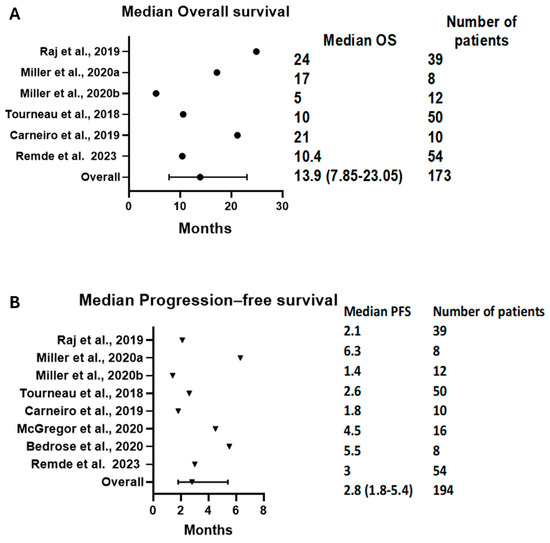
Figure 5.
Forest plots demonstrating (A) mOS and (B) mPFS. The pooled mOS was 13.9 (95% CI: 7.85–23.05), and the pooled mPFS was 2.8 (95% CI: 1.8–5.4). mOS: median overall survival, mPFS: median progression-free survival [16,17,23,24,29,31,32].
3.3.4. Adverse Events
The pooled grade 3/4 AEs rate were reported in eight trials and was 22% (95% CI = 9–45%, I2 = 60%); see Figure S5. Due to the heterogeneity of reporting AEs, we implemented unweighted pooled statistics for selected AEs, shown in Table 2. Fatigue was the most commonly reported all-grade adverse event in 24.6% of the patients, while elevated liver enzymes/hepatitis was the most common grade 3/4 adverse event.

Table 2.
Adverse events in ICI-based regimens. Adverse events reported in at least three studies were only pooled.
3.3.5. Publication Bias
Based on the DCR results, we found no evidence of publication bias (Begg’s p-value = 0.531 and Egger’s p-value = 0.213). However, the results based on the ORR showed the risk of publication bias (Begg’s p-value = 0.085 and Egger’s p-value = 0.01); see Figure 6A,B. Thus, we conclude that publication bias may influence our results.
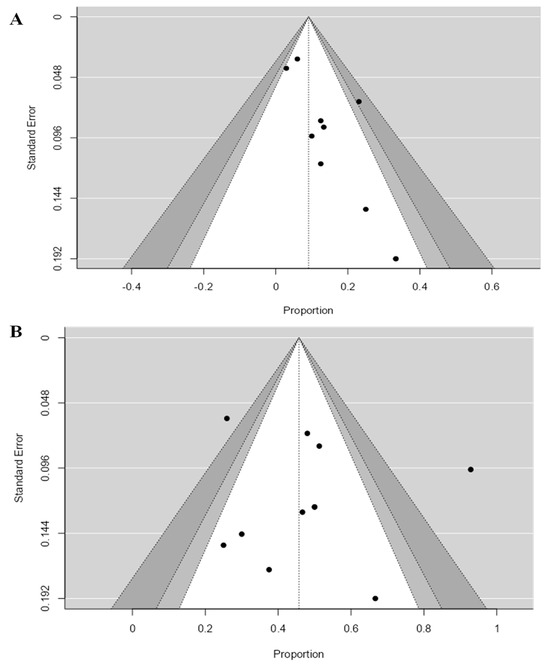
Figure 6.
Funnel plots evaluating publication bias of published studies that reported (A) ORR and (B) DCR. Egger’s test revealed the presence of publication bias in the case of ORR (p-value = 0.01) but no publication bias in the case of DCR (p-value = 0.213). ORR: objective response rate, DCR: disease control rate.
