Proton Treatment Suppresses Exosome Production in Head and Neck Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Cell Culture
2.2.1. Reagents and Chemicals
2.2.2. Cal27 Cell Culture
2.2.3. Generation of Primary HNSCC Patient-Derived Keratinocyte Cell Cultures
2.3. Irradiation of HNSCC Cells
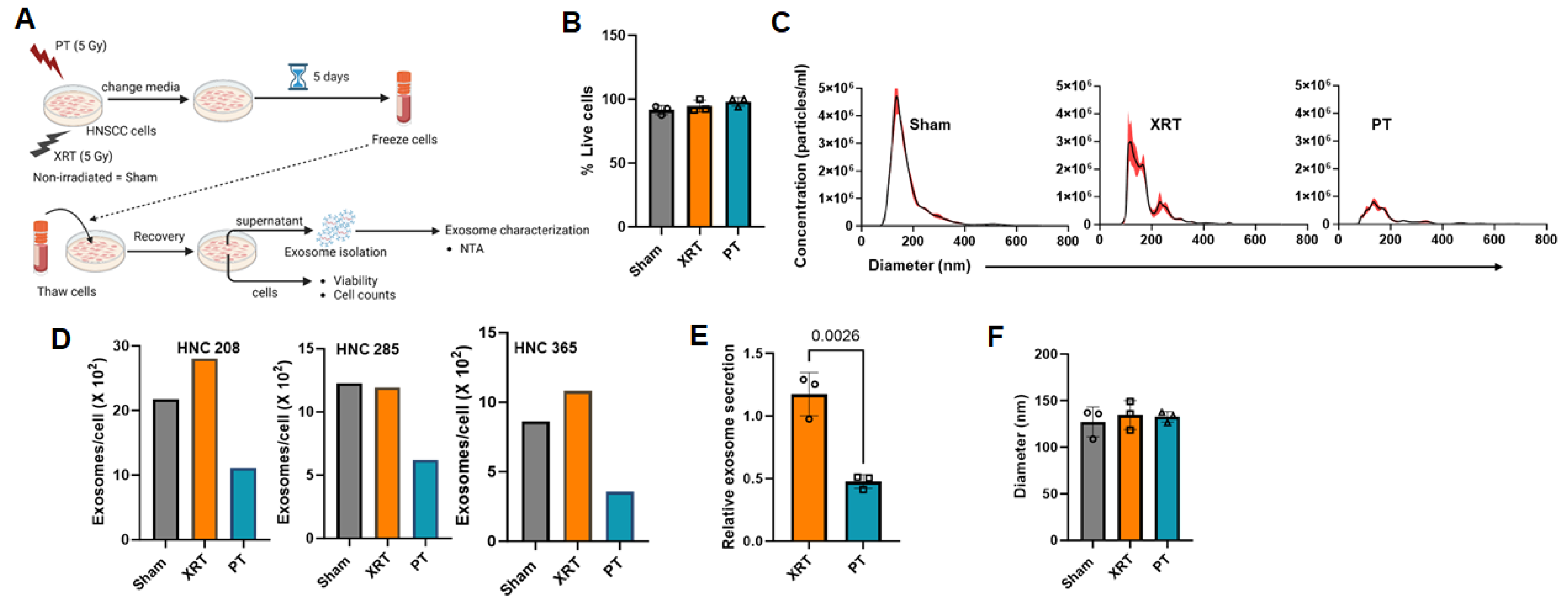
2.4. Cell Viability
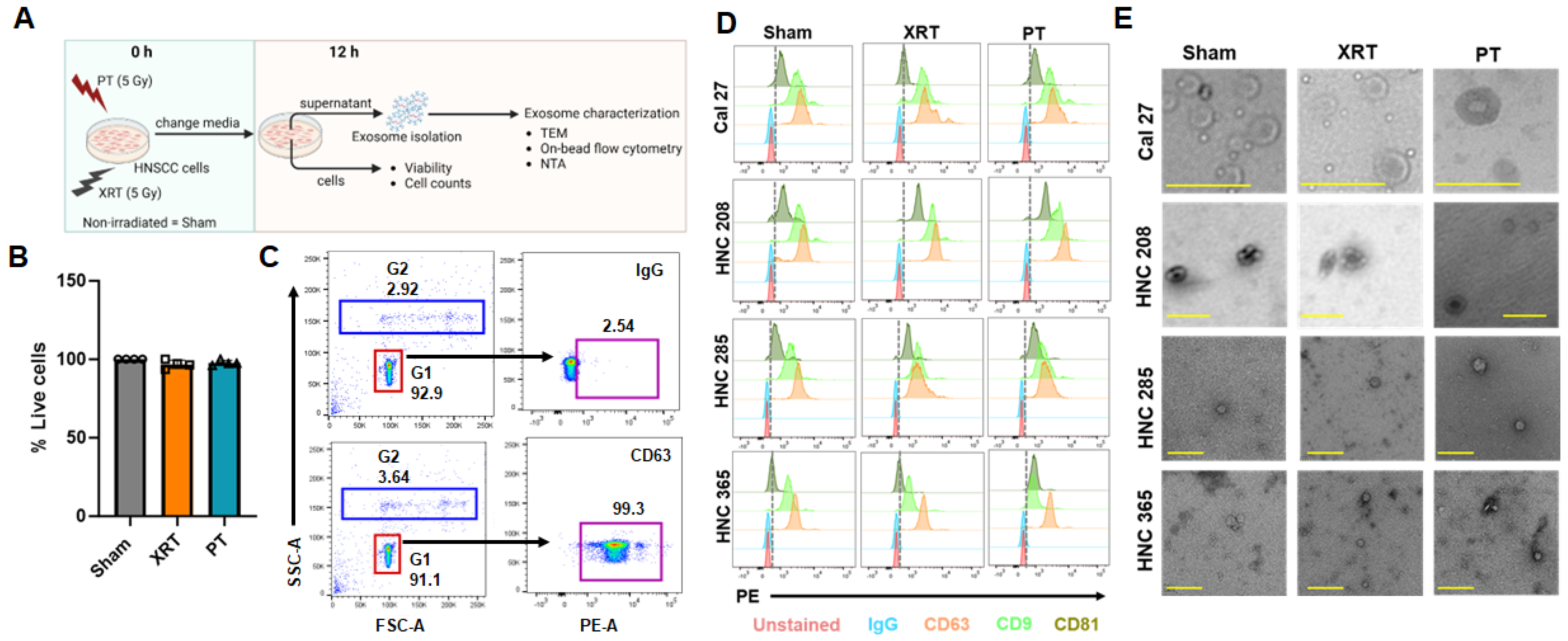
2.5. Exosome Isolation
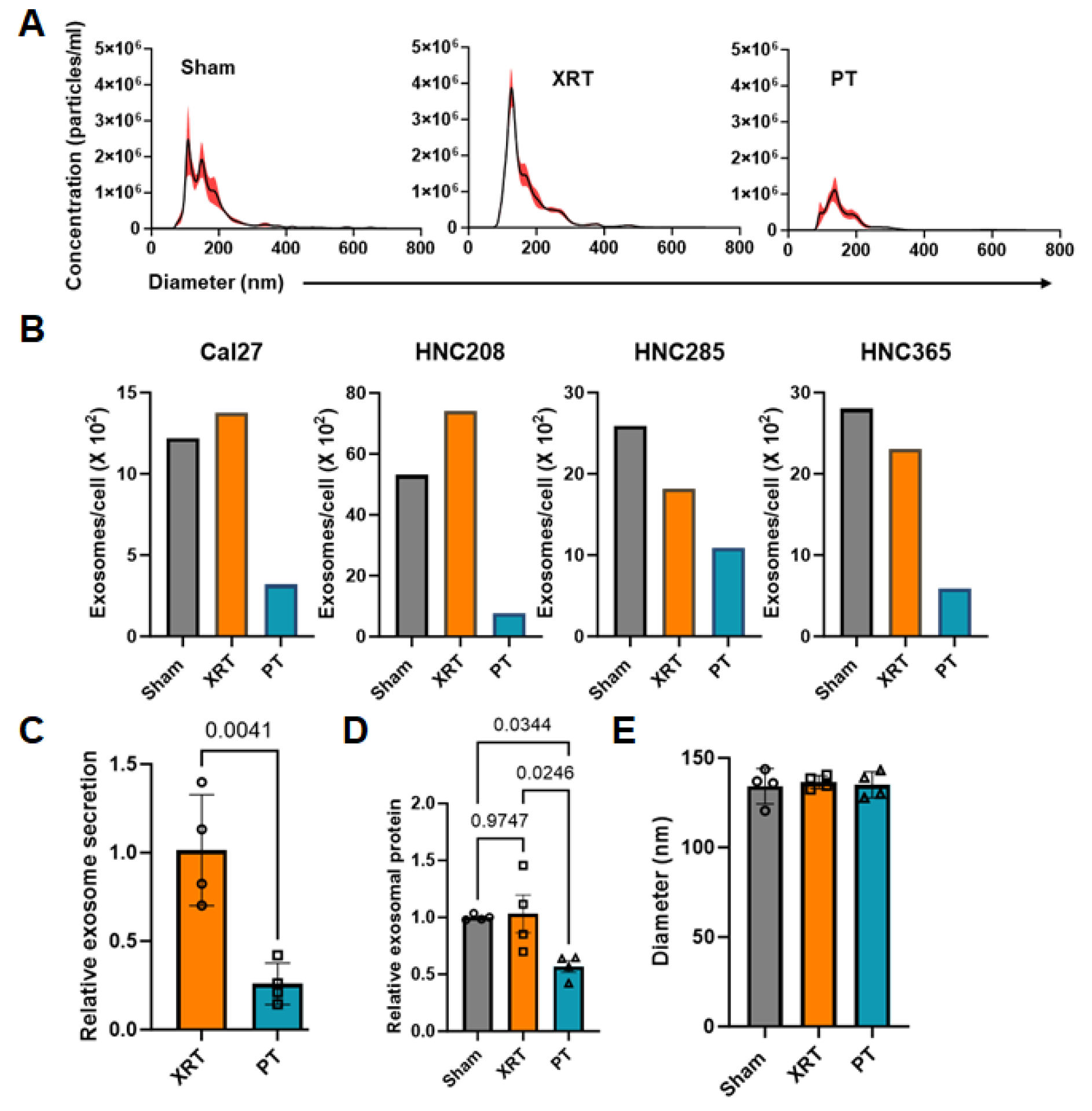
2.6. Nanoparticle Tracking Analysis
2.7. Exosome Protein Determination
2.8. On-Bead Flow Cytometry
