Expanding Broad Molecular Reflex Testing in Non-Small Cell Lung Cancer to Squamous Histology
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. DNA-Based Next-Generation Sequencing
2.3. RNA-Based Next-Generation Sequencing
2.4. Validation of Assays for DNA and RNA-Based Analyzes
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. DNA-Based NGS Results
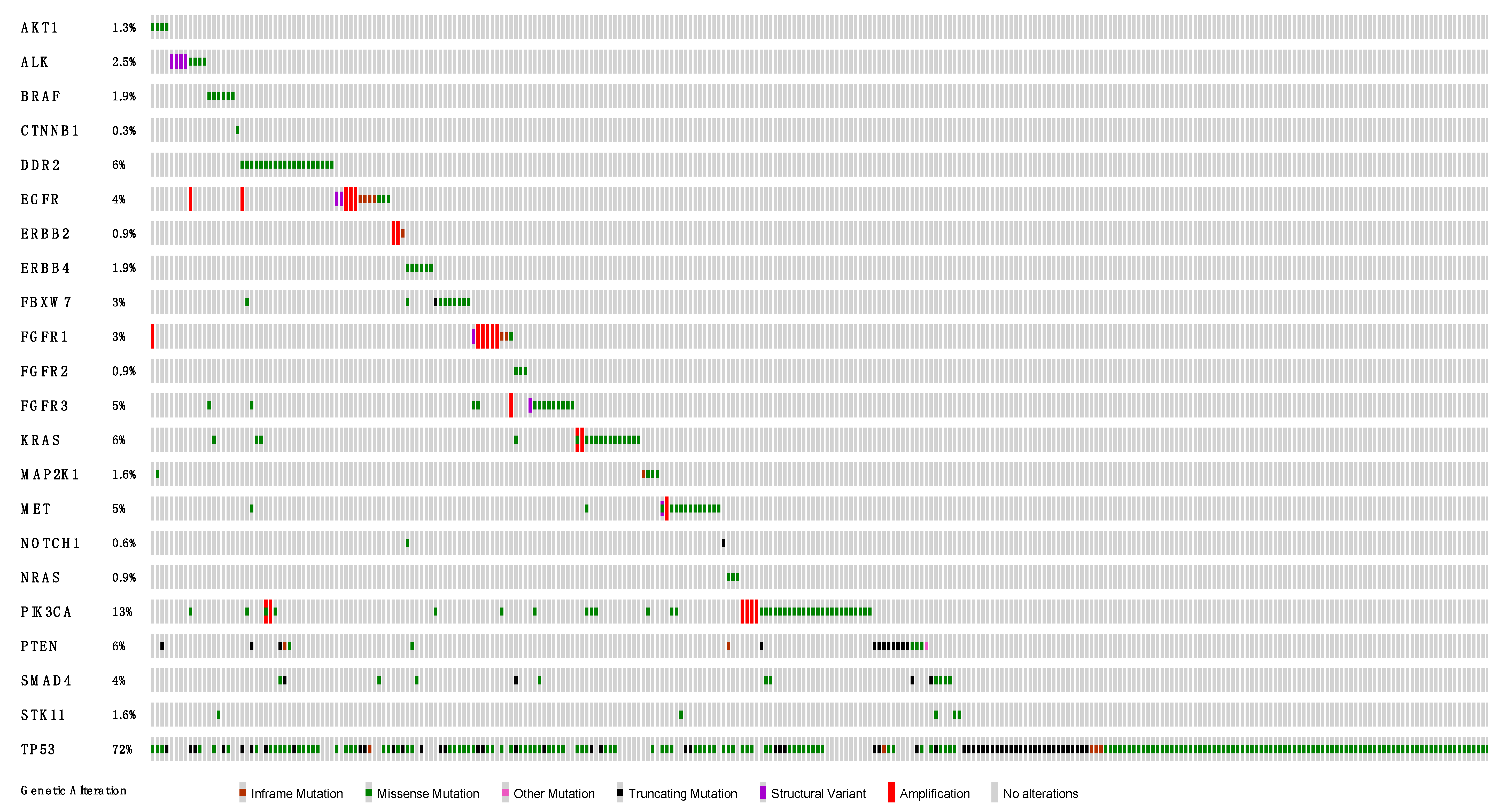
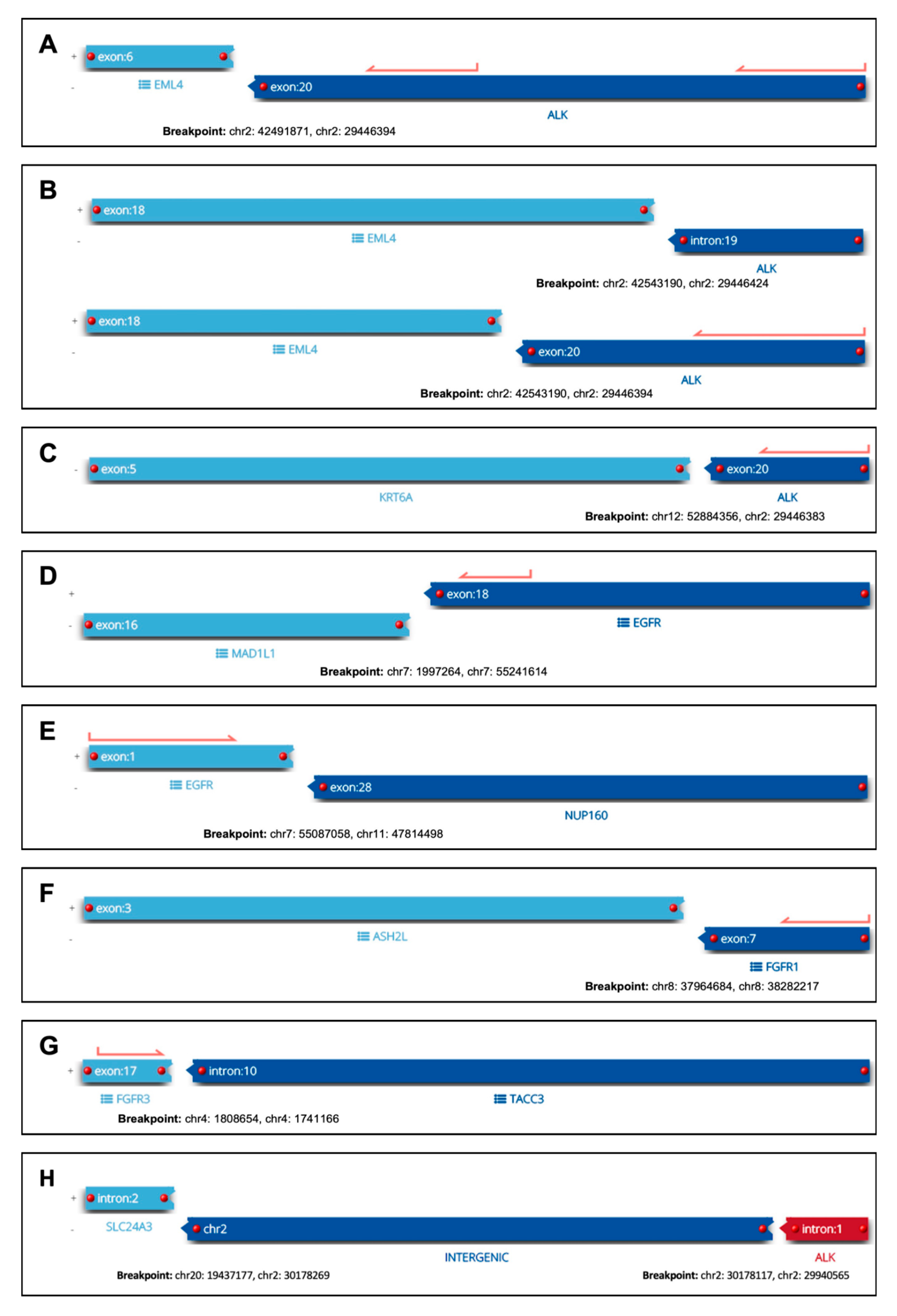
3.3. RNA-Based NGS Results
3.4. Detection of Already Established Therapeutic Targets in NSCLC
3.5. Detection of Emerging Therapeutic Targets in NSCLC
3.6. Correlation of PD-L1 Status with Established and Emerging Molecular Biomarkers
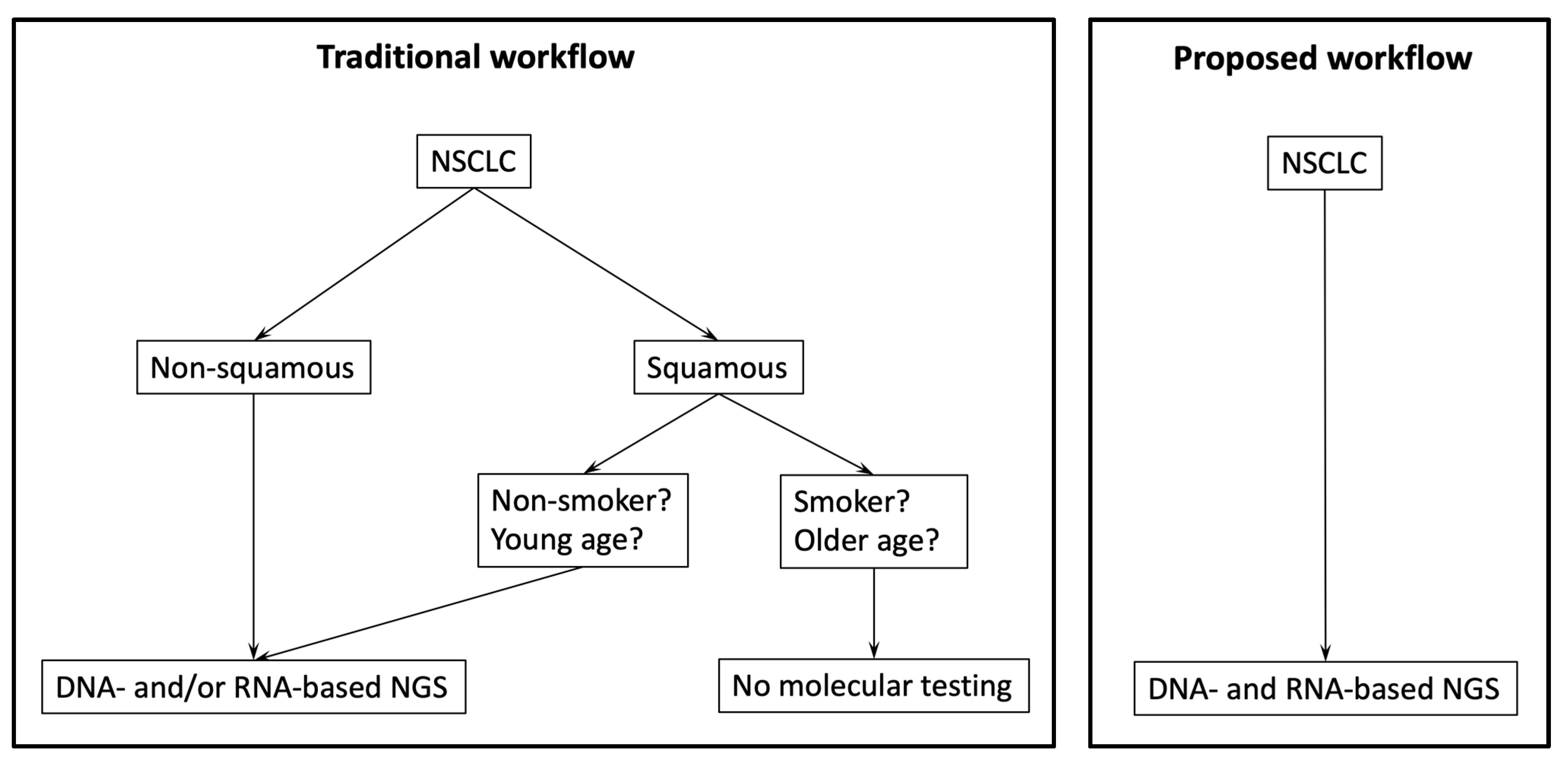
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camidge, D.R.; Doebele, R.C.; Kerr, K.M. Comparing and Contrasting Predictive Biomarkers for Immunotherapy and Targeted Therapy of NSCLC. Nat. Rev. Clin. Oncol. 2019, 16, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.M.; Bibeau, F.; Thunnissen, E.; Botling, J.; Ryška, A.; Wolf, J.; Öhrling, K.; Burdon, P.; Malapelle, U.; Büttner, R. The Evolving Landscape of Biomarker Testing for Non-Small Cell Lung Cancer in Europe. Lung Cancer 2021, 154, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Weir, B.A.; Woo, M.S.; Getz, G.; Perner, S.; Ding, L.; Beroukhim, R.; Lin, W.M.; Province, M.A.; Kraja, A.; Johnson, L.A.; et al. Characterizing the Cancer Genome in Lung Adenocarcinoma. Nature 2007, 450, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Devarakonda, S.; Morgensztern, D.; Govindan, R. Genomic Alterations in Lung Adenocarcinoma. Lancet Oncol. 2015, 16, e342–e351. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Lindeman, N.I.; Cagle, P.T.; Aisner, D.L.; Arcila, M.E.; Beasley, M.B.; Bernicker, E.H.; Colasacco, C.; Dacic, S.; Hirsch, F.R.; Kerr, K.; et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors Guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J. Thorac. Oncol. 2018, 13, 323–358. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non–Small Cell Lung Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 497–530. [Google Scholar] [CrossRef]
- Zacharias, M.; Absenger, G.; Kashofer, K.; Wurm, R.; Lindenmann, J.; Terbuch, A.; Konjic, S.; Sauer, S.; Gollowitsch, F.; Gorkiewicz, G.; et al. Reflex Testing in Non-Small Cell Lung Carcinoma Using DNA- and RNA-Based next-Generation Sequencing—A Single-Center Experience. Transl. Lung Cancer Res. 2021, 10, 4221–4234. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Saalfeld, F.C.; Wenzel, C.; Aust, D.E.; Wermke, M. Targeted Therapy in BRAF p.K601E-Driven NSCLC: Case Report and Literature Review. JCO Precis. Oncol. 2020, 4, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Su, P.-L.; Lin, C.-Y.; Chen, Y.-L.; Chen, W.-L.; Lin, C.-C.; Su, W.-C. Durable Response to Combined Dabrafenib and Trametinib in a Patient with BRAF K601E Mutation-Positive Lung Adenocarcinoma: A Case Report. JTO Clin. Res. Rep. 2021, 2, 100202. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.; Berezowska, S.; Kazdal, D.; Mograbi, B.; Ilié, M.; Stenzinger, A.; Hofman, V. Current Challenges and Practical Aspects of Molecular Pathology for Non-Small Cell Lung Cancers. Virchows Arch. 2023, 1–14. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Gosney, J.R.; Paz-Ares, L.; Jänne, P.; Kerr, K.M.; Leighl, N.B.; Lozano, M.D.; Malapelle, U.; Mok, T.; Sheffield, B.S.; Tufman, A.; et al. Pathologist-Initiated Reflex Testing for Biomarkers in Non-Small-Cell Lung Cancer: Expert Consensus on the Rationale and Considerations for Implementation. ESMO Open 2023, 8, 101587. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Varghese, A.M.; Sima, C.S.; Moreira, A.L.; Ladanyi, M.; Kris, M.G.; Rekhtman, N. Response to Erlotinib in Patients with EGFR Mutant Advanced Non–Small Cell Lung Cancers with a Squamous or Squamous-like Component. Mol. Cancer Ther. 2012, 11, 2535–2540. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef]
- Pirker, R.; Prosch, H.; Popper, H.; Klepetko, W.; Dieckmann, K.; Burghuber, O.C.; Klikovits, T.; Hoda, M.A.; Zöchbauer-Müller, S.; Filipits, M. Lung Cancer in Austria. J. Thorac. Oncol. 2021, 16, 725–733. [Google Scholar] [CrossRef]
- Lau, S.C.M.; Pan, Y.; Velcheti, V.; Wong, K.K. Squamous Cell Lung Cancer: Current Landscape and Future Therapeutic Options. Cancer Cell 2022, 40, 1279–1293. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Wang, G.; Cui, Z.; Xiong, G.; Jiang, X.; Li, Y.; Li, W.; Han, B.; Chen, S.; Shi, B. FGFR3 Destabilizes PD-L1 Via NEDD4 to Control T Cell-Mediated Bladder Cancer Immune Surveillance. Cancer Res. 2021, 82, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Griesinger, F.; Eberhardt, W.; Nusch, A.; Reiser, M.; Zahn, M.