4. Discussion
The management of advanced/metastatic ACC remains a major challenge, and there is an urgent need for a novel treatment paradigm. ICIs have revolutionized the treatment of different malignancies. To the best of our knowledge, this is the first systematic review and meta-analysis to summarize the efficacy and tolerability of different ICI agents in patients with advanced/metastatic ACC. We found that the ORR and DCR were 14% and 43%, respectively. The use of combination strategies did not correlate with higher responses compared with monotherapy. Although the type of ICI did not significantly affect the response, avelumab had the lowest ORR of 6%. The current standard of care is mitotane and EDP, which has a 23% ORR based on the results of the FIRM-ACT trial. Interestingly, the pooled mPFS of ICIs in our study was numerically inferior to in the FIRM-ACT trial in the second-line setting (5.6 months vs. 2.8 months). However, the mOS of ICIs was similar to that of mitotane and EDP in the FIRM-ACT trial (14.8 months vs. 13.9 months). Although comparing ICI-based strategies with mitotane and EDP needs a well-conducted randomized clinical trial, we believe that ICIs could be used either after mitotane and EDP progression or for unacceptable adverse events. Other ongoing immunotherapy-based trials are summarized in Table 3.

Table 3.
Ongoing immunotherapy clinical trials. Trial status as of 29 September 2023. ACC: adrenocortical carcinoma, AEs: adverse events, DLT: dose-limiting toxicity, ORR: objective response rate, OS: overall survival, PFS: progression-free survival.
Although we aimed to study predictive biomarkers for ICI response, the scarcity and heterogeneity of reporting for these biomarkers prevented such analysis. In 2017, the FDA approved the use of pembrolizumab for the treatment of microsatellite-instability-high (MSI-H) solid malignancies [39]. Raj et al. found that out of the six patients treated with pembrolizumab, two patients achieved PR and another two achieved SD [16]. However, they found that tumor microsatellite status did not predict response. Interestingly, one patient with MSI-H died just one month after starting pembrolizumab. Similarly, one of the six cases in the Klein et al. study also had rapidly progressive disease and died within 12 weeks of the initiation of ipilimumab and nivolumab treatment [25]. We are not sure whether these patients fit the criteria of hyperprogression phenomena. However, oncologists should suspect hyperprogression phenomena in patients with rapidly progressive disease after ICIs [40].
No included trial found PD-L1 as a predictive biomarker for ACC. In theory, anti-PD-1 treatment can shut down both the PD-1/PD-L1 and PD-1/PD-L2 axes [41]. Clinically, anti-PD-1 treatment shows very similar responses to anti-PD-L1 treatment [42]. The use of avelumab (anti-PD-L1) had the worst ORR and DCR results. It is unclear why avelumab was associated with a poor response compared to other ICIs. A possible hypothesis could be that a high expression of PD-L1 assessed according to immunohistochemistry is only 3–11% while PD-L2 expression is about 44% in these tumors, which may mean that anti-PD-1 treatment may offer better blockage of the PD L1/L2 axis compared to anti-PD-L1 treatment [43,44]. Thus, future studies should also assess the expression and predictive role of PD-L2. In 2020, the FDA approved pembrolizumab for the treatment of high TMB solid tumors [45]. However, Raj et al. and Remde et al. found that TMB did not correlate with response in ACC [16,31]. Mota et al. reported two cases of TMB-H ACC treated with pembrolizumab. The patient who responded had an additional MSH2 mutation while the other who did not respond did not have any known deleterious mutations [33]. One of the cases in the Edenfield et al. study had a PD-L1 expression of 1%, MSS, a low TMB, and no actionable mutations in metastatic ACC, receiving durvalumab with tremelimumab, and had a sustained complete response at 18 months [27]. These results suggest the classical biomarkers of ICI response are of low benefit in ACC.