2.9. Negative Staining and Transmission Electron Microscope Imaging
2.10. PBMC Isolation
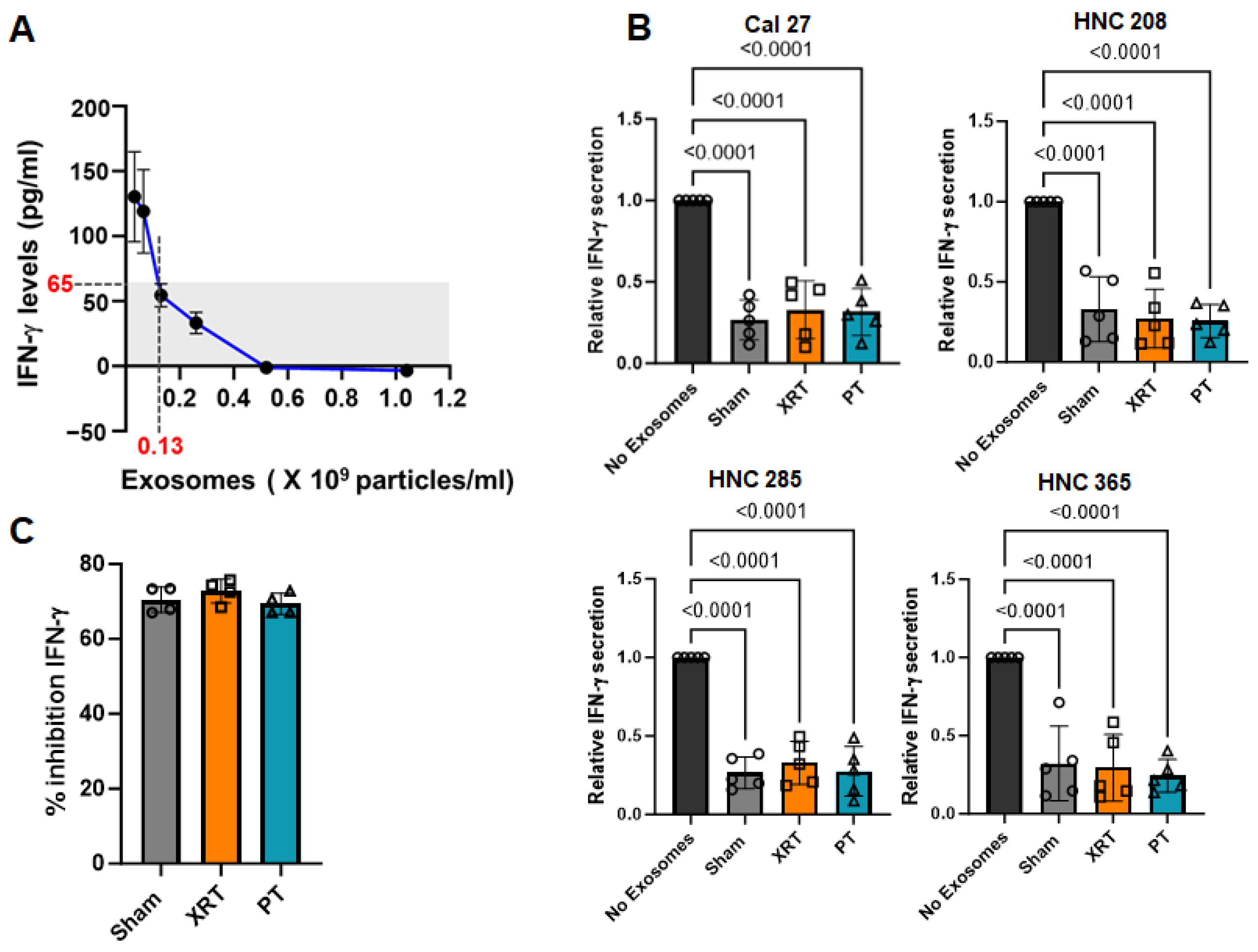
2.11. IFN-γ ELISA
2.12. Statistical Analysis
3. Results and Discussion
3.1. Proton Radiation Decrease Exosome Production by HNSCC Cells
3.2. Exosomes Derived from Irradiated HNSCC Cells Inhibited IFN-γ Production from PBMCs Irrespective of the Radiation Modality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nuyts, S.; Bollen, H.; Ng, S.P.; Corry, J.; Eisbruch, A.; Mendenhall, W.M.; Smee, R.; Strojan, P.; Ng, W.T.; Ferlito, A. Proton Therapy for Squamous Cell Carcinoma of the Head and Neck: Early Clinical Experience and Current Challenges. Cancers 2022, 14, 2587. [Google Scholar] [CrossRef] [PubMed]
- Elmusrati, A.; Wang, J.; Wang, C.-Y. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int. J. Oral Sci. 2021, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fossati, P.; Paganetti, H.; Ma, L.; Gillison, M.; Myers, J.N.; Hug, E.; Frank, S.J. The Biological Basis for Enhanced Effects of Proton Radiation Therapy Relative to Photon Radiation Therapy for Head and Neck Squamous Cell Carcinoma. Int. J. Part. Ther. 2021, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Franzese, C.; Lillo, S.; Cozzi, L.; Teriaca, M.A.; Badalamenti, M.; Di Cristina, L.; Vernier, V.; Stefanini, S.; Dei, D.; Pergolizzi, S.; et al. Predictive value of clinical and radiomic features for radiation therapy response in patients with lymph node-positive head and neck cancer. Head Neck 2023, 45, 1184–1193. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network: NCCN Guidelines for Head and Neck Cancer: Version 2. 2024. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437 (accessed on 23 February 2024).