-O.; Maintz, C.; Bernhardt, C.; Losem, C.; Stenzinger, A.; Heukamp, L.C.; et al. Biomarker Testing in Non-Small Cell Lung Cancer in Routine Care: Analysis of the First 3,717 Patients in the German Prospective, Observational, Nation-Wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021, 152, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Adib, E.; Nassar, A.H.; Alaiwi, S.A.; Groha, S.; Akl, E.W.; Sholl, L.M.; Michael, K.S.; Awad, M.M.; Jӓnne, P.A.; Gusev, A.; et al. Variation in Targetable Genomic Alterations in Non-Small Cell Lung Cancer by Genetic Ancestry, Sex, Smoking History, and Histology. Genome Med. 2022, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Sands, J.M.; Nguyen, T.; Shivdasani, P.; Sacher, A.G.; Cheng, M.L.; Alden, R.S.; Jänne, P.A.; Kuo, F.C.; Oxnard, G.R.; Sholl, L.M. Next-Generation Sequencing Informs Diagnosis and Identifies Unexpected Therapeutic Targets in Lung Squamous Cell Carcinomas. Lung Cancer 2020, 140, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kron, A.; Scheffler, M.; Heydt, C.; Ruge, L.; Schaepers, C.; Eisert, A.-K.; Merkelbach-Bruse, S.; Riedel, R.; Nogova, L.; Fischer, R.N.; et al. Genetic Heterogeneity of MET-Aberrant NSCLC and Its Impact on the Outcome of Immunotherapy. J. Thorac. Oncol. 2021, 16, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Zanwar, S.; Noronha, V.; Patil, V.M.; Chougule, A.; Kumar, R.; Janu, A.; Mahajan, A.; Kapoor, A.; Prabhash, K. EGFR Mutation in Squamous Cell Carcinoma of the Lung: Does It Carry the Same Connotation as in Adenocarcinomas? OncoTargets Ther. 2017, 10, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Thunnissen, E.; Weynand, B.; Udovicic-Gagula, D.; Brcic, L.; Szolkowska, M.; Hofman, P.; Smojver-Ježek, S.; Anttila, S.; Calabrese, F.; Kern, I.; et al. Lung Cancer Biomarker Testing: Perspective from Europe. Transl. Lung Cancer Res. 2020, 9, 887–897. [Google Scholar] [CrossRef]