Cortisol-secreting ACC has been associated with CD8+ T cell suppression and decreased immune cell trafficking and tumor cell recognition [46,47,48]. High CTNNB1 expression of the Wnt pathway has also been linked to higher cortisol levels and immunotherapy resistance [47,49]. Thus, targeting WNT/β-catenin might bypass the adverse cortisol role and enhance immunotherapy response. Several preclinical studies have shown superior ICI responses when combined with WNT/β-catenin inhibitors [50,51,52]. Currently, we are waiting for the results of several early-phase clinical trials using ICI and WNT/β-catenin inhibitor combinations in advanced solid tumors (NCT03447470, NCT02675946, NCT04166721). In the Carneiro et al. study, all four patients with hormone-secreting ACC had a PD [23]. Habra et al. reported seven patients with hormone-secreting disease, with one patient achieving PR and another three achieving SD, while the other three had a PD [22]. Casey et al. reported a case of rapid progression in a 58-year-old female Lynch syndrome patient with cortisol-secreting ACC treated with pembrolizumab [34]. On the other hand, Weng et al. reported sustained stable disease in hormone-secreting ACC for about two years using a unique combination of mitotane, paraplatin, sintilimab, and etoposide [37]. In the Remde et al. study, four patients out of seven patients achieving PR had glucocorticoid-secreting tumors [31]. However, no association between glucocorticoid excess and OS was found. Alam et al. reported an interesting case of non-hormonally active ACC treated with pembrolizumab and mitotane and achieving a complete radiological response [36]. This case suggests a possible synergistic effect between mitotane and pembrolizumab even in the settings of hormonally inactive disease. In addition, many patients receive exogenous glucocorticoids as a supplement especially if they are being treated with mitotane. This might partially explain the global low response to ICIs. No study detailed patients’ glucocorticoid supplements. Although discounting these supplements is not realistic, dose modifications might be considered when using ICIs to balance the vital bodily need for glucocorticoids and effective disease control using ICIs.
In a systematic review and meta-analysis conducted by Zhou et al., the occurrence of adverse events was associated with a better ICI response [53]. Raj et al. noticed that all the responders in their study also had immune-related hepatitis [16]. Similarly, Klein et al. reported that all patients who achieved disease control from ICIs also had autoimmune hepatitis [25]. Importantly, mitotane can also cause elevated liver enzymes, and monitoring the LFT in patients receiving either mitotane, ICIs, or a combination of both is necessary [40]. Due to the typically limited sample size in ACC trials, it is difficult to investigate such a hypothesis.
Our study has several limitations. First, there is a lot of heterogeneity between studies in terms of the study designs and treatment strategies. Second, the number of patients was small. Third, publication bias was detected in studies reporting the ORR. Lastly, many studies did not report the OS or PFS or on possible predictive biomarkers such as PD-L1, TMB, and MSI. Thus, we could not perform a meta-analysis of them.
5. Conclusions
In conclusion, our systematic review and meta-analysis showed that immune checkpoint inhibitors (ICIs) have modest efficacy in patients with advanced adrenocortical carcinoma (ACC), with an ORR of 14% and a DCR of 49%. The use of combination strategies did not correlate with higher responses compared with monotherapy. Although the type of ICI did not significantly affect the response, avelumab had the lowest ORR. We could not find any predictive factor for ICI response. ICIs could be used either after mitotane and EDP progression or in patients with poor tolerance to EDP. Designing interventional studies for rare cancers like ACC can be challenging and often below the feasibility threshold of prospective clinical trials. Based on our data, we could postulate that a combination of ICI-based therapies could be a promising therapeutic option in this disease space.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers16050900/s1, Figure S1: ORR based on the used treatment [16,17,21,23,24,25,29,30,31,32]. Figure S2: ORR based on the used treatment [16,17,21,23,24,25,29,30,31,32]. Figure S3: 6-month overall survival [17,23]. Figure S4: 6-month progression-free survival [16,17,23]. Figure S5: Grade III/IV adverse events [16,17,23,25,30,31,32]. Table S1: Search algorithm. Table S2: Quality appraisal of included studies according to the MINORS assessment tool. Table S3: Quality appraisal of included case report studies according to JBI critical appraisal checklist [33,34,35,36,37,38].
Author Contributions
Conceptualization, O.A.; methodology, O.A.; formal analysis, O.A.; investigation, A.G., M.A. and A.B.-H.; data curation, A.G., M.A. and S.A.M.; writing—draft preparation, O.A., M.A., A.B.-H. and A.S.; writing—review and editing, N.A. and A.S.; review of data and analysis, review of the manuscript, visualization, O.A. and M.A.; supervision, N.A. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon reasonable request.
Conflicts of Interest
Aditya Shreenivas is an advisory board member for Taiho Oncology and Iylon Pvt. Ltd., receives research support from Natera and Caris, and is a member of the virtual tumor board Target Cancer Foundation. All the other authors declare no conflicts of interest.