- Gordon, K.B.; Smyk, D.I.; Gulidov, I.A. Proton Therapy in Head and Neck Cancer Treatment: State of the Problem and Development Prospects (Review). Sovrem Tekhnologii Med. 2021, 13, 70–80. [Google Scholar] [CrossRef]
- Baumann, B.C.; Mitra, N.; Harton, J.G.; Xiao, Y.; Wojcieszynski, A.P.; Gabriel, P.E.; Zhong, H.; Geng, H.; Doucette, A.; Wei, J.; et al. Comparative Effectiveness of Proton vs Photon Therapy as Part of Concurrent Chemoradiotherapy for Locally Advanced Cancer. JAMA Oncol. 2020, 6, 237–246. [Google Scholar] [CrossRef]
- Jumaniyazova, E.; Smyk, D.; Vishnyakova, P.; Fatkhudinov, T.; Gordon, K. Photon- and Proton-Mediated Biological Effects: What Has Been Learned? Life 2022, 13, 30. [Google Scholar] [CrossRef]
- Mirjolet, C.; Nicol, A.; Limagne, E.; Mura, C.; Richard, C.; Morgand, V.; Rousseau, M.; Boidot, R.; Ghiringhelli, F.; Noel, G.; et al. Impact of proton therapy on antitumor immune response. Sci. Rep. 2021, 11, 13444. [Google Scholar] [CrossRef]
- Parmar, K.; Mohamed, A.; Vaish, E.; Thawani, R.; Cetnar, J.; Thein, K.Z. Immunotherapy in head and neck squamous cell carcinoma: An updated review. Cancer Treat. Res. Commun. 2022, 33, 100649. [Google Scholar] [CrossRef]
- American Society for Radiation Oncology Model Policies: Proton Beam Therapy (pbt). Available online: https://www.astro.org/ASTRO/media/ASTRO/Daily%20Practice/PDFs/ASTROPBTModelPolicy.pdf (accessed on 8 September 2023).
- Lupu-Plesu, M.; Claren, A.; Martial, S.; N’Diaye, P.D.; Lebrigand, K.; Pons, N.; Ambrosetti, D.; Peyrottes, I.; Feuillade, J.; Hérault, J.; et al. Effects of proton versus photon irradiation on (lymph)angiogenic, inflammatory, proliferative and anti-tumor immune responses in head and neck squamous cell carcinoma. Oncogenesis 2017, 6, e354. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Han, S.; Zhu, J.; Li, Y.; Wang, Z.; Fan, Y.H.; Lin, E.; Zhang, R.; Sahoo, N.; et al. Patterns of protein expression in human head and neck cancer cell lines differ after proton vs photon radiotherapy. Head Neck 2020, 42, 289–301. [Google Scholar] [CrossRef]
- Du, J.; Kageyama, S.I.; Hirata, H.; Motegi, A.; Nakamura, M.; Hirano, Y.; Okumura, M.; Yamashita, R.; Tsuchihara, K.; Hojo, H.; et al. Comparative analysis of the immune responses in cancer cells irradiated with X-ray, proton and carbon-ion beams. Biochem. Biophys. Res. Commun. 2021, 585, 55–60. [Google Scholar] [CrossRef]
- Chirra, M.; Newton, H.S.; Gawali, V.S.; Wise-Draper, T.M.; Chimote, A.A.; Conforti, L. How the Potassium Channel Response of T Lymphocytes to the Tumor Microenvironment Shapes Antitumor Immunity. Cancers 2022, 14, 3564. [Google Scholar] [CrossRef]
- Chimote, A.A.; Balajthy, A.; Arnold, M.J.; Newton, H.S.; Hajdu, P.; Qualtieri, J.; Wise-Draper, T.; Conforti, L. A defect in KCa3.1 channel activity limits the ability of CD8(+) T cells from cancer patients to infiltrate an adenosine-rich microenvironment. Sci. Signal. 2018, 11, eaaq1616. [Google Scholar] [CrossRef]
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Whiteside, T.L. Evaluating tumor cell- and T cell-derived extracellular vesicles as potential biomarkers of cancer and immune cell competence. Expert Rev. Mol. Diagn. 2023, 23, 109–122. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Yerneni, S.; Gooding, W.E.; Ohr, J.; Clump, D.A.; Bauman, J.E.; Ferris, R.L.; Whiteside, T.L. Circulating exosomes measure responses to therapy in head and neck cancer patients treated with cetuximab, ipilimumab, and IMRT. Oncoimmunology 2019, 8, 1593805. [Google Scholar] [CrossRef]
- Ludwig, S.; Floros, T.; Theodoraki, M.N.; Hong, C.S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of Lymphocyte Functions by Plasma Exosomes Correlates with Disease Activity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef]