- Chaft, J.E.; Rekhtman, N.; Ladanyi, M.; Riely, G.J. ALK-Rearranged Lung Cancer: Adenosquamous Lung Cancer Masquerading as Pure Squamous Carcinoma. J. Thorac. Oncol. 2012, 7, 768–769. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, Y.; Ye, T.; Zhao, Y.; Gao, Z.; Yuan, H.; Zheng, D.; Zheng, S.; Li, H.; Li, Y.; et al. Detection of Novel NRG1, EGFR, and MET Fusions in Lung Adenocarcinomas in the Chinese Population. J. Thorac. Oncol. 2019, 14, 2003–2008. [Google Scholar] [CrossRef]
- Konduri, K.; Gallant, J.-N.; Chae, Y.K.; Giles, F.J.; Gitlitz, B.J.; Gowen, K.; Ichihara, E.; Owonikoko, T.K.; Peddareddigari, V.; Ramalingam, S.S.; et al. EGFR Fusions as Novel Therapeutic Targets in Lung Cancer. Cancer Discov. 2016, 6, 601–611. [Google Scholar] [CrossRef]
- Sperandio, R.C.; Tostes, F.L.T.; Campregher, P.V.; Paes, V.R.; Moura, F.; Schvartsman, G. EGFR-RAD51 Fusion in Lung Adenocarcinoma with Systemic and Intracranial Response to Osimertinib: A Case Report and Review of the Literature. Lung Cancer 2022, 166, 94–97. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, W.; Xu, C.; Song, Z.; Du, K.; Chen, G.; Lv, T.; Song, Y. EGFR-RAD51 Fusion Variant in Lung Adenocarcinoma and Response to Erlotinib: A Case Report. Lung Cancer 2018, 115, 131–134. [Google Scholar] [CrossRef]
- Kazdal, D.; Hofman, V.; Christopoulos, P.; Ilié, M.; Stenzinger, A.; Hofman, P. Fusion-positive Non-small Cell Lung Carcinoma: Biological Principles, Clinical Practice, and Diagnostic Implications. Genes Chromosom. Cancer 2022, 61, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P.; Calabrese, F.; Kern, I.; Adam, J.; Alarcão, A.; Alborelli, I.; Anton, N.T.; Arndt, A.; Avdalyan, A.; Barberis, M.; et al. Real-World EGFR Testing Practices for Non-Small-Cell Lung Cancer by Thoracic Pathology Laboratories across Europe. ESMO Open 2023, 8, 101628. [Google Scholar] [CrossRef] [PubMed]
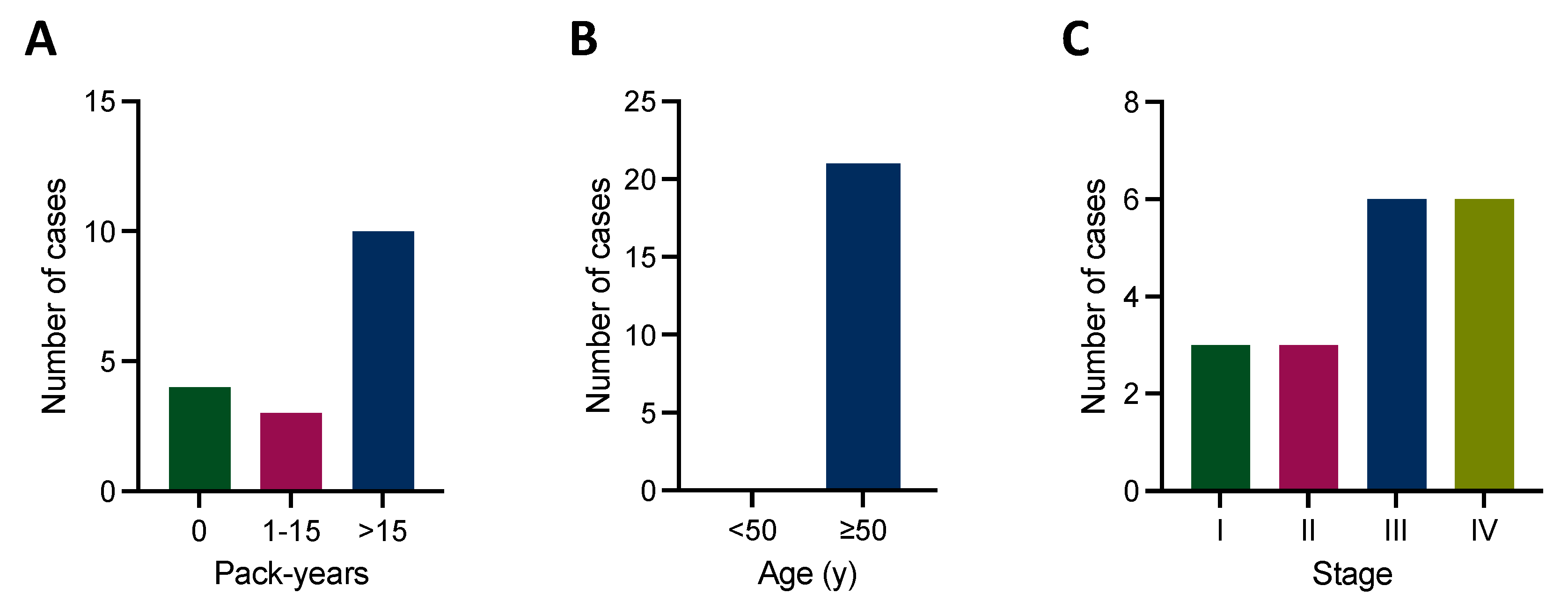

| Basic Characteristics | n = 316 |
|---|---|
| Age—median (range) | 68 (36–85) |
| Female | 105 (33.2%) |
| Male | 211 (66.8%) |
| Smoking status known | n = 242 |
| Smoker | 237 (97.9%) |
| Current smoker | 151 (63.7%) |
| Former smoker | 86 (36.3%) |
| Never smoker | 5 (2.1%) |
| Pack-years known | n = 201 |
| ≤15 pack-years | 15 (7.5%) |
| >15 pack-years | 186 (92.5%) |
| Pack-years median (range) | 40 (0–200) |
| Staging known | n = 247 |
| Stage I | 52 (21.1%) |
| Stage II | 41 (16.6%) |
| Stage III | 88 (35.6%) |
| Stage IV | 66 (26.7%) |
| Sample type | n = 316 |
| Resection specimen | 56 (17.7%) |
| Biopsy | 255 (80.7%) |
| Cytology/Cell block | 5 (1.6%) |
| Sample origin | n = 316 |
| Lung | 271 (85.8%) |
| Pleura | 2 (0.6%) |
| Lymph node | 24 (7.6%) |
| Distant metastasis | 19 (6%) |
| Case | Sex | Age | Smoker | PY | Stage | Molecular Target | Co-Mutations | PD-L1 (TPS) | Tested Specimen (Site/Type) | Subsequent Sample | Subsequent Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | f | 72 | yes (former) | 40 | IIB | EML4-ALK fusion | no | 5 | lung biopsy | lung resection | SCC |
| 2 | f | 61 | no | 0 | IVA | EML4-ALK fusion | no | 0 | lung biopsy | adrenal resection | ASC |
| 3 | f | 74 | yes (former) | 30 | IVA | KRT6A-ALK fusion | no | 0 | lung biopsy | no | NA |