References
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef]
- Fassnacht, M.; Dekkers, O.M.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef]
- Fassnacht, M.; Terzolo, M.; Allolio, B.; Baudin, E.; Haak, H.; Berruti, A.; Welin, S.; Schade-Brittinger, C.; Lacroix, A.; Jarzab, B.; et al. Combination Chemotherapy in Advanced Adrenocortical Carcinoma. N. Engl. J. Med. 2012, 366, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, T.J.; Gillis, A.; Alobuia, W.M.; Wild, H.; Kebebew, E. Surgery for adrenocortical carcinoma: When and how? Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101408. [Google Scholar] [CrossRef] [PubMed]
- Paragliola, R.M.; Torino, F.; Papi, G.; Locantore, P.; Pontecorvi, A.; Corsello, S.M. Role of Mitotane in Adrenocortical Carcinoma—Review and State of the art. Eur. Endocrinol. 2018, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Nehs, M.; Kilbridge, K. Treatment of Adrenocortical Carcinoma. Surg. Pathol. Clin. 2019, 12, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.S.; Ahmad, T.; Groll, J.; Scherf-Clavel, O.; Kroiss, M.; Luxenhofer, R. The Challenging Pharmacokinetics of Mitotane: An Old Drug in Need of New Packaging. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 575. [Google Scholar] [CrossRef] [PubMed]
- Domènech, M.; Grau, E.; Solanes, A.; Izquierdo, A.; del Valle, J.; Carrato, C.; Pineda, M.; Dueñas, N.; Pujol, M.; Lázaro, C.; et al. Characteristics of Adrenocortical Carcinoma Associated with Lynch Syndrome. J. Clin. Endocrinol. Metab. 2021, 106, 318–325. [Google Scholar] [CrossRef]
- Gambichler, T.; Gambichler, T.; Finis, C.; Finis, C.; Abu Rached, N.; Abu Rached, N.; Scheel, C.H.; Scheel, C.H.; Becker, J.C.; Becker, J.C.; et al. Expression of DNA mismatch repair proteins in melanoma patients treated with immune checkpoint inhibitors. J. Cancer Res. Clin. Oncol. 2023, 149, 1241–1247. [Google Scholar] [CrossRef]
- Ellis, H.; Klempner, S.J.; Quintanilha, J.C.F.C.F.; Myer, P.; Lin, D.I.; Panarelli, N.; Fisher, V.; Li, G.; Huang, R.S.; Ross, J.S.; et al. Microsatellite instability (MSI), mismatch repair (MMR), and tumor mutational burden (TMB) as predictive biomarkers for immune checkpoint inhibitor (ICI) effectiveness in real-world patients with metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2023, 41, 46. [Google Scholar] [CrossRef]
- Yamauchi, M.; Ono, A.; Amioka, K.; Fujii, Y.; Nakahara, H.; Teraoka, Y.; Uchikawa, S.; Fujino, H.; Nakahara, T.; Murakami, E.; et al. Lenvatinib activates anti-tumor immunity by suppressing immunoinhibitory infiltrates in the tumor microenvironment of advanced hepatocellular carcinoma. Commun. Med. 2023, 3, 152. [Google Scholar] [CrossRef]
- Baechle, J.J.; Hanna, D.N.; Sekhar, K.R.; Rathmell, J.C.; Rathmell, W.K.; Baregamian, N. Integrative computational immunogenomic profiling of cortisol-secreting adrenocortical carcinoma. J. Cell. Mol. Med. 2021, 25, 10061–10072. [Google Scholar] [CrossRef]
- Landwehr, L.-S.; Altieri, B.; Schreiner, J.; Sbiera, I.; Weigand, I.; Kroiss, M.; Fassnacht, M.; Sbiera, S. Interplay between glucocorticoids and tumor-infiltrating lymphocytes on the prognosis of adrenocortical carcinoma. J. Immunother. Cancer 2020, 8, 469. [Google Scholar] [CrossRef]