- Hofmann, L.; Ludwig, S.; Vahl, J.M.; Brunner, C.; Hoffmann, T.K.; Theodoraki, M.N. The Emerging Role of Exosomes in Diagnosis, Prognosis, and Therapy in Head and Neck Cancer. Int. J. Mol. Sci. 2020, 21, 4072. [Google Scholar] [CrossRef]
- Aheget, H.; Mazini, L.; Martin, F.; Belqat, B.; Marchal, J.A.; Benabdellah, K. Exosomes: Their Role in Pathogenesis, Diagnosis and Treatment of Diseases. Cancers 2020, 13, 84. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29. [Google Scholar] [CrossRef]
- Chimote, A.A.; Alshwimi, A.O.; Chirra, M.; Gawali, V.S.; Powers-Fletcher, M.V.; Hudock, K.M.; Conforti, L. Immune and ionic mechanisms mediating the effect of dexamethasone in severe COVID-19. Front. Immunol. 2023, 14, 1143350. [Google Scholar] [CrossRef]
- Göttgens, E.L.; Ostheimer, C.; Span, P.N.; Bussink, J.; Hammond, E.M. HPV, hypoxia and radiation response in head and neck cancer. Br. J. Radiol. 2019, 92, 20180047. [Google Scholar] [CrossRef]
- Langevin, S.; Kuhnell, D.; Parry, T.; Biesiada, J.; Huang, S.; Wise-Draper, T.; Casper, K.; Zhang, X.; Medvedovic, M.; Kasper, S. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget 2017, 8, 82459–82474. [Google Scholar] [CrossRef]
- Mutschelknaus, L.; Peters, C.; Winkler, K.; Yentrapalli, R.; Heider, T.; Atkinson, M.J.; Moertl, S. Exosomes Derived from Squamous Head and Neck Cancer Promote Cell Survival after Ionizing Radiation. PLoS ONE 2016, 11, e0152213. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Ratajczak, M.Z. Lung cancer secreted microvesicles: Underappreciated modulators of microenvironment in expanding tumors. Int. J. Cancer 2009, 125, 1595–1603. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-Associated Exosome Release from Human Prostate Cancer Cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef]
- Tau, G.; Rothman, P. Biologic functions of the IFN-gamma receptors. Allergy 1999, 54, 1233–1251. [Google Scholar] [CrossRef]
| HNC208 | HNC285 | HNC365 | |
|---|---|---|---|
| Age | 77 | 72 | 56 |
| Gender | Female | Male | Male |
| p16 status | Negative | Negative | Negative |
| Smoking | Non-smoker | Former, 75 pack years | Former, 19 pack years |
| Tumor grade | Well to moderately differentiated | Moderately differentiated | Moderately differentiated |
| Tumor stage | pT4aN0M0 | pT4a pN2bcM0 | pT4a pN3b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimote, A.A.; Lehn, M.A.; Bhati, J.; Mascia, A.E.; Sertorio, M.; Lamba, M.A.; Ionascu, D.; Tang, A.L.; Langevin, S.M.; Khodoun, M.V.; et al. Proton Treatment Suppresses Exosome Production in Head and Neck Squamous Cell Carcinoma. Cancers 2024, 16, 1008. https://doi.org/10.3390/cancers16051008
Chimote AA, Lehn MA, Bhati J, Mascia AE, Sertorio M, Lamba MA, Ionascu D, Tang AL, Langevin SM, Khodoun MV, et al. Proton Treatment Suppresses Exosome Production in Head and Neck Squamous Cell Carcinoma. Cancers. 2024; 16(5):1008. https://doi.org/10.3390/cancers16051008
Chicago/Turabian StyleChimote, Ameet A., Maria A. Lehn, Jay Bhati, Anthony E. Mascia, Mathieu Sertorio, Michael A. Lamba, Dan Ionascu, Alice L. Tang, Scott M. Langevin, Marat V. Khodoun, and et al. 2024. "Proton Treatment Suppresses Exosome Production in Head and Neck Squamous Cell Carcinoma" Cancers 16, no. 5: 1008. https://doi.org/10.3390/cancers16051008
APA StyleChimote, A. A., Lehn, M. A., Bhati, J., Mascia, A. E., Sertorio, M., Lamba, M. A., Ionascu, D., Tang, A. L., Langevin, S. M., Khodoun, M. V., Wise-Draper, T. M., & Conforti, L. (2024). Proton Treatment Suppresses Exosome Production in Head and Neck Squamous Cell Carcinoma. Cancers, 16(5), 1008. https://doi.org/10.3390/cancers16051008