| 4 | f | 75 | yes (former) | 30 | IIA | SLC24A3-ALK fusion | no | 10 | lung biopsy | lung resection | SCC |
| 5 | m | 76 | yes (current) | 50 | IB | EGFR-NUP160 fusion | TP53 p.H179R | 10 | lung biopsy | lung resection | SCC |
| 6 | f | 69 | no | 0 | IIA | MAD1L1-EGFR fusion | no | 0 | lung biopsy | lung resection | ASC |
| 7 | f | 70 | yes (former) | 15 | IVB | EGFR p.S768_D770dup | TP53 p.E298X | 10 | brain resection | no | NA |
| 8 | f | 70 | NA | NA | IIIB | EGFR p.L747_P753delinsS | no | 1 | lung biopsy | lung resection | ASC |
| 9 | f | 60 | yes (former) | 8 | IIIA | EGFR p.746_750del | TP53 p.177_182del | 60 | lung biopsy | no | NA |
| 10 | f | 67 | yes (current) | 20 | IVB | EGFR p.L747_P753delinsS | TP53 p.M246fs | 90 | lung biopsy | no | NA |
| 11 | f | 81 | no (current) | 0 | IIIA | EGFR p.L858R | SMAD4 p.W524X | 20 | lung biopsy | lung resection | SCC |
| 12 | f | 69 | no | 0 | IB | EGFR p.L858R EGFR p.S768I | TP53 p.R213Q | 2 | lung biopsy | lung resection | SCC |
| 13 | m | 61 | NA | NA | NA | BRAF p.K601E | CDKN2A p.25_31del STK11 p.A398V | NA | lung biopsy | no | NA |
| 14 | f | 55 | NA | NA | NA | KRAS p.G12C | TP53 p.V157F | NA | soft tissue biopsy | no | NA |
| 15 | f | 67 | yes (former) | 10 | IIIB | KRAS p.G12C | PIK3CA p.H1047R TP53 V225fs | 1 | lung biopsy | no | NA |
| 16 | m | 61 | NA | NA | NA | KRAS p.G12C | TP53 p.V274F | 70 | adrenal biopsy | no | NA |
| 17 | m | 70 | yes (current) | 50 | IA3 | KRAS p.G12C | TP53 p.R213L | 100 | lung biopsy | lung resection | SCC |
| 18 | f | 63 | yes (current) | 45 | IVB | KRAS p.G12C | no | 90 | soft tissue biopsy | no | NA |