- Guven, D.C.; Stephen, B.; Sahin, T.K.; Cakir, I.Y.; Erul, E.; Aksoy, S. The efficacy of immune checkpoint inhibitors in rare tumors: A systematic review of published clinical trials. Crit. Rev. Oncol./Hematol. 2022, 174, 103700. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Consoli, F.; Ghidini, A.; Perego, G.; Luciani, A.; Mercurio, P.; Berruti, A.; Grisanti, S. Efficacy of Immune Checkpoint Inhibitors in Rare Tumours: A Systematic Review. Front. Immunol. 2021, 12, 720748. [Google Scholar] [CrossRef] [PubMed]
- Raj, N.; Zheng, Y.; Kelly, V.; Katz, S.S.; Chou, J.; Do, R.K.G.; Capanu, M.; Zamarin, D.; Saltz, L.B.; Ariyan, C.E.; et al. PD-1 Blockade in Advanced Adrenocortical Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 71–80. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Hoimes, C.; Zarwan, C.; Wong, D.J.; Bauer, S.; Claus, R.; Wermke, M.; Hariharan, S.; von Heydebreck, A.; Kasturi, V.; et al. Avelumab in patients with previously treated metastatic adrenocortical carcinoma: Phase 1b results from the JAVELIN solid tumor trial. J. ImmunoTherapy Cancer 2018, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Database Syst. Rev. Implement. Rep. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- McGrath, S.; Sohn, H.; Steele, R.; Benedetti, A. Meta-analysis of the difference of medians. Biom. J. Biom. Z. 2020, 62, 69–98. [Google Scholar] [CrossRef]
- Naing, A.; Meric-Bernstam, F.; Stephen, B.; Karp, D.D.; Hajjar, J.; Ahnert, J.R.; Piha-Paul, S.A.; Colen, R.R.; Jimenez, C.; Raghav, K.P.; et al. Phase 2 study of pembrolizumab in patients with advanced rare cancers. J. ImmunoTherapy Cancer 2020, 8, e000347. [Google Scholar] [CrossRef] [PubMed]
- Habra, M.A.; Stephen, B.; Campbell, M.; Hess, K.; Tapia, C.; Xu, M.; Rodon Ahnert, J.; Jimenez, C.; Lee, J.E.; Perrier, N.D.; et al. Phase II clinical trial of pembrolizumab efficacy and safety in advanced adrenocortical carcinoma. J. ImmunoTherapy Cancer 2019, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; Konda, B.; Costa, R.B.; Costa, R.L.B.; Sagar, V.; Gursel, D.B.; Kirschner, L.S.; Chae, Y.K.; Abdulkadir, S.A.; Rademaker, A.; et al. Nivolumab in Metastatic Adrenocortical Carcinoma: Results of a Phase 2 Trial. J. Clin. Endocrinol. Metab. 2019, 104, 6193–6200. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.A.; Campbell, M.T.; Xie, W.; Farah, S.; Bilen, M.A.; Schmidt, A.L.; Sonpavde, G.P.; Kilbridge, K.L.; Choudhury, A.D.; Mortazavi, A.; et al. Results of a multicenter, phase 2 study of nivolumab and ipilimumab for patients with advanced rare genitourinary malignancies. Cancer 2021, 127, 840–849. [Google Scholar] [CrossRef]
- Klein, O.; Senko, C.; Carlino, M.S.; Markman, B.; Jackett, L.; Gao, B.; Lum, C.; Kee, D.; Behren, A.; Palmer, J.; et al. Combination immunotherapy with ipilimumab and nivolumab in patients with advanced adrenocortical carcinoma: A subgroup analysis of CA209-538. Oncoimmunology 2021, 10, 1908771. [Google Scholar] [CrossRef]
- Geoerger, B.; Kang, H.J.; Yalon-Oren, M.; Marshall, L.V.; Vezina, C.; Pappo, A.; Laetsch, T.W.; Petrilli, A.S.; Ebinger, M.; Toporski, J.; et al. Pembrolizumab in paediatric patients with advanced melanoma or a PD-L1-positive, advanced, relapsed, or refractory solid tumour or lymphoma (KEYNOTE-051): Interim analysis of an open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2020, 21, 121–133. [Google Scholar] [CrossRef]
- Edenfield, W.J.; Chung, K.; O’Rourke, M.; Cull, E.; Martin, J.; Bowers, H.; Smith, W.; Gluck, W.L. A Phase II Study of Durvalumab in Combination with Tremelimumab in Patients with Rare Cancers. Oncologist 2021, 26, e1499–e1507. [Google Scholar] [CrossRef]
- Sakamuri, D.; Glitza, I.C.; Cuellar, S.L.B.; Subbiah, V.; Fu, S.; Tsimberidou, A.M.; Wheler, J.J.; Hong, D.S.; Naing, A.; Falchook, G.S.; et al. Phase I dose-escalation study of anti–ctla-4 antibody ipilimumab and lenalidomide in patients with advanced cancers. Mol. Cancer Ther. 2018, 17, 671–676. [Google Scholar] [CrossRef]
- Miller, K.C.; Chintakuntlawar, A.V.; Hilger, C.; Bancos, I.; Morris, J.C.; Ryder, M.; Smith, C.Y.; Jenkins, S.M.; Bible, K.C. Salvage Therapy with Multikinase Inhibitors and Immunotherapy in Advanced Adrenal Cortical Carcinoma. J. Endocr. Soc. 2020, 4, bvaa069. [Google Scholar] [CrossRef]
- Head, L.; Kiseljak-Vassiliades, K.; Clark, T.J.; Somerset, H.; King, J.; Raeburn, C.; Albuja-Cruz, M.; Weyant, M.; Cleveland, J.; Wierman, M.E.; et al. Response to Immunotherapy in Combination with Mitotane in Patients with Metastatic Adrenocortical Cancer. J. Endocr. Soc. 2019, 3, 2295–2304. [Google Scholar] [CrossRef]
- Remde, H.; Schmidt-Pennington, L.; Reuter, M.; Landwehr, L.-S.; Jensen, M.; Lahner, H.; Kimpel, O.; Altieri, B.; Laubner, K.; Schreiner, J.; et al. Outcome of immunotherapy in adrenocortical carcinoma: A retrospective cohort study. Eur. J. Endocrinol. 2023, 188, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Bedrose, S.; Miller, K.C.; Altameemi, L.; Ali, M.S.; Nassar, S.; Garg, N.; Daher, M.; Eaton, K.D.; Yorio, J.T.; Daniel, D.B.; et al. Combined lenvatinib and pembrolizumab as salvage therapy in advanced adrenal cortical carcinoma. J. ImmunoTherapy Cancer 2020, 8, e001009. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.M.; Sousa, L.G.; Braghiroli, M.I.; Siqueira, L.T.; Neto, J.E.B.; Chapchap, P.; De Oliveira Hoff, A.A.; Hoff, P.M. Pembrolizumab for metastatic adrenocortical carcinoma with high mutational burden Two case reports. Medicine 2018, 97, e13517. [Google Scholar] [CrossRef] [PubMed]
- Casey, R.; Giger, O.; Seetho, I.; Marker, A.; Pitfield, D.; Boyle, L.; Gurnell, M.; Shaw, A.; Tischkowitz, M.; Maher, E.; et al. Rapid disease progression in a patient with mismatch repair-deficient and cortisol secreting adrenocortical carcinoma treated with pembrolizumab. Semin. Oncol. 2018, 45, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Caccese, M.; Ceccato, F.; Fassan, M.; Fassina, A.; Padovan, M.; Mammi, I.; Iacobone, M.; Scaroni, C.; Zagonel, V.; Lombardi, G. Letter to Editor: Reply to R.T. Casey (Semin Oncol. 2018 Jun;45(3):151-155). Semin. Oncol. 2019, 46, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Alam, W.; Bouferraa, Y.; Haibe, Y.; Shamseddine, A. Complete Radiological Response of Recurrent Metastatic Adrenocortical Carcinoma to Pembrolizumab and Mitotane. Clin. Med. Insights Oncol. 2021, 15, 11795549211007682. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Wang, L.; Wang, X.-Y.; Fan, X.-X.; Yan, L.; Li, Z.-H.; Zhang, S.-L. Case report: Remarkable response to a novel combination of mitotane, etoposide, paraplatin, and sintilimab in a patient with metastatic adrenocortical carcinoma. Front. Endocrinol. 2023, 14, 1115893. [Google Scholar] [CrossRef]