| 19 | f | 66 | yes (current) | 35 | IVB | KRAS p.G12C | TP53 p.P190fs KEAP1 p.Q217X | 0 | lung biopsy | no | NA |
| 20 | f | 63 | yes (current) | 30 | IIIA | KRAS p.G12C | no | 10 | lung biopsy | lung resection | SCC |
| 21 | m | 73 | yes (current) | 50 | IIIA | METex14 skipping | TP53 p.P190L MET p.T1010I | 70 | lung biopsy | no | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacharias, M.; Konjic, S.; Kratochwill, N.; Absenger, G.; Terbuch, A.; Jost, P.J.; Wurm, R.; Lindenmann, J.; Kashofer, K.; Gollowitsch, F.; et al. Expanding Broad Molecular Reflex Testing in Non-Small Cell Lung Cancer to Squamous Histology. Cancers 2024, 16, 903. https://doi.org/10.3390/cancers16050903
Zacharias M, Konjic S, Kratochwill N, Absenger G, Terbuch A, Jost PJ, Wurm R, Lindenmann J, Kashofer K, Gollowitsch F, et al. Expanding Broad Molecular Reflex Testing in Non-Small Cell Lung Cancer to Squamous Histology. Cancers. 2024; 16(5):903. https://doi.org/10.3390/cancers16050903
Chicago/Turabian StyleZacharias, Martin, Selma Konjic, Nikolaus Kratochwill, Gudrun Absenger, Angelika Terbuch, Philipp J. Jost, Robert Wurm, Jörg Lindenmann, Karl Kashofer, Franz Gollowitsch, and et al. 2024. "Expanding Broad Molecular Reflex Testing in Non-Small Cell Lung Cancer to Squamous Histology" Cancers 16, no. 5: 903. https://doi.org/10.3390/cancers16050903
APA StyleZacharias, M., Konjic, S., Kratochwill, N., Absenger, G., Terbuch, A., Jost, P. J., Wurm, R., Lindenmann, J., Kashofer, K., Gollowitsch, F., Gorkiewicz, G., & Brcic, L. (2024). Expanding Broad Molecular Reflex Testing in Non-Small Cell Lung Cancer to Squamous Histology. Cancers, 16(5), 903. https://doi.org/10.3390/cancers16050903