- Charles, R.; Madhu, D.; Powles, A.; Boyde, A.; Hughes, O.; Kumar, N.; Moorcraft, S.Y. Case Report: Response to ipilimumab and nivolumab in a patient with adrenocortical carcinoma. Front. Oncol. 2023, 13, 1242560. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Brabo, E.P.; Moraes, A.B.; Neto, L.V. The role of immune checkpoint inhibitor therapy in advanced adrenocortical carcinoma revisited: Review of literature. J. Endocrinol. Investig. 2020, 43, 1531–1542. [Google Scholar] [CrossRef]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 273409. [Google Scholar] [CrossRef] [PubMed]
- Tartarone, A.; Roviello, G.; Lerose, R.; Roudi, R.; Aieta, M.; Zoppoli, P. Anti-PD-1 versus anti-PD-L1 therapy in patients with pretreated advanced non-small-cell lung cancer: A meta-analysis. Future Oncol. 2019, 15, 2423–2433. [Google Scholar] [CrossRef]
- Tierney, J.F.; Vogle, A.; Poirier, J.; Min, I.M.; Finnerty, B.; Zarnegar, R.; Pappas, S.G.; Scognamiglio, T.; Ghai, R.; Gattuso, P.; et al. Expression of programmed death ligand 1 and 2 in adrenocortical cancer tissues: An exploratory study. Surgery 2019, 165, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Fay, A.P.; Signoretti, S.; Callea, M.; Telό, G.H.; McKay, R.R.; Song, J.; Carvo, I.; Lampron, M.E.; Kaymakcalan, M.D.; Poli-De-Figueiredo, C.E.; et al. Programmed death ligand-1 expression in adrenocortical carcinoma: An exploratory biomarker study. J. ImmunoTherapy Cancer 2015, 3, 3. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Grisanti, S.; Cosentini, D.; Laganà, M.; Volta, A.D.; Palumbo, C.; Tiberio, G.A.M.; Sigala, S.; Berruti, A. The long and winding road to effective immunotherapy in patients with adrenocortical carcinoma. Future Oncol. 2020, 16, 3017–3020. [Google Scholar] [CrossRef]
- Fiorentini, C.; Grisanti, S.; Cosentini, D.; Abate, A.; Rossini, E.; Berruti, A.; Sigala, S. Molecular Drivers of Potential Immunotherapy Failure in Adrenocortical Carcinoma. J. Oncol. 2019, 2019, 6072863. [Google Scholar] [CrossRef] [PubMed]
- Baechle, J.J.; Hanna, D.N.; Konjeti, S.R.; Rathmell, J.C.; Rathmell, W.K.; Baregamian, N. Multiplatform Integrative Analyses of Immunosuppressive Signatures in Cortisol-secreting Adrenocortical Carcinoma. bioRxiv 2021. [Google Scholar] [CrossRef]
- Liu, S.; Ding, G.; Zhou, Z.; Feng, C. β-Catenin-driven adrenocortical carcinoma is characterized with immune exclusion. OncoTargets Ther. 2018, 11, 2029–2036. [Google Scholar] [CrossRef]
- Ganesh, S.; Shui, X.; Craig, K.P.; Park, J.; Wang, W.; Brown, B.D.; Abrams, M.T. RNAi-Mediated β-Catenin Inhibition Promotes T Cell Infiltration and Antitumor Activity in Combination with Immune Checkpoint Blockade. Mol. Ther. 2018, 26, 2567. [Google Scholar] [CrossRef]
- Haas, M.S.; Kagey, M.H.; Heath, H.; Schuerpf, F.; Rottman, J.B.; Newman, W. mDKN-01, a Novel Anti-DKK1 mAb, Enhances Innate Immune Responses in the Tumor Microenvironment. Mol. Cancer Res. 2021, 19, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, F.; Cheng, X.; Wang, J.; Zhang, W.; Zhang, B.; Tang, Y.; Li, Q.; Zhou, C.; Tu, S. Wnt Inhibition Sensitizes PD-L1 Blockade Therapy by Overcoming Bone Marrow-Derived Myofibroblasts-Mediated Immune Resistance in Tumors. Front. Immunol. 2021, 12, 619209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).